Giant Thoracic Aneurysm Following Valve Replacement for Bicuspid Aortic Valve
Cao Tran, MD, Ehtesham Ul Haq, MD, Ngoc Nguyen, MD, Bassam Omar, MD, PhD
Division of Cardiology, University of South Alabama, Mobile, AL
Bicuspid aortic valve is a common congenital anomaly associated with aortopathy, which can cause aortic root dilatation, necessitating regular screening if the aortic root is > 4.0 cm. Despite the low absolute incidence of aortic complications associated with bicuspid aortic valve in the general population, the consequences of such complications for an individual patient can be devastating. Herein we propose a balanced algorithm that incorporates recommendations from the three major guidelines for follow-up imaging of the aortic root and ascending thoracic aorta in patients with a bicuspid aortic valve, maintaining the current recommendations with regard to surgical thresholds.
[ Rev Cardiovasc Med. 2015;16(4):255-260 doi: 10.3909/ricm0786 ]
© 2016 MedReviews®, LLC
Giant Thoracic Aneurysm Following Valve Replacement for Bicuspid Aortic Valve
Cao Tran, MD, Ehtesham Ul Haq, MD, Ngoc Nguyen, MD, Bassam Omar, MD, PhD
Division of Cardiology, University of South Alabama, Mobile, AL
Bicuspid aortic valve is a common congenital anomaly associated with aortopathy, which can cause aortic root dilatation, necessitating regular screening if the aortic root is > 4.0 cm. Despite the low absolute incidence of aortic complications associated with bicuspid aortic valve in the general population, the consequences of such complications for an individual patient can be devastating. Herein we propose a balanced algorithm that incorporates recommendations from the three major guidelines for follow-up imaging of the aortic root and ascending thoracic aorta in patients with a bicuspid aortic valve, maintaining the current recommendations with regard to surgical thresholds.
[ Rev Cardiovasc Med. 2015;16(4):255-260 doi: 10.3909/ricm0786 ]
© 2016 MedReviews®, LLC
Giant Thoracic Aneurysm Following Valve Replacement for Bicuspid Aortic Valve
Cao Tran, MD, Ehtesham Ul Haq, MD, Ngoc Nguyen, MD, Bassam Omar, MD, PhD
Division of Cardiology, University of South Alabama, Mobile, AL
Bicuspid aortic valve is a common congenital anomaly associated with aortopathy, which can cause aortic root dilatation, necessitating regular screening if the aortic root is > 4.0 cm. Despite the low absolute incidence of aortic complications associated with bicuspid aortic valve in the general population, the consequences of such complications for an individual patient can be devastating. Herein we propose a balanced algorithm that incorporates recommendations from the three major guidelines for follow-up imaging of the aortic root and ascending thoracic aorta in patients with a bicuspid aortic valve, maintaining the current recommendations with regard to surgical thresholds.
[ Rev Cardiovasc Med. 2015;16(4):255-260 doi: 10.3909/ricm0786 ]
© 2016 MedReviews®, LLC
KEY WORDS
Bicuspid aortic valve • Thoracic aneurysm • Aortic valve replacement
KEY WORDS
Bicuspid aortic valve • Thoracic aneurysm • Aortic valve replacement
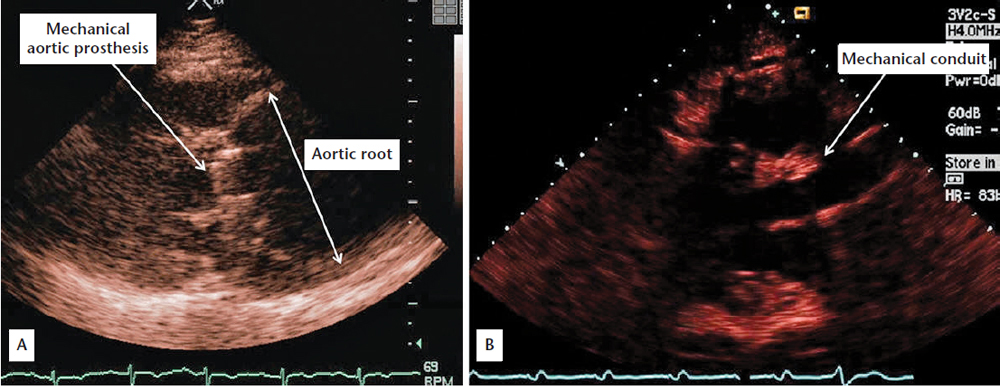
Figure 1. (A) Preoperative two-dimensional parasternal echocardiographic view showing dilated aortic root and mechanical aortic prosthesis. (B) Postoperative two-dimensional parasternal echocardiographic view showing mechanical conduit.

Figure 2. (A) Preoperative aortogram showing the massively dilated aortic root. (B) Extensive dissection along the descending thoracic aorta with darker opacification of the smaller true lumen, and lighter opacification of the larger false lumen.
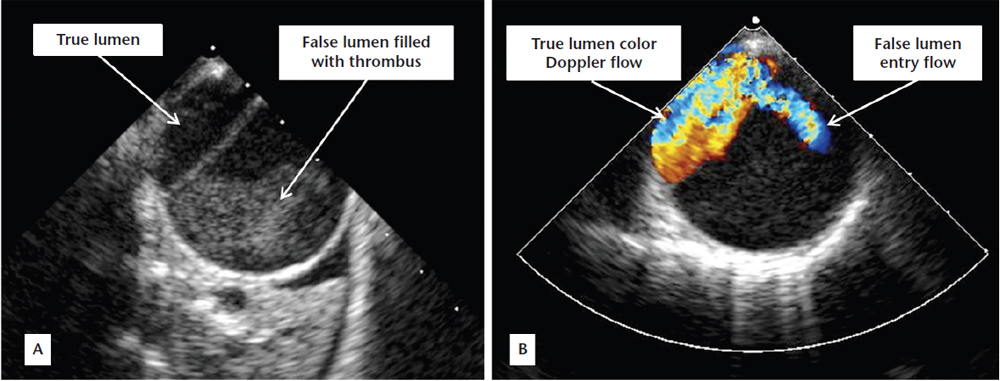
Figure 3. (A) Postoperative transesophageal echocardiographic view showing dissection within the descending thoracic aorta with a small true lumen and a larger thrombus-filled false lumen. (B) Color Doppler showing flow within the true lumen and an entry point to the false lumen.
… patients who were operated on for valve stenosis had significantly higher (93%) rate of 15-year freedom from late adverse aortic events (including proximal aortic intervention and aortic dissection/rupture) compared with those operated on for isolated aortic regurgitation (78%).
The incidence of aortic dissection over a mean of 16 years of followup in bicuspid aortic valve patients was approximately eight times higher than in the general population. However, despite this high relative risk, the absolute incidence of aortic dissection remained very low, at 3.1 cases per 10,000 patient-years.
Surgery is reasonable (Class IIa indication) with aortic dilation of 5.1 cm to 5.5 cm only if there is a family history of aortic dissection or rapid progression of dilation ($ 0.5 cm/y), and when the aortic diameter is . 4.5 cm in patients undergoing aortic valve replacement because of severe aortic valve stenosis or regurgitation.
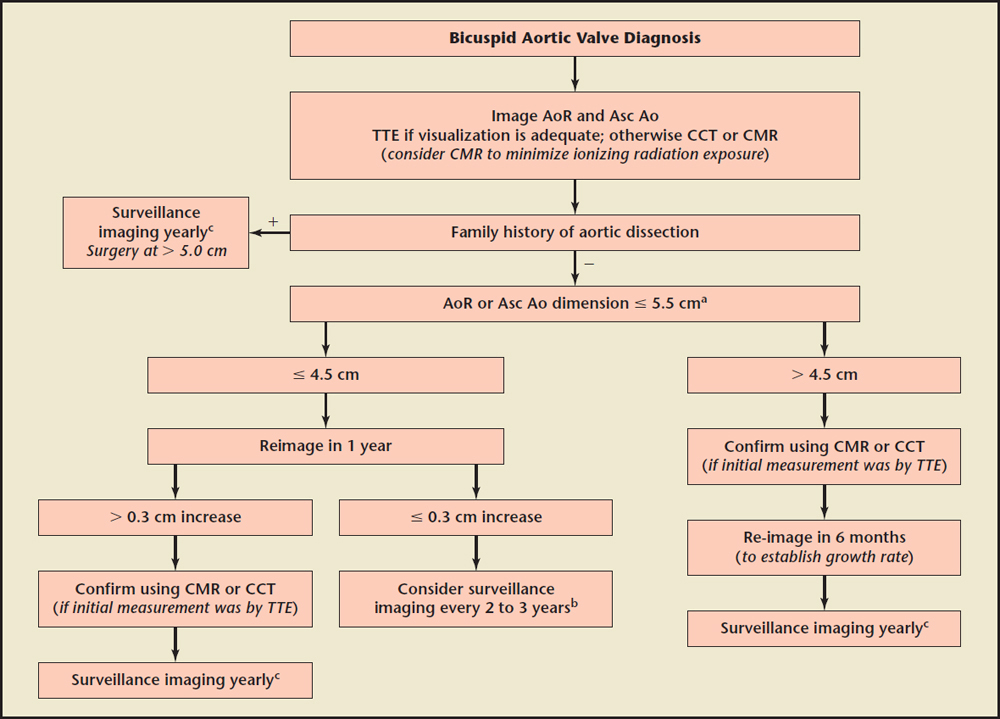
Figure 4. An algorithm for follow-up imaging on the aortic root and ascending aorta in patients with bicuspid aortic valve, incorporating recommendations from several guidelines. AoR, aortic root; Asc Ao, ascending aorta; CCT, cardiac computed tomography; CMR, cardiac magnetic resonance imaging; TTE, transthoracic echocardiogram. aSurgical dimension is . 5.5 cm, unless the rate of growth on a follow-up study is $ 0.5 cm/y, then surgical dimension should be . 5.0 cm. bPresence of risk factors such coarctation of the aorta and hypertension may warrant more frequent (yearly) surveillance imaging. cMay consider surveillance imaging every 6 months once within 0.5 cm of elective surgical dimension. Data from Nishimura RA et al,14 Hiratzka LF et al,15 and Erbel R et al.16
Main Points
• Bicuspid aortic valve is a common congenital valve anomaly and is heterogeneous in presentation and natural history. Despite the low absolute incidence of aortic complications associated with bicuspid aortic valve in the general population, the consequences of such complications for an individual patient can be devastating.
• There is aortic wall immaturity and increased susceptibility to dilation in bicuspid valves, which dictate different diagnostic and therapeutic approaches.
• Better employment of imaging techniques with a more sophisticated risk evaluation approach, incorporating aorta and aortic valve morphology, aortic stenosis severity, and the characteristics of aortic wall tissue, was suggested as an alternative to the limited aortic root dimensions recommended by guidelines for decisions regarding surgical intervention.
• The latest American College of Cardiology/American Heart Association guidelines for the management of patients with valvular heart disease acknowledge the limited data on the optimal timing of aortic surgery in bicuspid aortic valve patients. Despite a previously more aggressive approach of recommending aortic surgery on such patients when the aortic dilatation is . 5.0 cm, current guidelines consider the evidence supporting these previous recommendations very limited and anecdotal, and endorse a more individualized approach.
Main Points
• Bicuspid aortic valve is a common congenital valve anomaly and is heterogeneous in presentation and natural history. Despite the low absolute incidence of aortic complications associated with bicuspid aortic valve in the general population, the consequences of such complications for an individual patient can be devastating.
• There is aortic wall immaturity and increased susceptibility to dilation in bicuspid valves, which dictate different diagnostic and therapeutic approaches.
• Better employment of imaging techniques with a more sophisticated risk evaluation approach, incorporating aorta and aortic valve morphology, aortic stenosis severity, and the characteristics of aortic wall tissue, was suggested as an alternative to the limited aortic root dimensions recommended by guidelines for decisions regarding surgical intervention.
• The latest American College of Cardiology/American Heart Association guidelines for the management of patients with valvular heart disease acknowledge the limited data on the optimal timing of aortic surgery in bicuspid aortic valve patients. Despite a previously more aggressive approach of recommending aortic surgery on such patients when the aortic dilatation is . 5.0 cm, current guidelines consider the evidence supporting these previous recommendations very limited and anecdotal, and endorse a more individualized approach.
A60-year-old man who underwent aortic valve replacement with a mechanical prosthesis for severely incompetent bicuspid aortic valve and congestive heart failure 6 years earlier was referred for evaluation of dyspnea. Aortic root dimension at the time of surgery was reportedly < 4.5 cm, not meeting criteria for root replacement. He was maintained on warfarin and metoprolol since surgery. Echocardiography revealed massive aortic root dilatation, which measured 9.5 cm (Figure 1A). This was confirmed on aortography (Figure 2A), performed as part of angiography in preparation for surgery, which also revealed extensive dissection extending from the aortic arch down to the renal arteries (Figure 2B). He underwent repair of the arch and ascending aorta with a 28-mm Vascutek (Renfrewshire, Scotland, United Kingdom) tube graft to the arch, aortic root aneurysm repair, and aortic root replacement with a 24-mm St. Jude Medical (St. Paul, MN) valve mechanical conduit (Figure 1B) and reimplantation of coronary arteries. Postoperatively, transesophageal echocardiography revealed aortic dissection with thrombus in the false lumen of the descending aorta (Figure 3). The patient recovered well and was discharged with recommendations to undergo computed tomography angiography at 3 months, 6 months, and then yearly to assess the stability of the aortic repair and residual dissection.
Discussion
Bicuspid aortic valve is a common congenital valve anomaly and is heterogeneous in presentation and natural history. Schaefer and colleagues1 reviewed clinical data and transthoracic echocardiograms of 191 bicuspid aortic valve cases, assessing leaflet morphology, valve function, and aortic shape and dimensions; they identified three morphologies: type 1—fusion of right and left coronary cusp (80%); type 2—right and noncoronary cusp fusion (20%); and type 3—left and noncoronary cusp fusion (< 1%). They found a higher incidence of ascending aorta dilatation in type 2 and type 3 (when the noncoronary cusp is involved in the fusion) compared with the more common type 1.
Losenno and associates2 suggested that emerging evidence indicates a heterogeneous presentation of bicuspid aortic valve phenotypes related to complex congenital, genetic, and/or connective tissue abnormalities, causing variable degrees of aortic valvular abnormalities and ascending aortic dilatation. They proposed that optimal management requires careful assessment of various risk factors of the aortic valve and the aorta to determine ongoing surveillance, medical management, and operative intervention.
Grewal and associates3 examined ascending aortic wall biopsy specimens from bicuspid and tricuspid aortic valves. In tricuspid aortic valves the aortic wall showed more aspects of aging, whereas in bicuspid valves it had defective smooth muscle cell differentiation possibly linked to lower lamin A/C expression. They concluded that there is aortic wall immaturity and increased susceptibility to dilation in bicuspid valves, which dictate different diagnostic and therapeutic approaches.
Fedak and colleagues4 quoted a ≥ 50% echocardiographic evidence of aortic dilation in young patients with normally functioning bicuspid aortic valves. They argued that, despite ongoing controversy, the vascular complications of bicuspid aortic valve are not believed to be secondary to valvular dysfunction, and can therefore manifest in young adults without significant aortic stenosis or regurgitation, as well as in patients who underwent aortic valve replacement with prosthesis.
Charitos and coworkers5 investigated the evolution of aortic root and ascending aorta dimensions in patients with normal transvalvular hemodynamics after a subcoronary autograft aortic valve replacement (which preserved intact the native aortic wall). They reported no difference in aortic diameter increase rates among bicuspid and tricuspid aortic valve patients, or between bicuspid aortic valve phenotypes. They concluded that, for the first postoperative decade, transvalvular hemodynamics appeared to have a greater effect than the genetic component of bicuspid aortic valve on the development of aortopathy.
Conversely, Cohoon and colleagues6 reported a 22% incidence of ascending aortic aneurysm (> 4.5 cm) after 10-year follow-up, and a 12% death rate after 6-year follow-up, of bicuspid aortic valve patients who underwent elective aortic valve surgery with a mechanical St. Jude bioprosthesis. They concluded that lifelong serial monitoring of the ascending aorta in patients with bicuspid aortic valve should be the standard of care.
Girdauskas and coworkers7 reviewed a bicuspid aortic valve database of patients who underwent isolated aortic valve replacement. They reported that patients who were operated on for valve stenosis had significantly higher (93%) rate of 15-year freedom from late adverse aortic events (including proximal aortic intervention and aortic dissection/rupture) compared with those operated on for isolated aortic regurgitation (78%).
Girdauskas and coworkers8 studied patients with bicuspid aortic valve and dilated ascending aorta of ≥ 5.0 cm who underwent aortic valve replacement and simultaneous replacement of the proximal aorta. They reported nearly twice the elastic fiber loss in the ascending aorta in patients with aortic valve regurgitation compared with those with aortic valve stenosis, with a lower 10-year event-free survival of 64% compared with 93%, respectively.
Kallenbach and colleagues9 debated the need for prophylactic aortic surgery at ascending aortic aneurysm diameters of < 5.0 cm in patients with bicuspid aortic valve, to avoid acute type A aortic dissection, because 50% of such dissections have been reported with aortic diameters of < 5.5 cm. The success and lower mortality of surgical therapy to avoid such a devastating outcome may encourage earlier intervention. However, the low absolute incidence of aortic complications and the health implication of an earlier intervention may call for prudence. Overall, better employment of imaging techniques with a more sophisticated risk evaluation approach, incorporating aorta and aortic valve morphology, aortic stenosis severity, and possibly the characteristics of aortic wall tissue, was suggested as an alternative to the limited aortic root dimensions recommended by guidelines for decisions regarding surgical intervention.
Michelena and associates10 conducted a comprehensive assessment of aortic complications of patients with bicuspid aortic valve living in a population-based setting in Olmsted County, Minnesota. They reported an overall prevalence of 1.3 % of bicuspid aortic valve in the general population. The incidence of aortic dissection over a mean of 16 years of follow-up in bicuspid aortic valve patients was approximately eight times higher than in the general population. However, despite this high relative risk, the absolute incidence of aortic dissection remained very low, at 3.1 cases per 10,000 patient-years. An encouraging finding was that there was no excess mortality during 25 years of follow-up within this large bicuspid aortic valve community cohort.
Coady and associates11 analyzed data on patients with thoracic aortic aneurysms followed for nearly 12 years, and reported a median size at the time of rupture or dissection of 5.9 cm. Therefore, they recommended 5.5 cm as an acceptable size for elective operation on ascending aortic aneurysms. Svensson and coworkers,12 however, reported that, in patients with bicuspid aortic valve presenting with thoracic aortic dissection, 35% had an aortic size of ≤ 5.5 cm and 12.5% had an aortic size of < 5.0 cm. The authors, therefore, argued that, in the setting of a bicuspid aortic valve, thoracic aortic dissection may occur at a smaller size, and recommended elective operative repair at an aortic size of ≥ 4.5 cm. Observation from the International Registry of Acute Aortic Dissection reported a median ascending aortic diameter of 5.0 cm (mean 5.3 cm) in all patients presenting with type A dissection, casting further doubt on the adequacy of an aortic diameter of ≥ 5.5 cm as a good predictor of type A aortic dissection in the general population.13
The latest American College of Cardiology/American Heart Association (ACC/AHA) guidelines for the management of patients with valvular heart disease14 acknowledge the limited data on the optimal timing of aortic surgery in bicuspid aortic valve patients. Despite a previously more aggressive approach of recommending aortic surgery on such patients when the aortic dilatation is > 5.0 cm,15 current guidelines consider the evidence supporting these previous recommendations very limited and anecdotal, and endorse a more individualized approach. Surgery is reasonable (Class IIa indication) with aortic dilation of 5.1 cm to 5.5 cm only if there is a family history of aortic dissection or rapid progression of dilation (≥ 0.5 cm/y), and when the aortic diameter is > 4.5 cm in patients undergoing aortic valve replacement because of severe aortic valve stenosis or regurgitation. In all other patients, operation is indicated (Class I indication) if there is more severe dilation (5.5 cm). The writing committee also did not recommend the application of formulas to adjust the aortic diameter for body size. With regard to follow-up, the guidelines recommend (Class I indication) at least annual imaging in patients with significant aortic dilation (> 4.5 cm), a rapid rate of change, in aortic diameter, and in those with a family history of aortic dissection. In patients with milder dilation, no change on sequential studies and a negative family history of aortic dissection, a longer interval between imaging studies is considered appropriate. The 2014 European Society of Cardiology Guidelines on the diagnosis and treatment of aortic diseases16 recommend yearly imaging of the aortic root and ascending aorta when the dimension exceeds 4.5 cm or the growth rate exceeds 0.3 cm/y. These guidelines recommend surgery at a diameter of > 5.5 cm, with a stricter surgical threshold of > 5.0 cm in the presence of coarctation of the aorta, systemic hypertension, family history of dissection, or increase in aortic diameter of ≥ 0.3 cm/y (on repeated measurements using the same imaging technique, measured at the same aortic level, with side-by-side comparison and confirmed by another technique). Many of the guidelines' recommendations with regard to surveillance intervals of the aortic root and ascending aorta in bicuspid valve patients are level C recommendations, due to the absence of randomized clinical trials. We propose a balanced algorithm (Figure 4), which incorporates recommendations from the three major guidelines,14-16 for follow-up imaging of the aortic root and ascending thoracic aorta in patients with a bicuspid aortic valve, maintaining the ACC/AHA recommendations14 with regard to surgical thresholds. This algorithm may be applied in bicuspid aortic valve patients whether or not they have received isolated aortic valve replacement, as this has not been convincingly shown to alter the natural history of aortic dilation in this condition.
Conclusions
Bicuspid aortic valve is a common congenital anomaly associated with aortopathy. Despite the low absolute incidence of aortic complications associated with bicuspid aortic valve in the general population, the consequences of such complications for an individual patient can be devastating. Controversy is likely to continue with regard to the adequacy of the guideline recommendations in terms of the individual risk of aortic complications in bicuspid aortic valve patients, despite the low population risk of such complications. The risk obviously involves a complex interplay of hemodynamic, morphologic, and genetic factors. Improved future laboratory and diagnostic imaging techniques will better help characterize such risk and individualize therapy. Meanwhile, vigilance is recommended with adequate surveillance to avoid missing severe aortic dilatation, as we report in our patient who was lost to follow-up for years. ![]()
References
- Schaefer BM , Lewin MB, Stout KK, et al. The bicuspid aortic valve: an integrated phenotypic classification of leaflet morphology and aortic root shape. Heart. 2008;94:1634-1638.
- Losenno KL, Goodman RL, Chu MW. Bicuspid aortic valve disease and ascending aortic aneurysms: gaps in knowledge. Cardiol Res Pract. 2012;2012:145202.
- Grewal N, Gittenberger-de Groot AC, Poelmann RE, et al. Ascending aorta dilation in association with bicuspid aortic valve: a maturation defect of the aortic wall. J Thorac Cardiovasc Surg. 2014;148:1583-1590.
- Fedak PW, Verma S, David TE, et al. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation. 2002;106:900-904.
- Charitos EI, Stierle U, Petersen M, et al. The fate of the bicuspid valve aortopathy after aortic valve replacement. Eur J Cardiothorac Surg. 2014;45:e128-e135.
- Cohoon KP, Foley J, Dieter RS, et al. The development of ascending aortic aneurysms after elective aortic valve replacement with St Jude mechanical valve prosthesis in the bicuspid patient: a pilot study. Angiology. 2013;64:379-384.
- Girdauskas E, Disha K, Secknus M, et al. Increased risk of late aortic events after isolated aortic valve replacement in patients with bicuspid aortic valve insufficiency versus stenosis. J Cardiovasc Surg (Torino). 2013;54:653-659.
- Girdauskas E, Rouman M, Borger MA, Kuntze T. Comparison of aortic media changes in patients with bicuspid aortic valve stenosis versus bicuspid valve insufficiency and proximal aortic aneurysm. Interact Cardiovasc Thorac Surg. 2013;17:931-936.
- Kallenbach K, Sundt TM, Marwick TH. Aortic surgery for ascending aortic aneurysms under 5.0 cm in diameter in the presence of bicuspid aortic valve. JACC Cardiovasc Imaging. 2013;6:1321-1326.
- Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA. 2011;306:1104-1112.
- Coady MA, Rizzo JA, Hammond GL, et al. Surgical intervention criteria for thoracic aortic aneurysms: a study of growth rates and complications. Ann Thorac Surg. 1999;67:1922-1926.
- Svensson LG, Kim KH, Lytle BW, Cosgrove DM. Relationship of aortic cross-sectional area to height ratio and the risk of aortic dissection in patients with bicuspid aortic valves. J Thorac Cardiovasc Surg. 2003;126:892-893.
- Pape LA, Tsai TT, Isselbacher EM, et al; International Registry of Acute Aortic Dissection (IRAD) Investigators. Aortic diameter ≥ 5.5 cm is not a good predictor of type A aortic dissection: observations from the International Registry of Acute Aortic Dissection (IRAD). Circulation. 2007;116: 1120-1127.
- Nishimura RA, Otto CM, Bonow RO, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63: 2438-2488.
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM Guidelines for the diagnosis and management of patients with thoracic aortic disease. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. J Am Coll Cardiol. 2010;55: e27-e129.
- Erbel R, Aboyans V, Boileau C, et al; ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. Eur Heart J. 2014;35: 2873-2926.