A Closer Look at Fractional Flow Reserve in Complex Anatomic Subsets: Left Main Disease, Bifurcation Lesions, and Saphenous Vein Grafts
David C. Lange, MD,1 Morton J. Kern, MD2
1Cedars-Sinai Medical Center, Division of Cardiology, Los Angeles, CA; 2University of California, Irvine and VA Long Beach, Irvine, CA
Fractional flow reserve (FFR) is a well-validated tool for determining the functional significance of a coronary artery stenosis, facilitating clinical decisions regarding the need for revascularization. FFR-guided revascularization improves clinical and economic outcomes. However, its application remains challenging in certain complex anatomic subsets, including left main coronary artery stenosis, bifurcation disease, and saphenous vein graft disease. This article reviews recent data supporting the use of FFR in these complex anatomic subsets.
[Rev Cardiovasc Med. 2016;17(1/2):7-15 doi: 10.3909/ricm0813]
© 2016 MedReviews®, LLC
A Closer Look at Fractional Flow Reserve in Complex Anatomic Subsets: Left Main Disease, Bifurcation Lesions, and Saphenous Vein Grafts
David C. Lange, MD,1 Morton J. Kern, MD2
1Cedars-Sinai Medical Center, Division of Cardiology, Los Angeles, CA; 2University of California, Irvine and VA Long Beach, Irvine, CA
Fractional flow reserve (FFR) is a well-validated tool for determining the functional significance of a coronary artery stenosis, facilitating clinical decisions regarding the need for revascularization. FFR-guided revascularization improves clinical and economic outcomes. However, its application remains challenging in certain complex anatomic subsets, including left main coronary artery stenosis, bifurcation disease, and saphenous vein graft disease. This article reviews recent data supporting the use of FFR in these complex anatomic subsets.
[Rev Cardiovasc Med. 2016;17(1/2):7-15 doi: 10.3909/ricm0813]
© 2016 MedReviews®, LLC
A Closer Look at Fractional Flow Reserve in Complex Anatomic Subsets: Left Main Disease, Bifurcation Lesions, and Saphenous Vein Grafts
David C. Lange, MD,1 Morton J. Kern, MD2
1Cedars-Sinai Medical Center, Division of Cardiology, Los Angeles, CA; 2University of California, Irvine and VA Long Beach, Irvine, CA
Fractional flow reserve (FFR) is a well-validated tool for determining the functional significance of a coronary artery stenosis, facilitating clinical decisions regarding the need for revascularization. FFR-guided revascularization improves clinical and economic outcomes. However, its application remains challenging in certain complex anatomic subsets, including left main coronary artery stenosis, bifurcation disease, and saphenous vein graft disease. This article reviews recent data supporting the use of FFR in these complex anatomic subsets.
[Rev Cardiovasc Med. 2016;17(1/2):7-15 doi: 10.3909/ricm0813]
© 2016 MedReviews®, LLC
KEY WORDS
Fractional flow reserve • Left main disease • Bifurcation disease • Saphenous vein graft disease
KEY WORDS
Fractional flow reserve • Left main disease • Bifurcation disease • Saphenous vein graft disease

… FFR provides an accurate assessment of the functional significance of an LM stenosis involving the ostium, mid segment, or distal LM bifurcation.
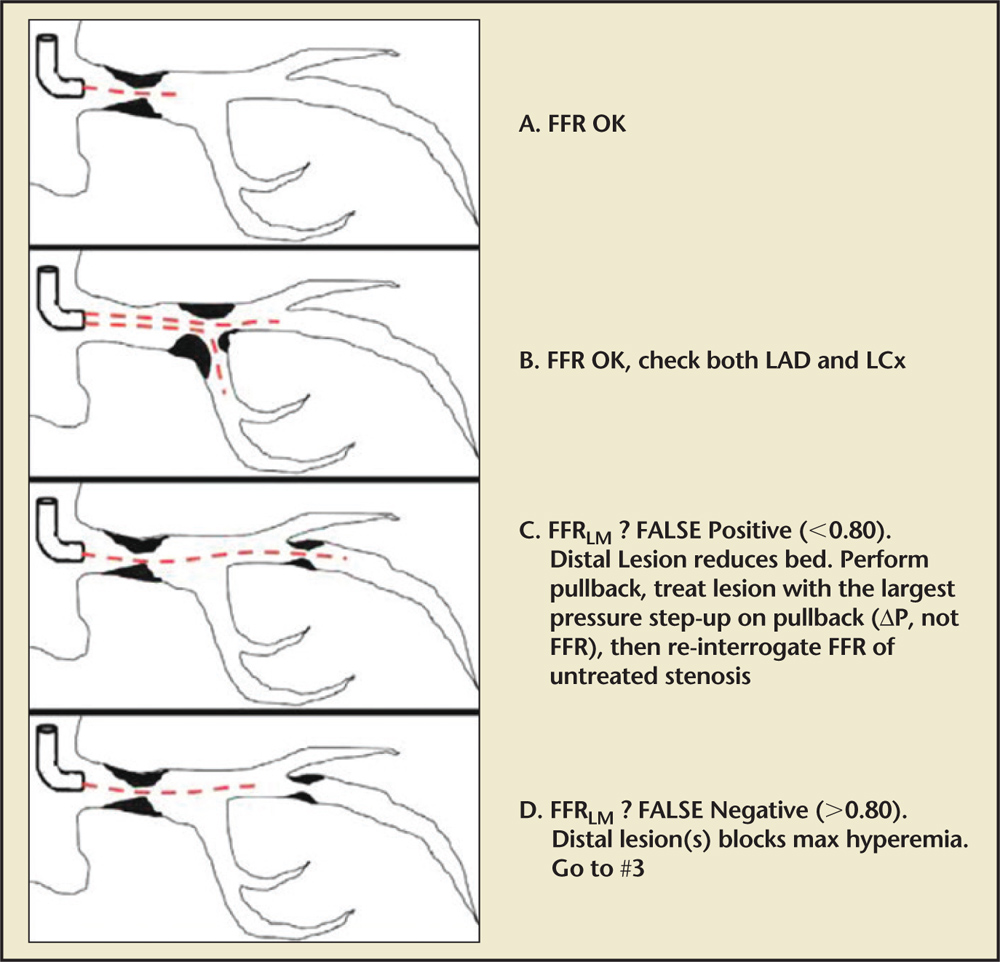
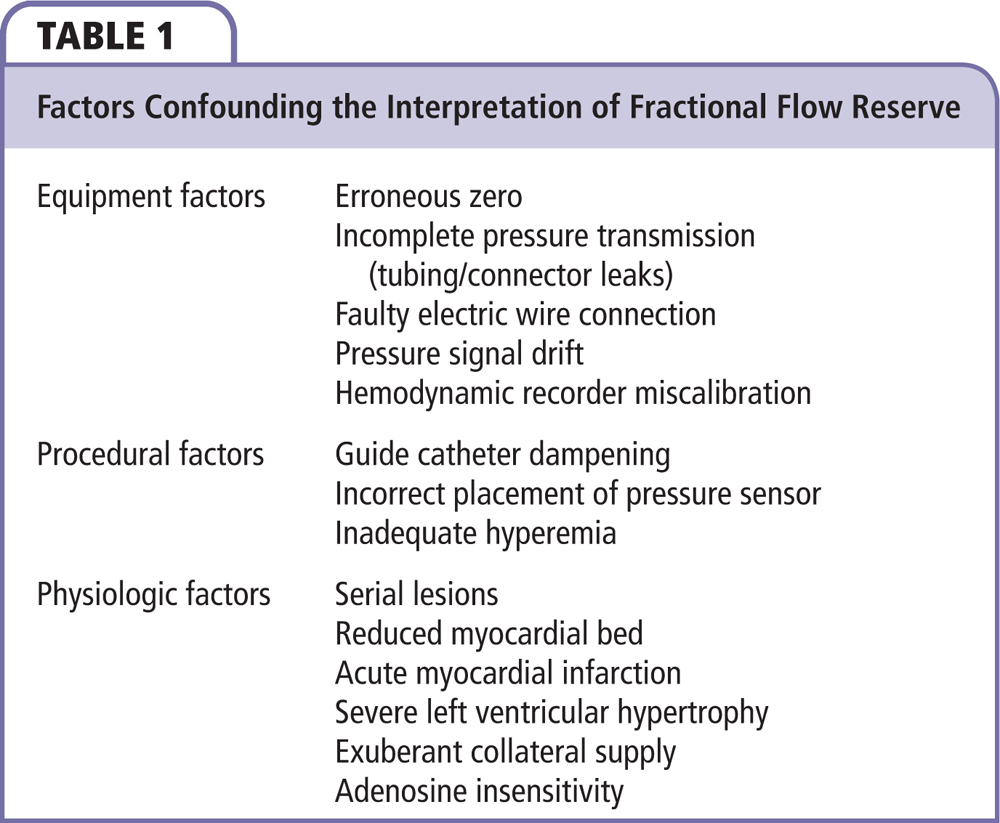
Figure 1. Performing FFR of LM disease. (A) Isolated LM stenosis: FFR can be performed per standard technique, revascularization recommended if FFRLM is ≤ 0.8. (B) LM bifurcation stenosis: FFR can be performed per standard technique but should be performed in both branch vessels (LAD and LCx), revascularization should be considered if FFRLM is ≤ 0.8 in either branch vessel. (C) LM stenosis with downstream disease: the summed FFR (LM + LAD = FFRepicardial) is measured to determine the need for treatment. If FFRepicardial is ≤ 0.8, a pressure pullback is performed to determine which lesion to treat first. The lesion with the larger pressure step-up on pullback (△P, not FFR) is treated first. Subsequently, the untreated lesion is again interrogated with FFR, with FFR ≤ 0.8 representing functionally significant lesions which warrant revascularization. (D) Inappropriate interrogation of FFRLM: the pressure drop across each lesion blunts the hyperemia of the other, thus, simple pressure ratios are no longer an accurate reflection of the functional significance of each individual lesion. LAD, left anterior descending artery; LCx, left circumflex artery; FFR, fractional flow reserve; FFRepicardial, summed FFR (LM + LAD); FFRLM, measurement of FFR across an LM stenosis; LM, left main coronary artery.

Figure 1. Performing FFR of LM disease. (A) Isolated LM stenosis: FFR can be performed per standard technique, revascularization recommended if FFRLM is ≤ 0.8. (B) LM bifurcation stenosis: FFR can be performed per standard technique but should be performed in both branch vessels (LAD and LCx), revascularization should be considered if FFRLM is ≤ 0.8 in either branch vessel. (C) LM stenosis with downstream disease: the summed FFR (LM + LAD = FFRepicardial) is measured to determine the need for treatment. If FFRepicardial is ≤ 0.8, a pressure pullback is performed to determine which lesion to treat first. The lesion with the larger pressure step-up on pullback (△P, not FFR) is treated first. Subsequently, the untreated lesion is again interrogated with FFR, with FFR ≤ 0.8 representing functionally significant lesions which warrant revascularization. (D) Inappropriate interrogation of FFRLM: the pressure drop across each lesion blunts the hyperemia of the other, thus, simple pressure ratios are no longer an accurate reflection of the functional significance of each individual lesion. LAD, left anterior descending artery; LCx, left circumflex artery; FFR, fractional flow reserve; FFRepicardial, summed FFR (LM + LAD); FFRLM, measurement of FFR across an LM stenosis; LM, left main coronary artery.
Figure 2. Lesions with downstream disease: the summed FFR (a + b) is measured to determine the need for treatment. If FFR (a + b)l is ≤ 0.8 a pressure pullback is performed to determine which lesion to treat first. The lesion with the larger pressure step-up on pullback (△P, not FFR) is treated first. Subsequently, the untreated lesion is again interrogated with FFR, with FFR ≤ 0.8 representing functionally significant lesions which warrant revascularization. FFR, fractional flow reserve; Pd/Pa, resting distal coronary pressure to aortic pressure ratio.

… functional assessment of side branch stenosis with FFR after main branch PCI can reduce unnecessary complex interventions and associated complications.
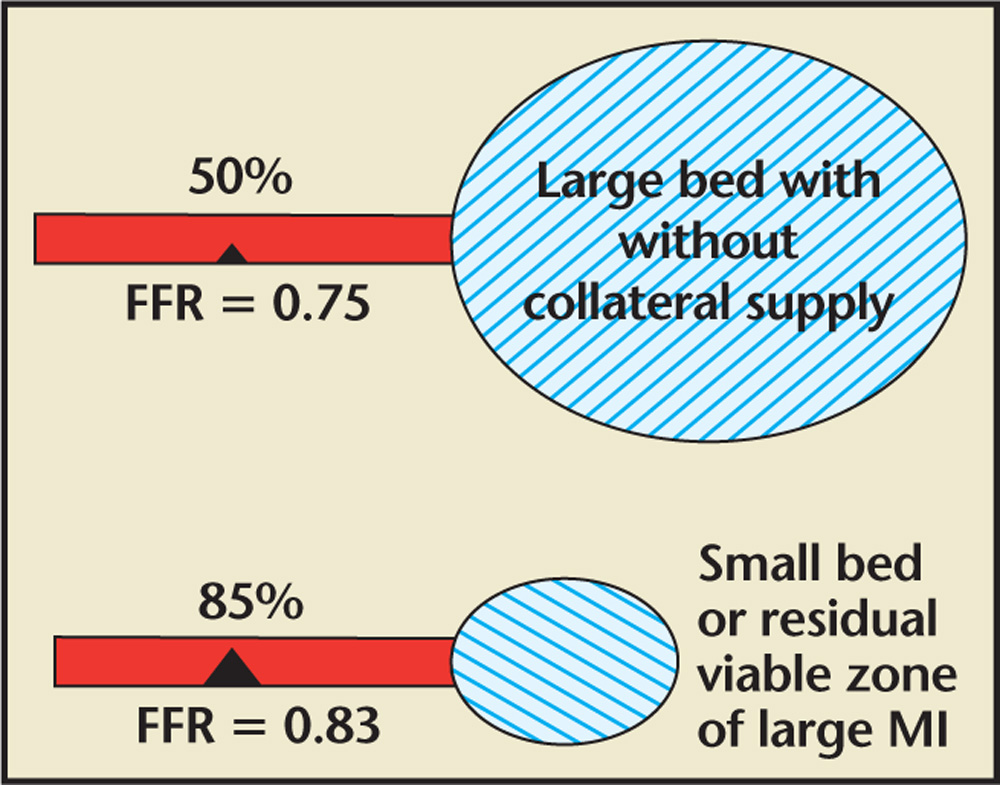
Figure 3. The visual-functional mismatch myocardial mass and FFR. A stenosis that appears severe by angiography but supplies a limited myocardial bed may not be functionally significant; however, less severe stenoses that supply a large myocardial bed without collaterals may be functionally significant. FFR, fractional flow reserve; MI, myocardial infarction.
… consideration should be given to the vessel caliber, angiographic severity, myocardial bed size, and operator experience prior to attempting FFR of a side branch lesion after PCI.
… FFR can be useful in deciding whether to treat SVG lesions.
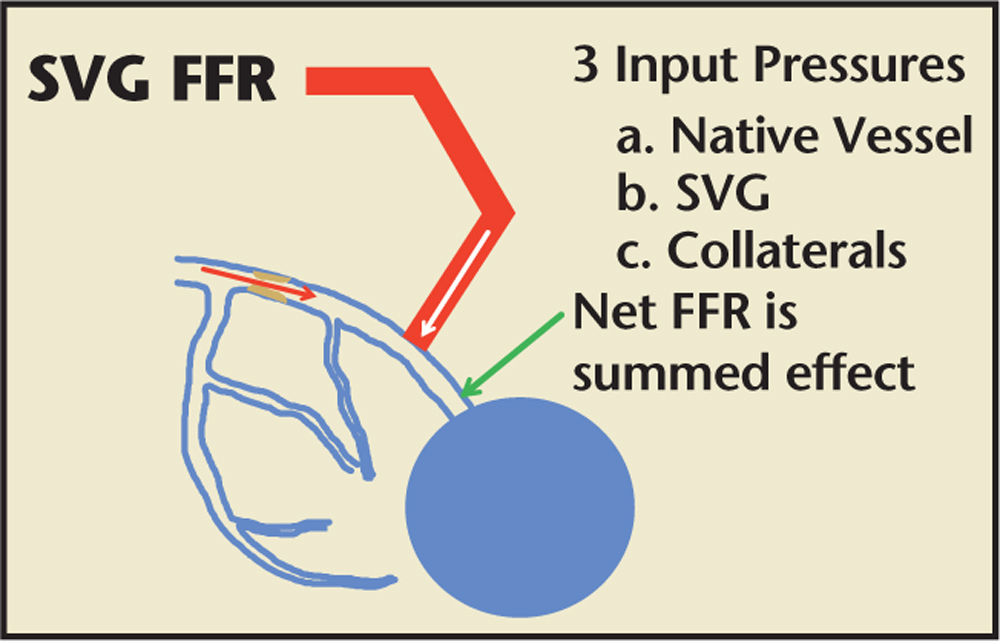
Figure 4. FFR assessment of an SVG. FFR assessment of a SVG must take into account (1) competing flow (and pressure) from the native and conduit vessels; (2) the presence of collaterals; and (3) the potential microvascular disease due to is chemic fibrosis, scarring, prior myocardial infarction, or chronic low-flow ischemia. FFR, fractional flow reserve; SVG, saphenous vein graft.

Figure 4. FFR assessment of an SVG. FFR assessment of a SVG must take into account (1) competing flow (and pressure) from the native and conduit vessels; (2) the presence of collaterals; and (3) the potential microvascular disease due to is chemic fibrosis, scarring, prior myocardial infarction, or chronic low-flow ischemia. FFR, fractional flow reserve; SVG, saphenous vein graft.
With appropriate understanding and application of the physiology, FFR-guided PCI of SVG lesions is safe and effective, and may reduce the need for unnecessary interventions of these complex lesions.
… variability in technique and equipment complicate the interpretation of these FFR studies. However, several large, randomized controlled trials have demonstrated convincing benefits to using an FFR-guided revascularization strategy.
Main Points
• Fractional flow reserve (FFR), defined as the ratio of coronary pressure beyond a stenosis to the central aortic pressure during maximal hyperemia, is a measure of ischemia and is a validated tool for determining the physiologic significance of a stenosis. FFR-guided revascularization improves clinical and economic outcomes.
• The presence of left main (LM) coronary artery stenosis has serious clinical implications, and decisions regarding revascularization of intermediate LM disease based solely on angiography are unreliable. FFR provides an accurate assessment of the functional significance of an LM stenosis involving the ostium, mid segment, or distal LM bifurcation.
• Because of the limitations of two-dimensional imaging with angiography, bifurcation disease is one of the most challenging anatomic subsets in the field of percutaneous coronary intervention (PCI). Functional assessment of side branch stenosis with FFR after main branch PCI can reduce unnecessary complex interventions and associated complications.
• When bypass graft angiography reveals saphenous vein graft (SVG) disease, it is often difficult to determine the functional significance of the disease. The functional assessment of an SVG must take into account (1) competing flow (and pressure) from the native and conduit vessels; (2) the presence of collaterals; and (3) the potential microvascular disease due to ischemic fibrosis, scarring, prior myocardial infarction, or chronic low-flow ischemia. In spite of these challenges, FFR can be useful in deciding whether to treat SVG lesions.
Main Points
• Fractional flow reserve (FFR), defined as the ratio of coronary pressure beyond a stenosis to the central aortic pressure during maximal hyperemia, is a measure of ischemia and is a validated tool for determining the physiologic significance of a stenosis. FFR-guided revascularization improves clinical and economic outcomes.
• The presence of left main (LM) coronary artery stenosis has serious clinical implications, and decisions regarding revascularization of intermediate LM disease based solely on angiography are unreliable. FFR provides an accurate assessment of the functional significance of an LM stenosis involving the ostium, mid segment, or distal LM bifurcation.
• Because of the limitations of two-dimensional imaging with angiography, bifurcation disease is one of the most challenging anatomic subsets in the field of percutaneous coronary intervention (PCI). Functional assessment of side branch stenosis with FFR after main branch PCI can reduce unnecessary complex interventions and associated complications.
• When bypass graft angiography reveals saphenous vein graft (SVG) disease, it is often difficult to determine the functional significance of the disease. The functional assessment of an SVG must take into account (1) competing flow (and pressure) from the native and conduit vessels; (2) the presence of collaterals; and (3) the potential microvascular disease due to ischemic fibrosis, scarring, prior myocardial infarction, or chronic low-flow ischemia. In spite of these challenges, FFR can be useful in deciding whether to treat SVG lesions.
Myocardial ischemia is an important risk factor for adverse clinical outcomes.1-3 Clinical outcomes and functional status can be improved with revascularization of a coronary stenosis that induces ischemia.3-5 However, revascularization of a coronary stenosis that does not include ischemia does not appear to be beneficial. For these patients, medical therapy is likely equally as effective as revascularization.6,7 Fractional flow reserve (FFR), defined as the ratio of coronary pressure beyond a stenosis to the central aortic pressure during maximal hyperemia, is a measure of ischemia and is a validated tool for determining the physiologic significance of a stenosis. FFR-guided revascularization improves clinical and economic outcomes.8-10 However, as with any diagnostic test, the utility of the test is dependent not only on the quality of the testing method, but also on the clinical context to which it is applied. Common factors that can confound the interpretation of FFR are listed in Table 1.11 Beyond these common confounders, there are certain clinical circumstances in which FFR measurements can be misinterpreted. These include, but are not limited to, left main (LM) coronary artery stenosis with downstream disease, complex bifurcation lesions, especially after stenting, and saphenous vein graft (SVG) lesions. In this article, we review the data surrounding the application of FFR to these complex anatomic subsets.
Complex Left Main Coronary Artery Disease
The presence of LM coronary artery stenosis has serious clinical implications, and decisions regarding revascularization of intermediate LM disease based solely on angiography are unreliable.12,13 As is the case for non-LM lesions, FFR provides an accurate assessment of the functional significance of an LM stenosis involving the ostium, mid segment, or distal LM bifurcation.11-18 For disease confined to the LM segment, measuring FFR across an LM stenosis (FFRLM) without additional lesions in the left anterior descending (LAD) or left circumflex (LCx) arteries (downstream disease) is similar to FFR of any other vessel. After appropriate zeroing and matching pressure to the aortic pressure, the FFR sensor is advanced beyond the stenosis and positioned into either the LAD or LCx artery, hyperemia is induced and FFR is measured. Most cardiac catheterization laboratories use intravenous adenosine infusions to induce hyperemia; however, intracoronary adenosine and intracoronary sodium nitroprusside, in the absence of contraindications, are acceptable alternatives.19 As with other vessels, an FFR ≤0.8 is indicative of a functionally significant stenosis. In the more complicated scenario of distal LM disease involving the bifurcation of the LM to LAD and LCx, FFR should be measured in both branches. Revascularization should be considered if FFRLM is ≤0.8 in either branch vessel.
Interpreting FFRLM in the more complex setting of downstream disease requires explanation in understanding the true FFRLM. In order to appropriately interpret the findings of FFRLM in this scenario, one must understand the physiology of FFR as it pertains to (1) lesions in series, and (2) reduced LM blood flow and myocardial bed size as a function of the degree of the downstream obstruction.11 In the presence of downstream disease, the pressure drop across each lesion blunts the hyperemia of the other; thus, simple pressure ratios are no longer an accurate reflection of the functional significance of each individual lesion.15,16 Clinically, this scenario is treated in a stepwise fashion: (1) the FFR wire is again appropriately zeroed and advanced across both lesions into the distal downstream vessel; (2) hyperemia is induced; and (3) the summed FFR (LM + 1 LAD = 5 FFRepicardial) is measured to determine the need for treatment. If FFRepicardial is ≤ 0.8, a pressure pullback is performed to determine which lesion to treat first. The lesion with the larger pressure step-up on pullback (ΔP, not FFR) is treated first (Figures 1 and 2). Subsequently, the untreated lesion is again interrogated with FFR, with FFR ≤0.8 representing functionally significant lesions that warrant revascularization. Kim and colleagues16 demonstrated the safety and efficacy of this pullback method in their case series of 131 patients with multiple intermediate stenoses of the same coronary artery.
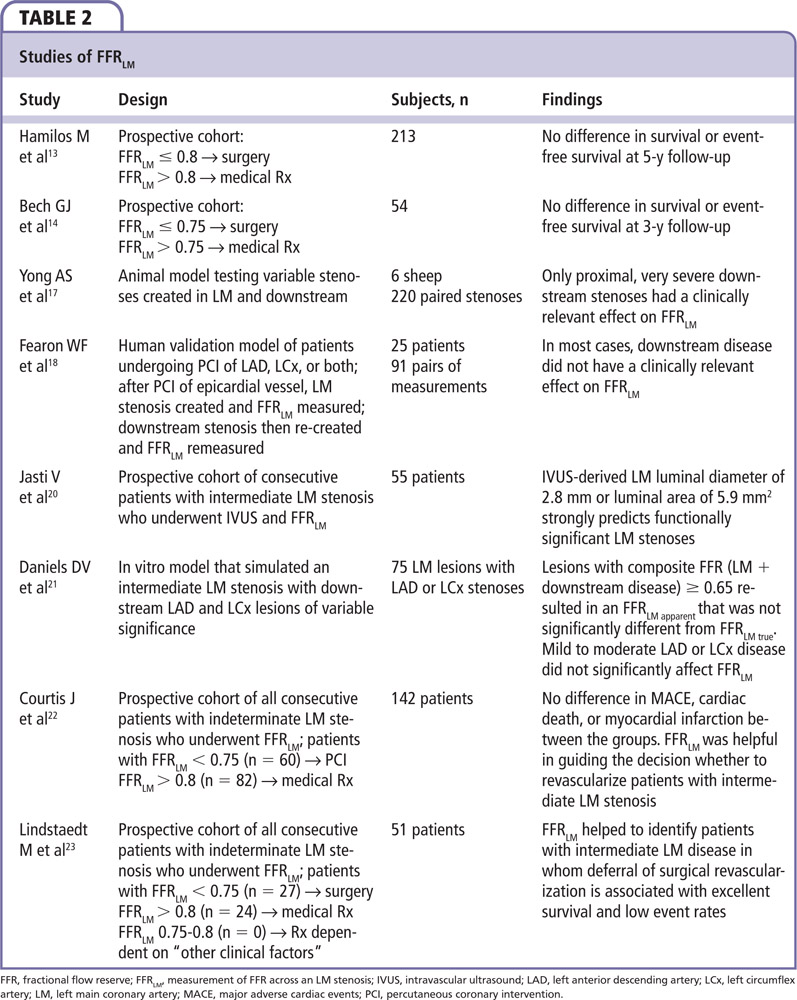
As coronary atherosclerosis is often a diffuse process, one can assume that many LAD lesions corrupt FFR interrogation of LM lesions. However, that does not appear to be the case. Yong and colleagues17 used an experimental animal model to demonstrate that only severe, proximal LAD lesions influence FFRLM. Using pressure sensor wires and balloon tip catheters they created an LM obstruction and downstream stenoses of variable severity in different locations. They demonstrated that FFRLM with no LAD stenosis (FFRtrue) and FFRLM with an LAD stenosis (FFRapparent) correlated directly with increasing severity of the LAD stenosis. For the entire cohort, the mean difference between FFRtrue and FFRapparent was 0.035 and was only > 0.05 when the FFRepicardial was < 0.5. They also demonstrated that proximal LAD stenoses had a greater effect on FFRapparent when compared with mid-LAD stenoses. Additionally, there were no cases in which FFRtrue was < 0.75 and FFRapparent was > 0.8, concluding that only severe proximal LAD stenoses influenced FFR true in this model.17 Fearon and colleagues18 expanded upon these findings by translating a similar model to patients. They interrogated 91 lesions in 25 patients (71 LAD lesions, 20 LCx lesions) after percutaneous coronary intervention (PCI) of the downstream lesion. An intermediate stenosis of the LM was created using a balloon catheter. FFR was then measured in the LAD and LCx before and after re-creation of a downstream stenosis, also with a balloon catheter, within the stent that had just been deployed. They then compared FFRtrue,, measured prior to re-creation of the downstream stenosis, to FFRapparent, after the downstream stenosis had been re-created and found that FFRtrue was significantly lower than FFRapparent (0.81 ± 6 0.08 vs 0.83 ± 6 0.08; P < .001), and that the difference correlated to the severity of the disease. The authors concluded that, because of the small absolute difference in values, downstream disease does affect FFRtrue, but does not have a clinically significant impact on FFRLM if the wire is positioned in the nondiseased branch. Several sentinel works that examined the role of FFR in LM disease are summarized in Table 2.13,14,17,18,20-23 In conclusion, FFRLM > 0.8 portends an excellent prognosis with medical therapy alone, whereas patients with FFRLM ≤0.8 will likely benefit from revascularization. Furthermore, the presence of downstream disease rarely has a clinically significant effect on FFRLM, except when the stenosis is proximal and severe.17,18
Bifurcation Disease
Because of the limitations of two-dimensional imaging with angiography, bifurcation disease is one of the most challenging anatomic subsets in the field of PCI. Currently, provisional PCI for side branch lesions is the most common approach to bifurcation lesions, perhaps related to prior clinical trials that failed to show benefit with empiric side branch stenting as compared with balloon angioplasty or medical therapy. Visual-functional mismatch is common in bifurcation disease, and angiographic assessment tends to overestimate the severity of side branch lesions (Figure 3).24-28 Therefore, functional assessment of side branch stenosis with FFR after main branch PCI can reduce unnecessary complex interventions and associated complications. Koo and colleagues25 demonstrated the feasibility of FFR interrogation of jailed side branch lesions, and found that no stenosis < 75% had an FFR < 0.75. They also demonstrated no difference in cardiac event rates of patients treated with FFR-guided PCI of jailed side branches as compared with a similar subset of patients treated without FFR-guided PCI.26 In the FFR cohort of the Nordic Bifurcation study, Kumsars and colleagues29 demonstrated that kissing balloon inflation (KBI) significantly increased the FFR of the jailed side branch. However, at 8-month follow-up, this difference was no longer evident. FFR interrogation of a side branch vessel after PCI can be technically challenging and may be complicated by plaque shifting, failure to pass the wire through stent struts, ostial side branch dissection, and late positive or negative remodeling of the side branch stenosis. Furthermore, the side branches can often be small caliber vessels that supply a limited myocardial bed. Therefore, consideration should be given to the vessel caliber, angiographic severity, myocardial bed size, and operator experience prior to attempting FFR of a side branch lesion after PCI (Figure 3).
The Double Kissing Crush Versus Provisional Stenting Technique for Treatment of Coronary Bifurcation Lesions VI (DKCRUSH-VI) trial was the first randomized controlled trial to compare FFR-guided side branch intervention versus angiography-guided side branch intervention in 320 patients with true bifurcation lesions (Medina classification 1,1,1 or 0,1,1).30 To standardize the approach, the FFR-guided group underwent FFR of the side branch after main branch stent deployment. If FFR ≤0.8, balloon angioplasty to the side branch was performed with KBI. If FFR after KBI remained ≤0.8, a stent was deployed in the side branch with final KBI. In contrast, the angiography group underwent KBI if the thrombolysis in myocardial infarction flow was < 3, there was more than a Type A dissection, or an ostial stenosis > 70% after the main branch stent was present. If any of these parameters persisted after KBI, a side branch stent was pursued. The authors found that 1-year target vessel revascularization and stent thrombosis rates were similar between both groups, with identical 1-year major adverse cardiac event (MACE) rates of 18.1% (hazard ratio [HR] 0.91, 95% confidence interval [CI], 0.48-1.88; P = 5 1.00).30
As with all lesions, FFR of a side branch stenosis is influenced by the degree of stenosis, lesion length, lesion morphology, vessel size, upstream and downstream stenoses, and the size of the myocardial bed supplied by the side branch. Furthermore, FFR of the side branch is vulnerable to the influence of the main-branch stenosis. Therefore, when approaching bifurcation lesions where both branches have angiographic stenoses > 70%, FFR and pressure pull back should be performed under maximal hyperemia for both branches. If FFR of both branches is ≤0.8, a two-stent approach is likely indicated. However, angiographic severity, myocardial bed size, and the presence of upstream and downstream disease should all be considered prior to pursuing FFR interrogation of a side branch vessel. When applied appropriately, FFR provides an objective measurement of the severity of bifurcation lesions, and can help to guide the approach to intervention.
Saphenous Vein Grafts
The average SVG patency is estimated to be 7 to 10 years. Atherosclerotic plaque and neointimal hyperplasia are the main causes of graft degeneration. PCI of SVG lesions depends on a number of factors because the SVG contribution to myocardial perfusion is only one of three potential sources. Myocardial flow may occur through residual native flow, collateral flow, and SVG flow. In addition, stenting of the SVG is associated with reduced long-term patency and increased periprocedural complications.31
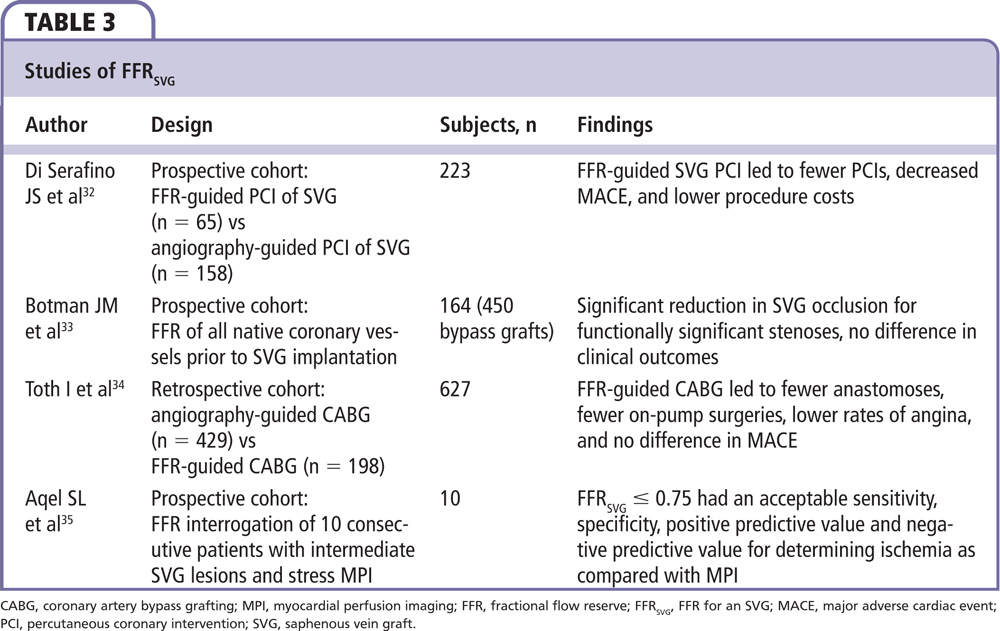
When bypass graft angiography reveals SVG disease, it is often difficult to determine the functional significance of the disease. The functional assessment of an SVG must take into account (1) competing flow (and pressure) from the native and conduit vessels; (2) the presence of collaterals; and (3) the potential microvascular disease due to ischemic fibrosis, scarring, prior myocardial infarction, or chronic low-flow ischemia (Figure 4). In spite of these challenges, FFR can be useful in deciding whether to treat SVG lesions. A recent study by Di Serafino and colleagues32 examined FFR-guided PCI in coronary artery bypass grafts. They studied 223 patients with stable or unstable angina and an intermediate stenosis of a bypass graft; 65 of these patients underwent FFR-guided PCI of their bypass graft, whereas 158 patients had angiographically guided PCI of their bypass graft. Despite similar baseline characteristics, a multivariate analysis demonstrated that the primary endpoint of MACE occurred in 28% of the FFR-guided PCI group versus 51% of the angiography-guided PCI group (HR 0.33, 95% CI, 0.11-0.96; P = .043). Furthermore, procedure costs were reduced in the FFR-guided PCI group, perhaps related to lower rates of PCI (35% in the FFR-guided group vs 57% in the angiography-guided group; P < .01).32 This and other relevant studies of FFR in SVG lesions is summarized in Table 3.32-35
The theory of FFR for an SVG (FFRSVG) is the same as for other lesions. To measure FFRSVG, the pressure sensor is zeroed and subsequently positioned distal to the anastomosis of the graft into the native vessel. Hyperemia is then induced and FFR is measured. If the native vessel is occluded, the FFRSVG reflects only the functional significance of the SVG lesion.31 Based on limited data, an FFRSVG ≤ 0.75 can be used as the cutoff for a functionally significant stenosis.35 However, if the native vessel still provides antegrade flow, the SVG and native vessel lesions act as lesions in series, and a pressure pullback during maximal hyperemia should be performed on each lesion. Here again, the lesion with the greatest pressure differential (ΔP, not FFR) should be treated first. Subsequently, the untreated lesion can again be interrogated with FFR, with consideration for revascularization if the FFR remains ≤0.8. With appropriate understanding and application of the physiology, FFR-guided PCI of SVG lesions is safe and effective, and may reduce the need for unnecessary interventions of these complex lesions.
Limitations and Alternatives
The utility of FFR in any given situation is dependent on the operator’s technical skill as well as his or her understanding of the physiology, application, and limitations of FFR. Although multiple studies have examined the role of FFR in various clinical situations, many of these studies are small and have limited outcome data. Furthermore, variability in technique and equipment complicate the interpretation of these FFR studies. However, several large, randomized controlled trials have demonstrated convincing benefits to using an FFR-guided revascularization strategy.7-10 Other adjunctive diagnostic modalities such as intravascular ultrasound and optical coherence tomography may also be effective in guiding the approach to LM and bifurcation disease.20,36-38 Furthermore, noninvasive imaging modalities that apply computational fluid dynamics, such as FFR derived from coronary computed tomography angiography (FFRCT), may provide functional information about presumed stenoses prior to the patient’s arrival in the catheterization laboratory.39 An integrated approach that considers patient factors, operator experience, and equipment availability should be applied to each case in which adjunctive diagnostic testing is required.
Conclusions
Recent studies have proven that functional evaluation of coronary stenoses can be applied to complex anatomic substrates including LM disease, bifurcation disease, and SVGs, with similar results. Future developments, including instantaneous flow reserve and FFRCT, may provide alternatives to FFR that obviate the need for hyperemia. Other invasive techniques such as catheter-based FFR systems allow operators to use a coronary guidewire of their choice, and to perform pullback measurements without sacrificing wire position. Based on the long-term favorable outcome studies of FFR-guided revascularization, the application of FFR will continue to grow.40 FFR is a critical tool in deciding whether to revascularize coronary artery disease in stable patients and may extend to revascularization of nonculprit lesions in acute coronary syndromes. With due consideration of the physiologic principles, FFR can be applied in almost any anatomic substrate. FFR can facilitate timely, clinically, and economically sound decision making to direct revascularization options to optimize patient outcomes. ![]()
References
- Beller GA, Zaret BL. Contributions of nuclear cardiology to diagnosis and prognosis of patients with coronary artery disease. Circulation. 2000;101:1465-1478.
- Shaw LJ, Iskandrian AE. Prognostic value of gated myocardial perfusion SPECT. J Nucl Cardiol. 2004;11:171-185.
- Shaw LJ, Berman DS, Maron DJ, et al; COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283-1291.
- Davies RF, Goldberg AD, Forman S, et al. Asymptomatic Cardiac Ischemia Pilot (ACIP) study two-year follow-up: outcomes of patients randomized to initial strategies of medical therapy versus revascularization. Circulation. 1997;95:2037-2043.
- Erne P, Schoenenberger AW, Burckhardt D, et al. Effects of percutaneous coronary interventions in silent ischemia after myocardial infarction: the SWISSI II randomized controlled trial. JAMA. 2007; 297:1985-1991.
- Boden WE, O’Rourke RA, Teo KK, et al; COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503-1516.
- Pijls NH, van Schaardenburgh P, Manoharan G, et al. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5-year follow-up of the DEFER Study. J Am Coll Cardiol 2007; 49:2105-2111.
- Tonino PA, De Bruyne B, Pijls NH, et al; FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213-224.
- De Bruyne B, Pijls NH, Kalesan B, et al; FAME 2 Trial Investigators. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991-1001.
- Berger A, Botman KJ, MacCarthy PA, et al. Long-term clinical outcome after fractional flow reserve-guided percutaneous coronary intervention in patients with multivessel disease. J Am Coll Cardiol. 2005;46: 438-442.
- Kern MJ. When does a left anterior descending stenosis alter flow across a left main segment? Interpreting left main fractional flow reserve with downstream obstruction. Circ Cardiovasc Interv. 2013;6:128-130.
- Caracciolo EA, Davis KB, Sopko G, et al, for the CASS Investigators. Comparison of surgical and medical group survival in patients with left main coronary artery disease. Circulation. 1995;91:2325-2334.
- Hamilos M, Muller O, Cuisset T, et al. Long-term clinical outcome after fractional flow reserve-guided treatment in patients with angiographically equivocal left main coronary artery stenosis. Circulation. 2009;120:1505-1512.
- Bech GJ, Drotse H, Piljs NH, et al. Value of fractional flow reserve in making decisions about bypass surgery for equivocal left main coronary artery disease. Heart. 2001;86:547-552.
- Pijls NH, De Bruyne B, Bech GJ, et al. Coronary pressure measurement to assess the hemodynamic significance of serial stenoses within one coronary artery: validation in humans. Circulation. 2000;102:2371-2377.
- Kim HL, Koo BK, Nam CW, et al. Clinical and physiological outcomes of fractional flow reserve-guided percutaneous coronary intervention in patients with serial stenoses within one coronary artery. JACC Interv. 2012;5:1013-1018.
- Yong AS, Daniels D, De Bruyne B, et al. Fractional flow reserve assessment of left main stenosis in the presence of downstream coronary stenoses. Circ Cardiovasc Interv. 2013;6:161-165.
- Fearon WF, Yong AS, Lenders G, et al. The impact of downstream coronary stenosis on fractional flow reserve assessment of intermediate left main coronary artery disease. JACC Interv. 2015;8:398-403.
- Leone AM, Porto I, De Caterina AR, et al. Maximal hyperemia in the assessment of fractional flow reserve: intracoronary adenosine versus intracoronary sodium nitroprusside versus intravenous adenosine: the NASCI (Nitroprussiato versus Adenosina nelle Stenosi Coronariche Intermedie) study. JACC Interv. 2012;5:402-408.
- Jasti V, Ivan E, Yalamanchili V, et al. Correlations between fractional flow reserve and intravascular ultrasound in patients with an ambiguous left main coronary artery stenosis. Circulation. 2004;110:2831-2836.
- Daniels DV, van’t Veer M, Pijls NH, et al. The impact of downstream coronary stenoses on fractional flow reserve assessment of intermediate left main disease. JACC Interv. 2012;5:1021-1025.
- Courtis J, Rodés-Cabau J, Larose E, et al. Usefulness of coronary fractional flow reserve measurements in guiding clinical decisions in intermediate or equivocal left main coronary stenosis. Am J Cardiol. 2009;103:943-949.
- Lindstaedt M, Yazar A, Germing A. Clinical outcome in patients with intermediate or equivocal left main coronary artery disease after deferral of surgical revascularization on the basis of fractional flow reserve measurements. Am Heart J. 2009;152:156.e1-156.e9
- Steigen TK, Maeng M, Wiseth R, et al Nordic PCI Study Group. Randomization on simple versus complex stenting of coronary artery bifurcation lesions: the Nordic bifurcation study. Circulation. 2006;114:1955-1961.
- Koo BK, Kang HJ, Youn TJ, et al. Physiologic assessment of jailed side branch lesions using fractional flow reserve. J Am Coll Cardiol. 2005;46:633-637.
- Koo BK, Park KW, Kang HJ, et al. Physiological evaluation of the provisional side-branch intervention strategy for bifurcation lesions using fractional flow reserve. Eur Heart J. 2008;29:726-732.
- Koh JS, Koo BK, Kim JH, et al. Relationship between fractional flow reserve and angiographic and intravascular ultrasound parameters in ostial lesions: major epicardial vessel versus side branch ostial lesions. JACC Cardiovasc Interv. 2012;5:409-415.
- Ahn JM, Lee JY, Kang SJ, et al. Functional assessment of jailed side branches in coronary bifurcation lesions using fractional flow reserve. JACC Cardiovasc Interv. 2012;5:155-161.
- Kumsars I, Narbute I, Niemela M, et al; Nordic-Baltic PCI study group. Side-branch fractional flow reserve measurements after main vessel stenting: a Nordic-Baltic bifurcation study III substudy. Eurointervention. 2012;7:1155-1161.
- Chen SL, Ye F, Zhang JJ, et al. Randomized Comparison of FFR-Guided and Angiography-Guided Provisional Stenting of True Coronary Bifurcation Lesions: The DKCRUSH-VI Trial (Double Kissing Crush Versus Provisional Stenting Technique for Treatment of Coronary Bifurcation Lesions VI). JACC Cardiovasc Interv. 2015;8:536-546.
- Kern MJ. Evaluation of coronary artery bypass graft lesions in the cath lab. Cath Lab Digest website. http://www.cathlabdigest.com/articles/Evaluation-Coronary-Artery-Bypass-Graft-Lesions-Cath-Lab. Accessed April 9, 2016.
- Di Sarafino L, De Bruyne B, Mangiacapra F, et al. Long-term clinical outcome after fractional-flow reserve- versus angio-guided percutaneous coronary intervention in patients with intermediate stenosis of coronary artery bypass grafts. Am Heart J. 2013;166:110-118.
- Botman CJ, Schonberger J, Koolen S, et al. Does stenosis severity of native vessels influence bypass graft patency? A prospective fractional flow reserve-guided study. Ann Thorac Surg. 2007;83:2093-2097.
- Toth G, De Bruyne B, Casselman F, et al. Fractional flow reserve-guided versus angiography-guided coronary artery bypass graft surgery. Circulation. 2013;128:1405-1411.
- Aqel R, Zoghbi GJ, Hage F, et al. Hemodynamic evaluation of coronary artery bypass graft lesions using fractional flow reserve. Cathet Cardiovasc Interv. 2008;72:479-485.
- Puri R, Kapadia SR, Nicholls SJ, et al. Optimizing outcomes during left main percutaneous coronary intervention with intravascular ultrasound and fractional flow reserve. JACC Interv. 2012;5:697-707.
- Parodi G, Maehara A, Giuliana G, et al. Optical coherence tomography in unprotected left main coronary stenting. Eurointervention. 2010;6:94-99.
- Koo BK, Waseda K, Kang HJ, et al. Anatomic and functional evaluation of bifurcation lesions undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2010;3:113-119.
- Nørgaard BL, Leipsic J, Gaur S, et al; NXT Trial Study Group. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J Am Coll Cardiol. 2014;63:1145-1155.
- van Nunen LX, Zimmermann FM, Tonino PA, et al; FAME Study Investigators. Fractional flow reserve versus angiography for guidance of PCI in patients with multivessel coronary artery disease (FAME): 5-year follow-up of a randomized controlled trial. Lancet. 2015;386:1853-1860.