Forty Years of Chest Pain: A Case Report and Contemporary Review of the Diagnostic and Therapeutic Options for Myocardial Bridging
Emily Tat,1 Richard Cheng, MD,1 Jeffrey S. Helfenstein, MD1,2
1Cedars-Sinai Heart Institute, Los Angeles, CA; 2The David Geffen School of Medicine at UCLA, Los Angeles, Los Angeles, CA
A 48-year-old woman with 40 years of intermittent squeezing chest pain presented with worsening symptoms. Results of an ambulatory electrocardiogram, echocardiogram, and exercise treadmill were unremarkable. Persistent symptoms prompted a computed tomography coronary angiogram (CTCA) that revealed mid-left anterior descending artery myocardial bridging (MB) that was not physiologically significant by exercise single-photon emission CT. Conservative treatment was pursued. Anatomic MB is prevalent in a large proportion of the general population and are increasingly identified by CTCA. The majority are benign, physiologically significant bridging is uncommon, but accelerated proximal atherosclerosis can occur. β-blockers and nondihydropyridine calcium-channel blockers are the primary treatment options, with surgical myomectomy, coronary artery bypass, and stenting reserved for patients refractory to medical therapy with demonstrable ischemia. Head-to-head evaluation of nonpharmacologic therapies is needed. Intracoronary techniques provide simultaneous anatomical and physiological assessment but CTCA fractional flow reserve and hybrid positron emission tomography with concomitant spatial imaging systems are evolving as noninvasive alternatives.
[Rev Cardiovasc Med. 2016;17(1/2):69-75 doi: 10.3909/ricm0814]
© 2016 MedReviews®, LLC
Forty Years of Chest Pain: A Case Report and Contemporary Review of the Diagnostic and Therapeutic Options for Myocardial Bridging
Emily Tat,1 Richard Cheng, MD,1 Jeffrey S. Helfenstein, MD1,2
1Cedars-Sinai Heart Institute, Los Angeles, CA; 2The David Geffen School of Medicine at UCLA, Los Angeles, Los Angeles, CA
A 48-year-old woman with 40 years of intermittent squeezing chest pain presented with worsening symptoms. Results of an ambulatory electrocardiogram, echocardiogram, and exercise treadmill were unremarkable. Persistent symptoms prompted a computed tomography coronary angiogram (CTCA) that revealed mid-left anterior descending artery myocardial bridging (MB) that was not physiologically significant by exercise single-photon emission CT. Conservative treatment was pursued. Anatomic MB is prevalent in a large proportion of the general population and are increasingly identified by CTCA. The majority are benign, physiologically significant bridging is uncommon, but accelerated proximal atherosclerosis can occur. β-blockers and nondihydropyridine calcium-channel blockers are the primary treatment options, with surgical myomectomy, coronary artery bypass, and stenting reserved for patients refractory to medical therapy with demonstrable ischemia. Head-to-head evaluation of nonpharmacologic therapies is needed. Intracoronary techniques provide simultaneous anatomical and physiological assessment but CTCA fractional flow reserve and hybrid positron emission tomography with concomitant spatial imaging systems are evolving as noninvasive alternatives.
[Rev Cardiovasc Med. 2016;17(1/2):69-75 doi: 10.3909/ricm0814]
© 2016 MedReviews®, LLC
Forty Years of Chest Pain: A Case Report and Contemporary Review of the Diagnostic and Therapeutic Options for Myocardial Bridging
Emily Tat,1 Richard Cheng, MD,1 Jeffrey S. Helfenstein, MD1,2
1Cedars-Sinai Heart Institute, Los Angeles, CA; 2The David Geffen School of Medicine at UCLA, Los Angeles, Los Angeles, CA
A 48-year-old woman with 40 years of intermittent squeezing chest pain presented with worsening symptoms. Results of an ambulatory electrocardiogram, echocardiogram, and exercise treadmill were unremarkable. Persistent symptoms prompted a computed tomography coronary angiogram (CTCA) that revealed mid-left anterior descending artery myocardial bridging (MB) that was not physiologically significant by exercise single-photon emission CT. Conservative treatment was pursued. Anatomic MB is prevalent in a large proportion of the general population and are increasingly identified by CTCA. The majority are benign, physiologically significant bridging is uncommon, but accelerated proximal atherosclerosis can occur. β-blockers and nondihydropyridine calcium-channel blockers are the primary treatment options, with surgical myomectomy, coronary artery bypass, and stenting reserved for patients refractory to medical therapy with demonstrable ischemia. Head-to-head evaluation of nonpharmacologic therapies is needed. Intracoronary techniques provide simultaneous anatomical and physiological assessment but CTCA fractional flow reserve and hybrid positron emission tomography with concomitant spatial imaging systems are evolving as noninvasive alternatives.
[Rev Cardiovasc Med. 2016;17(1/2):69-75 doi: 10.3909/ricm0814]
© 2016 MedReviews®, LLC
KEY WORDS
Myocardial bridging • Myocardial bridge • Myocardial ischemia • Myocardial perfusion • Chest pain
KEY WORDS
Myocardial bridging • Myocardial bridge • Myocardial ischemia • Myocardial perfusion • Chest pain
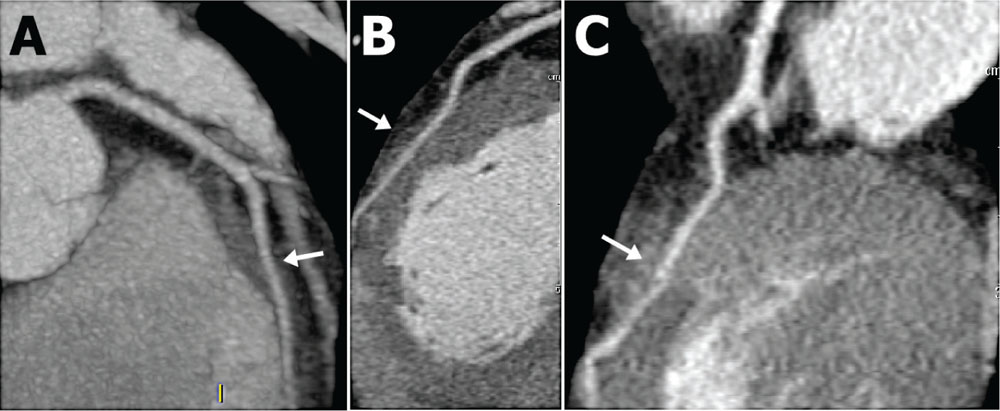
Figure 1. A) Intramyocardial course (arrow) of the left anterior descending artery. (B) Mid-left anterior descending myocardial bridge (arrow) during diastole. (C) Myocardial bridge (arrow) during systole showing trivial intraluminal narrowing.
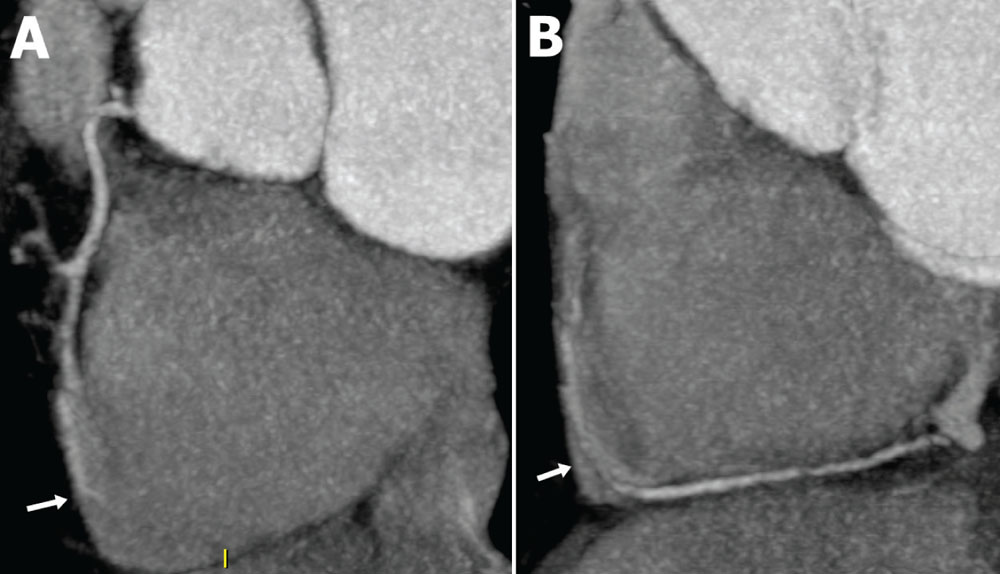
Figure 2. (A) Proximal and mid-right coronary artery showing an intracavitary bridge (arrow) in the mid-segment. (B) Mid and distal-right coronary artery showing the same intracavitary bridge (arrow).
Anatomic positioning is almost always in the mid-LAD but can be found on any epicardial artery.
Although most cases are benign, MB has been associated with clinical complications such as arrhythmia, coronary vasospasm, acute coronary syndromes, depressed left ventricular function, myocardial stunning, early death after cardiac transplantation, and sudden death.
The mainstay of treatment for symptomatic MB includes β-blockers and nondihydropyridine calcium channel blockers to relieve hemodynamic disturbances and effects of coronary vasospasm.


Main Points
• Anatomic myocardial bridges are common and increasingly detected by noninvasive imaging, but the majority are benign. In patients with ischemic symptoms, physiologic assessment with stress echocardiography, single-photon emission computed tomography, or positron emission tomography may be warranted.
• Accelerated atherosclerosis proximal to the bridging can occur and should be screened for in patients with risk factors for concomitant coronary artery disease.
• Patients with symptomatic and physiologically significant bridging can be treated with β-blockers or nondihydropyridine calcium channel blockers. Further testing with intracoronary Doppler, diastolic fractional flow reserve, and intravascular ultrasound can be considered for patients symptomatic on medical therapy in whom nonpharmacologic treatment options are being considered.
• Surgical myomectomy and coronary artery bypass grafting are the primary options for nonpharmacologic treatment of myocardial bridges. Percutaneous coronary intervention with first-generation drug-eluting stents are viable but less preferred, although outcomes data are still evolving for second-generation drug-eluting stents.
Main Points
• Anatomic myocardial bridges are common and increasingly detected by noninvasive imaging, but the majority are benign. In patients with ischemic symptoms, physiologic assessment with stress echocardiography, single-photon emission computed tomography, or positron emission tomography may be warranted.
• Accelerated atherosclerosis proximal to the bridging can occur and should be screened for in patients with risk factors for concomitant coronary artery disease.
• Patients with symptomatic and physiologically significant bridging can be treated with β-blockers or nondihydropyridine calcium channel blockers. Further testing with intracoronary Doppler, diastolic fractional flow reserve, and intravascular ultrasound can be considered for patients symptomatic on medical therapy in whom nonpharmacologic treatment options are being considered.
• Surgical myomectomy and coronary artery bypass grafting are the primary options for nonpharmacologic treatment of myocardial bridges. Percutaneous coronary intervention with first-generation drug-eluting stents are viable but less preferred, although outcomes data are still evolving for second-generation drug-eluting stents.
A 48-year-old woman presented to the cardiology clinic with 1 month of worsening symptoms and declared, “I have had chest pain for 40 years.” She recalls having spontaneous episodes of intermittent squeezing chest pain as a child that lasted 5 to 10 seconds in duration. Results of a pediatric workup were unrevealing and thought to be “growing pains.” However, the episodes continued into adulthood and past the births of two children. The chest pain occurred primarily at rest, at times accompanied by palpitations, without a clear trigger, and was not exacerbated by exercise. Over the past month the frequency of the symptoms has increased, and in one case lasted 15 minutes before subsiding. She has a history of attention deficit disorder for which she is intermittently treated with methylphenidate. Her cardiac symptoms preceded its use. She does not use illicit substances, and has a father who required a valve replacement.
Outpatient workup was initiated. An electrocardiogram (ECG) showed no accessory pathways or infarction; results of a 24-hour ambulatory ECG were unrevealing, and results of a transthoracic echocardiogram were unremarkable, notably without hypertrophy or mitral valve disease. She exercised 8.8 minutes and 10.1 METS on an exercise treadmill with no ischemic changes. Basic laboratory values were normal, with the exception of elevated cholesterol and C-reactive protein levels. She returned to the clinic complaining of persistent symptoms, and additional studies were ordered. A computed tomography coronary angiogram (CTCA) revealed a 3-cm long myocardial bridge (MB) in the mid-left anterior descending artery (LAD) without concomitant atherosclerotic plaque (Figure 1). Additionally, the mid-right coronary artery was intracavitary, traversing the right atrium (Figure 2). Exercise single-photon emission computed tomography (SPECT) demonstrated no defects, and magnetic resonance imaging (MRI) revealed no perfusion defects or scars. Given the lack of physiologic significance to the bridge, reassurance was provided to the patient; additional workup in the form of invasive intracoronary Doppler measurement with vasoreactivity testing to exclude vasospasm or microvascular disease was not pursued.
Discussion
MB is found in an unexpectedly large number of patients with angina without obstructive coronary artery disease, but their significance and the efficacy of treatment are not well established.1 Patients typically present with angina, syncope, or other symptoms of myocardial ischemia (Table 1), and MB should be especially considered in patients at low risk for atherosclerosis.2 MB is a congenital abnormality defined as an intramyocardial route of an epicardial coronary artery, and its prevalence varies by diagnostic modality. Coronary angiography, which has historically been the diagnostic standard, detects bridges in < 5% of cases, and has been outpaced by more sensitive modalities that more closely detect their prevalence in the general population, which is upwards of 86% as found by autopsy studies.3 CTCA studies report a 5.7% to 58% prevalence.4 The increased prevalence found by autopsy or CTCA can be attributed to anatomic evaluation of the bridge versus detection of systolic compression by coronary angiography, indicating that most bridges do not impact coronary perfusion. Anatomic positioning is almost always in the mid-LAD but can be found on any epicardial artery.5 Their prevalence is generally understood to be increased in patients with hypertrophic cardiomyopathy, with a 41% prevalence, although whether their presence increases the risk of cardiac death in this high-risk population has not been established.6,7
The pathophysiology of ischemia is classically attributed to systolic compression of the tunneled artery, although numerous studies have shown vessel compression extending into diastole causing increased flow velocities and reduced flow reserve.8-10 These hemodynamic effects may lead to endothelial damage, coronary vasospasm, and acute coronary syndrome.11 Degree of ischemia may also depend on the location, thickness, and length of the muscular bridge.12 Concomitant atherosclerosis proximal to the bridge due to hemodynamic disruptions occur in greater frequency, and an elevated C-reactive protein level may be an indicator of its development.8,10,13 Endothelial analysis at the proximal location showed structurally dysfunctional endothelial cells with a low shear stress state, allowing increased vascular expression for plaque development.14,15 In contrast, the tunneled segment of the artery is protected from atherosclerosis with structurally intact endothelium, laminar flow, and high shear.14 Although most cases are benign, MB has been associated with clinical complications such as arrhythmia, coronary vasospasm, acute coronary syndromes, depressed left ventricular function, myocardial stunning, early death after cardiac transplantation, and sudden death.16,17 It is still debated whether MB is the direct cause of these adverse events.18
Numerous imaging modalities can be used in the diagnosis of MB. Although coronary angiography has been historically used, CTCA shows promise as a noninvasive diagnostic tool, providing anatomic visualization of depth, length, and precise location of the tunneled artery.10,12 Deep MBs on CTCA have been found to be more greatly associated with mechanisms of ischemia, whereas superficial bridges are more often benign. Deep bridging is defined as > 2 mm of myocardial enclosure of the artery, and superficial bridging is defined as incomplete or < 2 mm depth of myocardial enclosure.19 Cardiac MRI is another noninvasive technique for visualization and functional assessment of MB.20,21 ECG, stress echocardiogram, SPECT, and positron emission tomography (PET) can be useful in the differential diagnosis for myocardial ischemia, and provide an alternative to intracoronary testing for the presence of functional ischemia in patients with MB. Exercise and dobutamine are preferred for stress testing.22-24 Patients with MB have a higher prevalence of a positive exercise ECG result,19,25 and septal wall abnormalities on stress echocardiogram have been shown to correlate with the presence of LAD bridges, a manifestation of increased velocity and decreased perfusion at the tunneled region of ischemia.26 Although stress imaging can provide useful physiologic information, its sensitivity and specificity for hemodynamically significant MB is incomplete and not fully established.23,24
In addition to coronary angiography, supplemental intracoronary diagnostic modalities include intravascular ultrasound (IVUS), intracoronary Doppler, and diastolic fractional flow reserve (FFR). Identification of systolic compression or an echolucent half-moon sign on IVUS confirms bridging.1 Diagnosis by intracoronary Doppler depends on identification of the characteristic early diastolic “fingertip” phenomenon, reduced systolic antegrade flow, decreased diastolic/systolic velocity ratio, and retrograde flow into the proximal segment.10 Additionally, diastolic FFR < 0.75 is an important measure of the hemodynamic significance of MB, indicating its association to ischemia.10 Noninvasive and intracoronary diagnostic modalities are summarized in Table 2.
The mainstay of treatment for symptomatic MB includes β-blockers and nondihydropyridine calcium channel blockers to relieve hemodynamic disturbances and the effects of coronary vasospasm. In contrast, nitrates should be used with caution due to reflex tachycardia that exacerbates ischemia from bridging.27-29 When symptoms sustain despite medical therapy, surgical options for treatment involve myotomy, resection of the overlying myocardium, or coronary artery bypass graft (CABG) surgery. Although both procedures have been shown to be comparable by clinical success, myotomy may be preferred for patients with substantial risk for myocardial infarction, ventricular tachycardia, or significant systolic compression. However, these surgical techniques have not been directly compared.30 Cases with deep or extensive MB likely favor CABG to eliminate the risk for ventricular perforation in myotomy. Percutaneous coronary intervention (PCI) has also been shown to resolve symptoms secondary to MB; however, there are numerous reports of coronary perforation and stent fraction, and reported rates of in-stent restenosis are suboptimal, although drug-eluting stents have reduced in-stent restenosis rates compared with bare-metal stents in this patient cohort.31 It is important to note that studies are lacking in the use of second-generation drug-eluting stents for treatment of bridges. Suggested steps in diagnosis and treatment of MB as informed by current literature and the Schwarz classification for MB and treatment are summarized in Table 3.10,29
Anatomic MB is prevalent in a large proportion of the general population and is increasingly identified by CTCA. Although the presence of a MB may account for some patients with angina without obstructive coronary artery disease, even in the absence of physiologic bridging, significant detectable ischemia is far less common as assessed by intracoronary and noninvasive diagnostic modalities such as intracoronary pressure wires and various forms of stress testing, and the vast majority are benign requiring only reassurance to the patient.22 β-blockers and nondihydropyridine calcium channel blockers are the primary treatment options; surgical myomectomy, CABG, and PCI are reserved for patients with demonstrable ischemia caused by bridging who are refractory to medical therapy. Recent advances in noninvasive imaging have both increased our appreciation of the prevalence of anatomic bridges and allowed us to assess for physiologic significance outside of the cardiac catheterization laboratory, but studies are needed to directly compare imaging modalities for their ability to detect ischemia in bridging. The arrival of CTCA fractional flow reserve and proliferation of hybrid PET and CT or MRI systems with intravenous contrast may also allow for noninvasive simultaneous anatomic and physiologic assessment of MB. Studies are needed to compare surgical myomectomy with CABG, and outcomes for PCI of bridges with second-generation drug-eluting stents are needed. ![]()
References
- Lee BK, Lim HS, Fearon WF, et al. Invasive evaluation of patients with angina in the absence of obstructive coronary artery disease. Circulation. 2015;131: 1054-1060.
- Ripa C, Melatini MC, Olivieri F, Antonicelli R. Myocardial bridging: a ‘forgotten’ cause of acute coronary syndrome - a case report. Int J Angiol. 2007;16: 115-118.
- Mohlenkamp S, Hort W, Ge J, Erbel R. Update on myocardial bridging. Circulation. 2002;106: 2616-2622.
- Nakanishi R, Rajani R, Ishikawa Y, et al. Myocardial bridging on coronary CTA: an innocent bystander or a culprit in myocardial infarction? J Cardiovasc Comput Tomogr. 2012;6:3-13.
- Qian JY, Zhang F, Dong M, et al. Prevalence and characteristics of myocardial bridging in coronary angiogram—data from consecutive 5525 patients. Chinese Med J (Engl). 2009;122:632-635.
- Shariat M, Thavendiranathan P, Nguyen E, et al. Utility of coronary CT angiography in outpatients with hypertrophic cardiomyopathy presenting with angina symptoms. J Cardiovasc Comput Tomogr. 2014;8: 429-437.
- Basso C, Thiene G, Mackey-Bojack S, et al. Myocardial bridging, a frequent component of the hypertrophic cardiomyopathy phenotype, lacks systematic association with sudden cardiac death. Eur Heart J. 2009;30:1627-1634.
- Duygu H, Zoghi M, Nalbantgil S, et al. High-sensitivity C-reactive protein may be an indicator of the development of atherosclerosis in myocardial bridging. Int J Cardiol. 2008;124:267-270.
- Klues HG, Schwarz ER, vom Dahl J, et al. Disturbed intracoronary hemodynamics in myocardial bridging: early normalization by intracoronary stent placement. Circulation. 1997;96:2905-2913.
- Corban MT, Hung OY, Eshtehardi P, et al. Myocardial bridging: contemporary understanding of pathophysiology with implications for diagnostic and therapeutic strategies. J Am Coll Cardiol. 2014;63:2346-2355.
- Maseri A, Beltrame JF, Shimokawa H. Role of coronary vasoconstriction in ischemic heart disease and search for novel therapeutic targets. Circ J. 2009;73:394-403.
- Ko SM, Choi JS, Nam CW, Hur SH. Incidence and clinical significance of myocardial bridging with ECG-gated 16-row MDCT coronary angiography. Int J Cardiovasc Imaging. 2008;24:445-452.
- Tian S, Li C, Song X, et al. Evaluation of association of myocardial bridge in the left anterior descending coronary with coronary atherosclerosis (stenosis > 50%) in the segment proximal to the site of bridge on coronary cta in hypertension subjects [Article in Chinese]. Zhonghua Yi Xue Za Zhi. 2014;94:1601-1604.
- Ishii T, Asuwa N, Masuda S, et al. Atherosclerosis suppression in the left anterior descending coronary artery by the presence of a myocardial bridge: an ultrastructural study. Mod Pathol. 1991;4:424-431.
- Cheng C, Tempel D, van Haperen R, et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744-2753.
- Alegria JR, Herrmann J, Holmes DR Jr, et al. Myocardial bridging. Eur Heart J. 2005;26:1159-1168.
- Bourassa MG, Butnaru A, Lespérance J, Tardif JC. Symptomatic myocardial bridges: overview of ischemic mechanisms and current diagnostic and treatment strategies. J Am Coll Cardiol. 2003;41:351-359.
- Rubinshtein R, Gaspar T, Lewis BS, et al. Long-term prognosis and outcome in patients with a chest pain syndrome and myocardial bridging: a 64-slice coronary computed tomography angiography study. Eur Heart J Cardiovasc Imaging. 2013;14:579-585.
- Jodocy D, Aglan I, Friedrich G, et al. Left anterior descending coronary artery myocardial bridging by multislice computed tomography: correlation with clinical findings. Eur J Radiol. 2010;73:89-95.
- Kelle S, Thouet T, Tangcharoen T, et al. Anatomical and functional evaluation of myocardial bridging on the left anterior descending artery by cardiovascular magnetic resonance imaging. J Cardiovasc Magn Reson. 2006;8:755-757.
- Canyigit M, Turkbey B, Hazirolan T, et al. Magnetic resonance imaging first-pass myocardial perfusion in evaluation of hemodynamic effects of myocardial bridging. J Comput Assist Tomogr. 2008;32:274-275.
- Gould KL, Johnson NP. Myocardial bridges: lessons in clinical coronary pathophysiology. JACC Cardiovasc Imaging. 2015;8:705-709.
- Gaibazzi N, Rigo F, Reverberi C. Severe coronary tortuosity or myocardial bridging in patients with chest pain, normal coronary arteries, and reversible myocardial perfusion defects. Am J Cardiol. 2011;108: 973-978.
- Gawor R, Kuśmierek J, Płachcińska A, et al. Myocardial perfusion GSPECT imaging in patients with myocardial bridging. J Nucl Cardiol. 2011;18: 1059-1065.
- Aksan G, Nar G, Inci S, et al. Exercise-induced repolarization changes in patients with isolated myocardial bridging. Med Sci Monit. 2015;21:2116-2124.
- Lin S, Tremmel JA, Yamada R, et al. A novel stress echocardiography pattern for myocardial bridge with invasive structural and hemodynamic correlation. J Am Heart Assoc. 2013;2:e000097.
- Schwarz ER, Klues HG, vom Dahl J, et al. Functional, angiographic and intracoronary Doppler flow characteristics in symptomatic patients with myocardial bridging: effect of short-term intravenous beta-blocker medication. J Am Coll Cardiol. 1996;27: 1637-1645.
- Alessandri N, Dei Giudici A, De Angelis S, et al. Efficacy of calcium channel blockers in the treatment of the myocardial bridging: a pilot study. Eur Rev Med Pharmacol Sci. 2012;16:829-834.
- Schwarz ER, Gupta R, Haager PK, et al. Myocardial bridging in absence of coronary artery disease: proposal of a new classification based on clinical-angiographic data and long-term follow-up. Cardiology. 2009;112:13-21.
- Wu QY, Xu ZH. Surgical treatment of myocardial bridging: report of 31 cases. Chin Med J (Engl). 2007;120:1689-1693.
- Lee CH, Kim U, Park JS, Kim YJ. Impact of myocardial bridging on the long-term clinical outcomes of patients with left anterior descending coronary artery disease treated with a drug-eluting stent. Heart Lung Circ. 2014;23:758-763.