Isolated Left Ventricular Noncompaction Cardiomyopathy: A Transient Disease?
Juan M. Vinardell, MD,1 Maria D. Avila, MD,2 Orlando Santana, MD2
1Department of Internal Medicine, Mount Sinai Medical Center, Miami Beach, FL; 2Columbia University Division of Cardiology at the Mount Sinai Heart Institute, Miami Beach, FL
Isolated left ventricular noncompaction is either a distinct cardiomyopathy or a morphologic trait shared by several different types of cardiomyopathies. Although there is no current gold standard for its diagnosis, cardiac imaging is the most commonly accepted modality. Described is a case of left ventricular noncompaction that resolved 2 years after the initial diagnosis.
[Rev Cardiovasc Med. 2016;17(1/2):80-84 doi: 10.3909/riu0817]
© 2016 MedReviews®, LLC
Isolated Left Ventricular Noncompaction Cardiomyopathy: A Transient Disease?
Juan M. Vinardell, MD,1 Maria D. Avila, MD,2 Orlando Santana, MD2
1Department of Internal Medicine, Mount Sinai Medical Center, Miami Beach, FL; 2Columbia University Division of Cardiology at the Mount Sinai Heart Institute, Miami Beach, FL
Isolated left ventricular noncompaction is either a distinct cardiomyopathy or a morphologic trait shared by several different types of cardiomyopathies. Although there is no current gold standard for its diagnosis, cardiac imaging is the most commonly accepted modality. Described is a case of left ventricular noncompaction that resolved 2 years after the initial diagnosis.
[Rev Cardiovasc Med. 2016;17(1/2):80-84 doi: 10.3909/riu0817]
© 2016 MedReviews®, LLC
Isolated Left Ventricular Noncompaction Cardiomyopathy: A Transient Disease?
Juan M. Vinardell, MD,1 Maria D. Avila, MD,2 Orlando Santana, MD2
1Department of Internal Medicine, Mount Sinai Medical Center, Miami Beach, FL; 2Columbia University Division of Cardiology at the Mount Sinai Heart Institute, Miami Beach, FL
Isolated left ventricular noncompaction is either a distinct cardiomyopathy or a morphologic trait shared by several different types of cardiomyopathies. Although there is no current gold standard for its diagnosis, cardiac imaging is the most commonly accepted modality. Described is a case of left ventricular noncompaction that resolved 2 years after the initial diagnosis.
[Rev Cardiovasc Med. 2016;17(1/2):80-84 doi: 10.3909/riu0817]
© 2016 MedReviews®, LLC
KEY WORDS
Ventricular noncompaction • Transient • Cardiomyopathy • Transthoracic echocardiogram
KEY WORDS
Ventricular noncompaction • Transient • Cardiomyopathy • Transthoracic echocardiogram
A two-layered structure of the myocardium with a noncompacted to compacted layer ratio > 2.0, when measured in end-systole, is diagnostic of LV noncompaction.
The authors concluded that noncompaction cardiomyopathy can either have a dynamic course or may be reversible, or that current morphologic criteria may occasionally misclassify a transient cardiomyopathy as noncompaction.
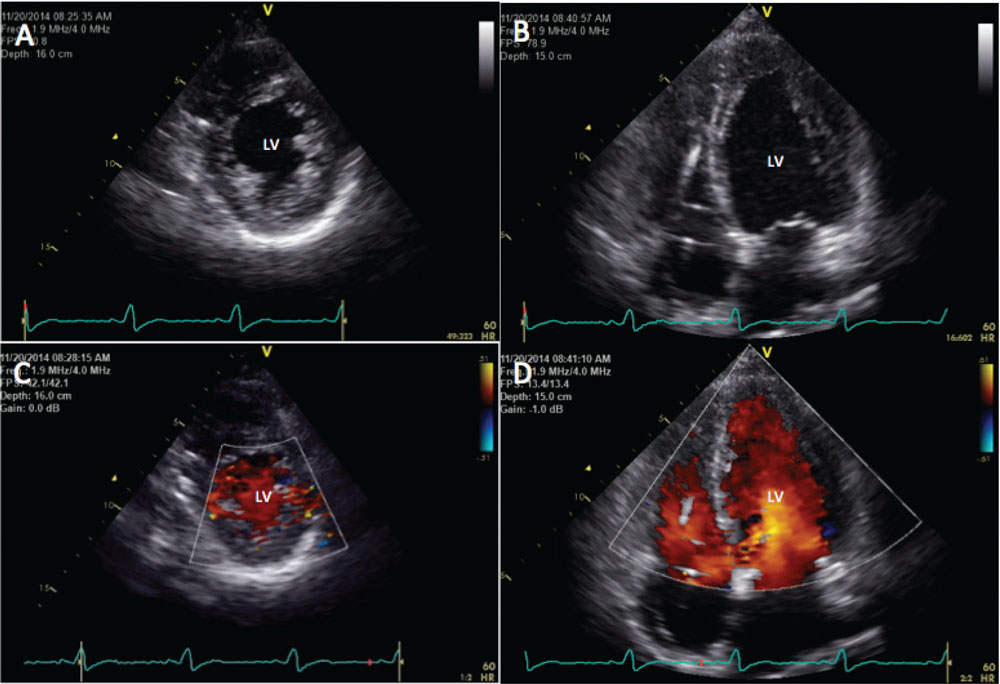
Figure 1. Transthoracic echocardiogram demonstrating complete resolution of noncompaction cardiomyopathy. (A) Short-axis view. (B) Long-axis view. (C) Short-axis view with color-flow Doppler. (D) Long-axis view with color-flow Doppler. LV, left ventricle.
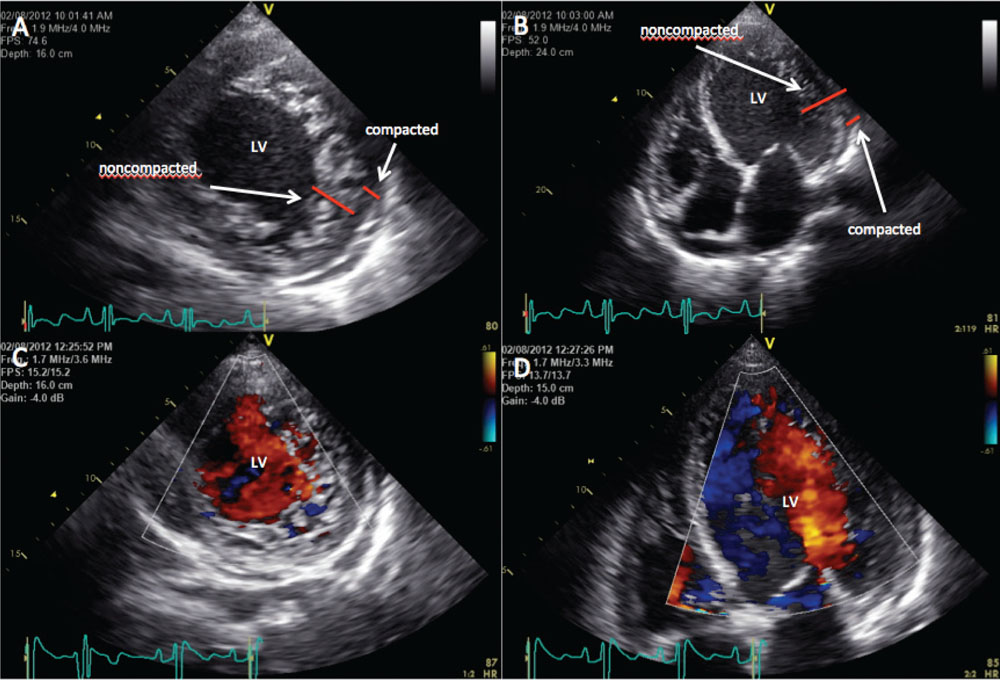
Figure 2. Transthoracic echocardiogram demonstrating deep trabeculations satisfying criteria for noncompaction cardiomyopathy. (A) Short-axis view. (B) Long-axis view. (C) Short-axis view with color-flow Doppler. (D) Long-axis view with color-flow Doppler. LV, left ventricle.
In our case, it is unclear if the regression of LV trabeculations is due to reversibility or nonspecific diagnostic criteria for this pathology.
Main Points
• Noncompaction cardiomyopathy is a rare cardiomyopathy, and is characterized by the persistence of fetal myocardium with excessive prominence of trabecular meshwork and deep intertrabecular recesses in the absence of structural abnormalities (eg, obstruction of the right or left ventricular [LV] outflow tracts, complex cyanotic congenital heart disease, and coronary artery anomalies).
• Although noncompaction cardiomyopathy has been classified as a primary cardiomyopathy of genetic origin, its definition, diagnostic criteria, and treatment modalities are still debated. It was suggested that it is caused by an arrest of the normal process of intrauterine endomyocardial and myocardial morphogenesis, with the trabeculations being found predominantly in the inferior and lateral portion of the apex and midventricular segments of the left ventricle.
• It remains to be determined whether the increased LV trabeculations are a de novo response to a physiologic or pathologic increase in the cardiac preload, an exaggeration of anomalies that were already present as a result of incomplete manifestation of LV noncompaction, or a part of a normal morphologic spectrum in healthy individuals.
Main Points
• Noncompaction cardiomyopathy is a rare cardiomyopathy, and is characterized by the persistence of fetal myocardium with excessive prominence of trabecular meshwork and deep intertrabecular recesses in the absence of structural abnormalities (eg, obstruction of the right or left ventricular [LV] outflow tracts, complex cyanotic congenital heart disease, and coronary artery anomalies).
• Although noncompaction cardiomyopathy has been classified as a primary cardiomyopathy of genetic origin, its definition, diagnostic criteria, and treatment modalities are still debated. It was suggested that it is caused by an arrest of the normal process of intrauterine endomyocardial and myocardial morphogenesis, with the trabeculations being found predominantly in the inferior and lateral portion of the apex and midventricular segments of the left ventricle.
• It remains to be determined whether the increased LV trabeculations are a de novo response to a physiologic or pathologic increase in the cardiac preload, an exaggeration of anomalies that were already present as a result of incomplete manifestation of LV noncompaction, or a part of a normal morphologic spectrum in healthy individuals.
Isolated left ventricular (LV) noncompaction cardiomyopathy was first described in 1984.1 Noncompaction cardiomyopathy is rare, with a prevalence of 0.014% in adults; it is characterized by the persistence of fetal myocardium with excessive prominence of trabecular meshwork and deep intertrabecular recesses in the absence of structural abnormalities (eg, obstruction of the right or LV outflow tracts, complex cyanotic congenital heart disease, and coronary artery anomalies).1-4 Although noncompaction cardiomyopathy has been classified as a primary cardiomyopathy of genetic origin, its definition, diagnostic criteria, and treatment modalities are still debated.5,6 The American Heart Association classifies it as a genetic cardiomyopathy,6 whereas the European Society of Cardiology considers it an unclassified cardiomyopathy.7 It was suggested that it is caused by an arrest of the normal process of intrauterine endomyocardial and myocardial morphogenesis, with the trabeculations being found predominantly in the inferior and lateral portion of the apex and midventricular segments of the left ventricle.3,6 Its clinical manifestations are highly variable, ranging from no symptoms to a progressive deterioration in cardiac function resulting in congestive heart failure, systemic thromboembolism, arrhythmias, and sudden cardiac death.8 The echocardiographic criteria for diagnosis are based on the presence of left ventricle myocardial trabeculations and a two-layer distinction with a ratio of 2:1 between the noncompacted and the compacted myocardium at the end of systole. Its management is mostly consistent in addressing atrial and ventricular arrhythmias, the need for an implantable cardioverter defibrillator (ICD) or biventricular pacemaker, as well as anticoagulation and (occasionally) heart transplantation.9,10 Although it is assumed to be a permanent disease process, we present a case of transient noncompaction cardiomyopathy.
Case Report
A 55-year-old black woman presented to the hospital after an episode of lightheadedness. She had no syncope, chest pain, palpitations, or shortness of breath. Her past medical history was remarkable for a history of noncompaction cardiomyopathy, ICD implantation, hypertension, and an ischemic cerebrovascular accident. The noncompaction cardiomyopathy was diagnosed 6 years prior by transthoracic echocardiogram (TTE), which demonstrated a noncompacted to compacted myocardium ratio > 2 with an ejection fraction (EF) < 20%. At that time, placement of an ICD was recommended for primary prevention based on EF < 30% and New York Heart Association (NYHA) Class II-III symptoms while on maximum guideline-determined medical therapy. However, the patient refused implantation of the ICD. Three years later, she was admitted to the hospital with an episode of congestive heart failure. A TTE was repeated and demonstrated the noncompaction cardiomyopathy features with an EF < 20%. These findings were confirmed by transesophageal echocardiogram. The patient was treated medically and discharged. One month later, she had a syncopal episode in the setting of ventricular fibrillation that required electrical cardioversion by emergency medical services. Cardiac magnetic resonance imaging (MRI) confirmed a noncompacted myocardium. Coronary angiography demonstrated minimal nonobstructive disease. The patient agreed to the implantation of an ICD and continued guideline-directed medical therapy as recommended by the American College of Cardiology Foundation/American Heart Association Task Force Heart Failure Guidelines.11 Four months later, her EF remained < 20%, her electrocardiogram demonstrated a left bundle branch block with a QRS width of 180 ms, and she continued to have NYHA Class II-III heart failure symptoms, satisfying criteria for a biventricular pacemaker/defibrillator. Her device was upgraded to cardiac resynchronization therapy as per guideline recommendations.10
Upon her arrival to the emergency department, her vital signs were stable and the physical examination was unremarkable. Electrocardiogram revealed an atrioventricular sequential biventricular-paced rhythm. Interrogation of the defibrillator did not demonstrate any arrhythmias. A TTE demonstrated a preserved EF = 60%, with normal left ventricle volumes, and mild LV hypertrophy with almost complete resolution in the size and number of the previously noted trabeculations, meaning she no longer met criteria for the diagnosis of a noncompaction cardiomyopathy (Figure 1).
Discussion
The diagnosis of LV noncompaction is made by the presence of a thick layer of prominent LV trabeculae, deep intertrabecular recesses, and a thin compacted layer of the left ventricle.5 A two-layered structure of the myocardium with a noncompacted to compacted layer ratio > 2.0, when measured in end-systole, is diagnostic of LV noncompaction.4 This patient satisfied the criteria for noncompaction cardiomyopathy in all her previous echocardiograms (Figure 2). However, the noncompaction cardiomyopathy resolved on the latest echocardiogram. Due to the presence of her ICD/biventricular pacemaker, which was not MRI compatible, MRI could not be performed.
Data on the reversibility of noncompaction cardiomyopathy are limited. There are two similar cases in the literature.12,13 The first case is that of a 58-year-old man with systolic heart failure (LVEF = 18%) who met criteria for LV noncompaction. The patient received guideline-directed medical therapy, and ICD implantation that was subsequently upgraded to a biventricular pacemaker. His LVEF improved to 51% and the morphologic features of noncompaction cardiomyopathy became less clear 6 months later. The authors concluded that noncompaction cardiomyopathy can either have a dynamic course or may be reversible, or that current morphologic criteria may occasionally misclassify a transient cardiomyopathy as noncompaction.
A second case is that of a 59-year-old man who presented with atypical chest pain following a viral illness, associated with progressively worsening breathlessness in the setting of elevated troponins, left bundle branch block, an EF < 19% and a two-layered myocardium consistent with isolated LV noncompaction. After initiation of standard medical therapy, the LV systolic dysfunction normalized and the two-layered myocardium resolved in 18 months. In this case, the authors concluded that isolated ventricular noncompaction diagnosis could not be made in an acute scenario and that follow-up imaging was warranted to look for possible resolution. Furthermore, a prospective longitudinal study of 102 primigravida pregnant women suggested that LV noncompaction may be a transient entity.14 In this study, none of the patients had increased LV trabeculations at baseline. Subsequently, 25% of the cohort developed increased LV trabeculations, and 9.8% had echocardiographic criteria of noncompaction cardiomyopathy during the pregnancy. In the postpartum period, seven cases of the above-mentioned 25% continued to display LV trabeculations, which turned less evident at 2 years postpartum in five of the cases and complete regression occurred in one case.
In our case, it is unclear if the regression of LV trabeculations is due to reversibility or nonspecific diagnostic criteria for this pathology. Further studies are needed to determine whether the increased LV trabeculations are a de novo response to a physiologic or pathologic increase in the cardiac preload, an exaggeration of anomalies that were already present as a result of incomplete manifestation of LV noncompaction, or a part of a normal morphologic spectrum in healthy individuals. ![]()
References
- Engberding R, Bender F. Identification of a rare congenital anomaly of the myocardium by two-dimensional echocardiography: Persistence of isolated myocardial sinusoids. Am J Cardiol. 1984;53:1733-1734.
- Ritter M, Oechslin E, Sütsch G, et al. Isolated noncompaction of the myocardium in adults. Mayo Clin Proc. 1997;72:26-31.
- Oechslin EN, Attenhofer Jost CH, Rojas JR, et al. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol. 2000;36:493-500.
- Jenni R, Oechslin E, Schneider J, et al. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart. 2001;86:666-671.
- Arbustini E, Weidemann F, Hall JL. Left ventricular noncompaction: a distinct cardiomyopathy or a trait shared by different cardiac diseases? J Am Coll Cardiol. 2014;64:1840-1850.
- Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807-1816.
- Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270-276.
- Ichida F, Hamamichi Y, Miyawaki T, et al. Clinical features of isolated noncompaction of the ventricular myocardium: long-term clinical course, hemodynamic properties, and genetic background. J Am Coll Cardiol. 1999;34:233-240.
- Udeoji DU, Philip KJ, Morrissey RP, et al. Left ventricular noncompaction cardiomyopathy: updated review. Ther Adv Cardiovasc Dis. 2013;7:260-273.
- Russo AM, Stainback RF, Bailey SR, et al. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2013;61:1318-1368.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62: e147-e239.
- Eurlings LW, Pinto YM, Dennert RM, Bekkers SC. Reversible isolated left ventricular non-compaction? Int J Cardiol. 2009;136:e35-e36.
- Luckie M, Khattar RS. Resolution of echocardiographic features of left ventricular non-compaction and systolic dysfunction following treatment for heart failure. Eur J Echocardiogr. 2010;11:E16.
- Gati S, Papadakis M, Papamichael ND, et al. Reversible de novo left ventricular trabeculations in pregnant women: implications for the diagnosis of left ventricular noncompaction in low-risk populations. Circulation. 2014;130: 475-483.