Renal Denervation: Past, Present, and Future
Bashar Hannawi, MD, Homam Ibrahim, MD, Colin M. Barker, MD
Houston Methodist Hospital, Houston, TX
Hypertension remains a leading cause of cardiovascular morbidity and mortality worldwide. It is estimated that 12.8% of hypertensive adults have resistant hypertension. The sympathetic nervous system is a well-known contributor to the pathophysiology of resistant hypertension. Renal denervation has emerged as an effective procedure to treat resistant hypertension by blocking the sympathetic nervous system. The medical device industry has developed various catheters in an effort to achieve better denervation in the absence of available testing to document adequate denervation. By adding a sham control group to the study design, researchers found that the results of the Renal Denervation in Patients With Uncontrolled Hypertension study (SYMPLICITY HTN-3) showed that renal denervation was not superior to placebo in decreasing systolic blood pressure. Although SYMPLICITY HTN-3 successfully addressed many issues that might have biased the previously published data, incomplete denervation caused by limited operator experience, catheter design, and the radiofrequency ablation technology may have accounted for the discrepancy of the results. This, along with differences in the study design and population, should direct future renal denervation studies. This article reviews the available literature and proposes future directions for renal denervation studies. It also provides a detailed comparison of the available catheters and their respective clinical data.
[Rev Cardiovasc Med. 2015;16(2):114-124 doi: 10.3909/ricm0755]
© 2015 MedReviews®, LLC
Renal Denervation: Past, Present, and Future
Bashar Hannawi, MD, Homam Ibrahim, MD, Colin M. Barker, MD
Houston Methodist Hospital, Houston, TX
Hypertension remains a leading cause of cardiovascular morbidity and mortality worldwide. It is estimated that 12.8% of hypertensive adults have resistant hypertension. The sympathetic nervous system is a well-known contributor to the pathophysiology of resistant hypertension. Renal denervation has emerged as an effective procedure to treat resistant hypertension by blocking the sympathetic nervous system. The medical device industry has developed various catheters in an effort to achieve better denervation in the absence of available testing to document adequate denervation. By adding a sham control group to the study design, researchers found that the results of the Renal Denervation in Patients With Uncontrolled Hypertension study (SYMPLICITY HTN-3) showed that renal denervation was not superior to placebo in decreasing systolic blood pressure. Although SYMPLICITY HTN-3 successfully addressed many issues that might have biased the previously published data, incomplete denervation caused by limited operator experience, catheter design, and the radiofrequency ablation technology may have accounted for the discrepancy of the results. This, along with differences in the study design and population, should direct future renal denervation studies. This article reviews the available literature and proposes future directions for renal denervation studies. It also provides a detailed comparison of the available catheters and their respective clinical data.
[Rev Cardiovasc Med. 2015;16(2):114-124 doi: 10.3909/ricm0755]
© 2015 MedReviews®, LLC
Renal Denervation: Past, Present, and Future
Bashar Hannawi, MD, Homam Ibrahim, MD, Colin M. Barker, MD
Houston Methodist Hospital, Houston, TX
Hypertension remains a leading cause of cardiovascular morbidity and mortality worldwide. It is estimated that 12.8% of hypertensive adults have resistant hypertension. The sympathetic nervous system is a well-known contributor to the pathophysiology of resistant hypertension. Renal denervation has emerged as an effective procedure to treat resistant hypertension by blocking the sympathetic nervous system. The medical device industry has developed various catheters in an effort to achieve better denervation in the absence of available testing to document adequate denervation. By adding a sham control group to the study design, researchers found that the results of the Renal Denervation in Patients With Uncontrolled Hypertension study (SYMPLICITY HTN-3) showed that renal denervation was not superior to placebo in decreasing systolic blood pressure. Although SYMPLICITY HTN-3 successfully addressed many issues that might have biased the previously published data, incomplete denervation caused by limited operator experience, catheter design, and the radiofrequency ablation technology may have accounted for the discrepancy of the results. This, along with differences in the study design and population, should direct future renal denervation studies. This article reviews the available literature and proposes future directions for renal denervation studies. It also provides a detailed comparison of the available catheters and their respective clinical data.
[Rev Cardiovasc Med. 2015;16(2):114-124 doi: 10.3909/ricm0755]
© 2015 MedReviews®, LLC
KEY WORDS
Renal denervation • Resistant hypertension • Interventional cardiology
KEY WORDS
Renal denervation • Resistant hypertension • Interventional cardiology
Renal denervation using intra-arterial catheter-based radiofrequency ablation was developed to simulate surgical splanchnicectomy.
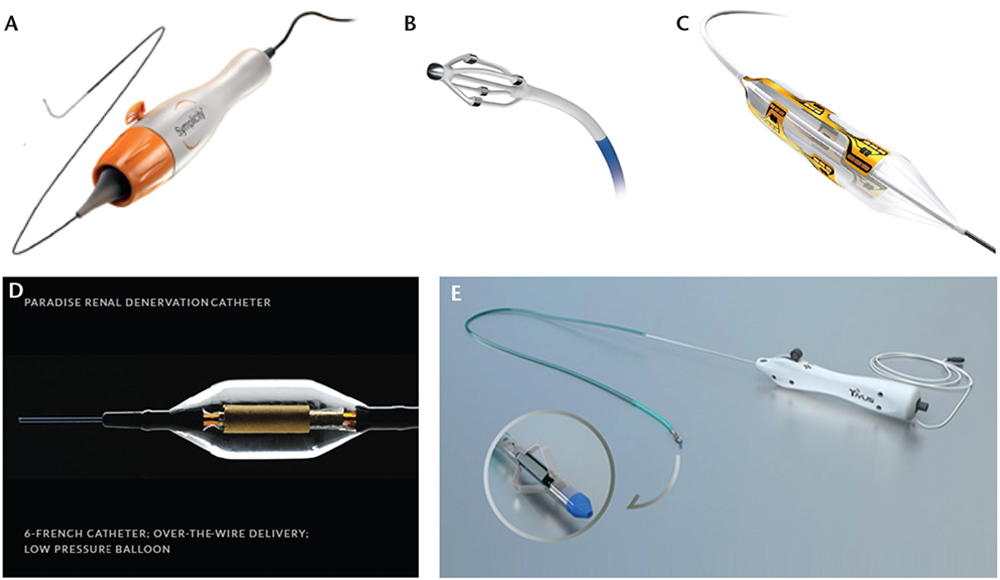
Figure 1. Renal denervation devices. (A) Medtronic Symplicity™ Flex catheter (Image provided courtesy of Medtronic, Minneapolis, MN). (B) EnligHTN™ Renal Artery Ablation Catheter (EnligHTN and St. Jude Medical are trademarks of St. Jude Medical, Inc. or its related companies. Reprinted with permission of St. Jude Medical, St. Paul, MN. © 2014. All rights reserved.). (C) Boston Scientific Vessix™ Vascular V2 system balloon catheter (Image provided courtesy of Boston Scientific, Marlborough, MA. © 2014 Boston Scientific Corporation or its affiliates. All rights reserved.). (D) ReCor Medical PARADISE™ catheter (Image used with permission of ReCor Medical, Ronkonkoma, NY). (E) CardioSonic TIVUS system (Image used with permission of CardioSonic, Tel Aviv, Israel).
… white coat hypertension may be a risk factor for the development of sustained hypertension.
In the currently available RF ablation catheters, the average depth of ablations extends only to 3 to 4 mm; thus, these catheters will theoretically miss at least 30% of nerves, and even more if intimal hyperplasia or atherosclerotic plaques are present.
Plasma renin and aldosterone activity also decline with age; thus, the renin-aldosterone system will have less implication in the pathogenesis of hypertension in elderly patients.
Procedural adverse effects that have been described in the literature were not directly related to the RDN procedure.
RDN decreased anxious and depressive mood, improved self-assessed physical and mental status, and reduced headache intensity.
Main Points
• Hypertension remains a leading cause of major cardiovascular diseases worldwide. Renal denervation (RDN) using intra-arterial catheter-based radiofrequency (RF) ablation was developed to simulate surgical splanchnicectomy, which had a high rate of significant complications.
• Different technologies have been developed to achieve RDN. The device should be able to achieve a maximal and irreversible destruction of the renal nerves, in a predictable pattern, while minimizing injury to the renal artery. The two technologies that have been used in humans thus far are RF ablation-based RDN and ultrasound energy-based RDN.
• Although many registries have found RDN to be an effective and safe procedure to control blood pressure in patients with resistant hypertension, SYMPLICITY HTN-3, the first blinded sham-controlled trial, failed to reach its primary efficacy endpoint, defined as a change in office systolic blood pressure at 6 months.
• Although SYMPLICITY HTN-3 successfully addressed many issues that might have biased the previously published data, incomplete denervation along with differences in the study design and population may have accounted for the discordance with prior studies. These factors should direct future renal denervation studies.
• Studies are underway to examine the effects of RDN on other conditions other than hypertension, such as insulin resistance, left ventricular hypertrophy, and atrial fibrillation.
Main Points
• Hypertension remains a leading cause of major cardiovascular diseases worldwide. Renal denervation (RDN) using intra-arterial catheter-based radiofrequency (RF) ablation was developed to simulate surgical splanchnicectomy, which had a high rate of significant complications.
• Different technologies have been developed to achieve RDN. The device should be able to achieve a maximal and irreversible destruction of the renal nerves, in a predictable pattern, while minimizing injury to the renal artery. The two technologies that have been used in humans thus far are RF ablation-based RDN and ultrasound energy-based RDN.
• Although many registries have found RDN to be an effective and safe procedure to control blood pressure in patients with resistant hypertension, SYMPLICITY HTN-3, the first blinded sham-controlled trial, failed to reach its primary efficacy endpoint, defined as a change in office systolic blood pressure at 6 months.
• Although SYMPLICITY HTN-3 successfully addressed many issues that might have biased the previously published data, incomplete denervation along with differences in the study design and population may have accounted for the discordance with prior studies. These factors should direct future renal denervation studies.
• Studies are underway to examine the effects of RDN on other conditions other than hypertension, such as insulin resistance, left ventricular hypertrophy, and atrial fibrillation.
Hypertension remains a leading cause of major cardiovascular diseases worldwide. It is estimated that 26.4% of adults, approximately 972 million, had hypertension in 2000. This number is predicted to increase to a total of 1.5 billion in 2025.1 The data from the National Health and Nutrition Examination Survey from 2003 through 2008 estimates that 12.8% of hypertensive adults have resistant hypertension, defined by blood pressure (BP) ≥ 140/90 mm Hg while using at least three different classes of medication or using four different classes of medication regardless of BP.2 The sympathetic nervous system is now a well-known contributor to the initiation, maintenance, and progression of hypertension. The kidneys receive efferent sympathetic nervous signals from the central nervous system through the preganglionic nerves located in the spinal cord, then through the splanchnic nerves to the mesenteric ganglia, from which signals are relayed again through the renal artery adventitia to innervate all structures of the kidneys. Activation of the efferent sympathetic system results in an increase in sodium and water retention, renin release and activation of the renin-angiotensin-aldosterone system, and renal arteriolar vasoconstriction. The kidneys also monitor their hydrostatic pressure and interstitial chemical compositions and relay signals through the afferent sympathetic nerves to the central nervous system. Activation of this afferent arm results in vasoconstriction and fluid retention. The splanchnicectomy procedure was developed to test the hypothesized utility of sympathetic denervation to treat hypertension. The procedure, comprising denervation of thoracic and abdominal organs, was effective in decreasing BP; however, given the high rate of complications, including refractory postural hypotension, this procedure was abandoned. Renal denervation (RDN) using intra-arterial catheter-based radiofrequency (RF) ablation was developed to simulate surgical splanchnicectomy. The procedure has emerged as an effective and safe procedure to control BP in patients with resistant hypertension. Some evidence also supports the use of RDN in patients with heart failure, atrial fibrillation, and metabolic syndrome. This review summarizes the evidence behind use of RDN, discusses employment of RDN in patients with conditions other than uncontrolled hypertension, and summarizes the currently available technology for RDN.
RDN and Available Evidence for its Use
Krum and colleagues3 published one of the first case reports about RDN, describing its use in a 59-year-old man with a long-standing history of hypertension despite being on seven different antihypertensive medications. RDN resulted in a significant and sustained drop in BP from 161/107 mm Hg at baseline to 140/91 mm Hg and 127/81 mm Hg at 1 and 12 months, respectively. RDN also resulted in a reduction in norepinephrine spillover by 48% from the left kidney and by 75% from the right kidney, along with a halving of renin activity and a reduction in muscle sympathetic nerve activity.
The first in-human, proof-of-concept study of RDN use was a prospective cohort study that enrolled 50 patients, of whom 45 underwent RDN. Mean BP decreased from a mean baseline of 177/101 mm Hg by 14/10 mm Hg, 21/10 mm Hg, 22/11 mm Hg, and 27/17 mm Hg, at 1, 3, 6, and 12 months, respectively. Only 6 of the 45 patients (16%) showed a decrease in systolic BP (SBP) by < 10 mm Hg and were defined as nonresponders.3 These promising results, with no complications directly related to the RDN procedure, led to the Renal Denervation in Patients With Refractory Hypertension study (SYMPLICITY HTN-1), which was the first open-label study to evaluate the long-term efficacy of RDN.
SYMPLICITY HTN-1 explored whether anatomic regrowth of renal nerves led to functional renervation. The study recruited 153 patients with resistant hypertension, employing an initial follow-up period of 24 months,4 followed by an extended follow-up period of 36 months.5 By 36 months, data were available for 88 patients (94% of patients who consented to be followed for up to 36 months). Reductions in SBP and diastolic BP (DBP), which were seen by 12 months, persisted and were progressively increased at 36 months (mean change of SBP/DBP from baseline was −26.5/−13.5 mm Hg and −32.0/−14.4 mm Hg at 12 and 36 months, respectively).4,5 The proportion of patients who achieved target SBP < 140 mm Hg increased progressively over the study follow-up period, up to 36 months.4 Response to RDN, as defined previously,3 increased from 69% at 1 month follow-up to 93% at 36 months.4
Because it was an open-label study, the SYMPLICITY HTN-1 was subject to many factors that might have biased its results. The SYMPLICITY investigators then performed the first RDN randomized controlled trial, randomizing 106 patients to either RDN or observation after 2 weeks of compliance with antihypertensive medications. The study found a decrease in office SBP/DBP by 32/12 mm Hg at 6 months from a baseline of 178/96 mm Hg (P < .0001), compared with a change of +1/0 mm Hg in the control group (P = NS),6 that persisted at 12-month follow-up with a 28.1/−9.7 mm Hg drop in SBP/DBP compared with baseline (P < .001)7 RDN was effective in decreasing SBP by ≥ 10 mm Hg in 83.7% and 78.7% of patients at 6- and 12-month follow-up, respectively.6,7 At 6 months, patients in the control group were given the option to receive RDN, which was successful in reducing SBP/DBP by 23.7/8.4 mm Hg after 6 months in eligible patients (n = 35; P < .001).7
The results of the recently published meta-analysis (12 studies, 561 patients) were consistent with these findings. Analyzing three controlled studies, the meta-analysis found a significant change in mean SBP/DBP by −28.9/−11.0 mm Hg at 6 months compared with medical therapy alone (P < .0001). Conversely, the mean change in SBP/DBP at 6 months was −25.0/−10.0 mm Hg in uncontrolled studies.8
White coat hypertension, first described in 1988, is used to describe patients who have elevated office BP but normal readings throughout their usual daily activities.9 White coat hypertension, as defined by an office SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg, and a mean awake SBP ≤ 135 mm Hg and DBP ≤ 90 mm Hg, carries a significantly lower risk for cardiovascular events than that seen for patients who have true hypertension.10,11 Nonetheless, white coat hypertension may be a risk factor for the development of sustained hypertension.11
Although it was available only in 20 patients (< 50%) in Renal Denervation in Patients With Uncontrolled Hypertension (SYMPLICITY HTN-2), ambulatory BP changed in parallel with office-based SBP by a mean decrease of 11/7 mm Hg. In a recent large, prospective study recruiting 346 patients with resistant hypertension as defined by the original criteria, patients were separated into true (n = 303) and pseudo (n = 43) resistant hypertension, defined by a mean ambulatory 24-hour SBP < 130 mm Hg. Although RDN achieved a comparable decrease in office SBP/DBP in both groups, it was effective in reducing ambulatory BP only in patients with true resistant hypertension at 3-, 6-, and 12-month follow-up (changes in SBP and DBP of −10.1/−10.2/−11.7 mm Hg and −4.8/−4.9/−7.4 mm Hg, respectively).12
Although these discrepancies between office and ambulatory BP response to RDN might simply be due to white coat hypertension, there are other factors that might explain this finding, including the design of the studies and bias effect. In a recent meta-analysis of 31 drug trials enrolling 4121 patients, the authors found that pressure reductions were 5.6 mm Hg greater on office measurements than ambulatory BP monitoring (P < .0001), and, by randomization and blinding, office BP reductions were identical to ambulatory BP reductions. Because there was no randomized blinded RDN trial at that time, the authors predict that, as RDN trial designs improve, office and ambulatory pressure drops will converge.13
Most recently, SYMPLICITY investigators reported the results of the first randomized sham-control trial of RDN, SYMPLICITY HTN-3. The study was carefully designed to overcome the drawbacks of the previous RDN studies, specifically by adding a sham procedure and excluding patients with white coat hypertension by adding mean ambulatory BP > 135 mm Hg as one of the inclusion criteria.14 A total of 535 patients underwent randomization in a 2:1 ratio. The study showed a reduction in SBP at 6 months of 14.13 ± 23.93 mm Hg in the RDN group compared with 11.74 ± 25.94 mm Hg reduction in the sham-procedure group, for a difference of −2.39 mm Hg between groups (P = .26 for superiority with a margin of 5 mm Hg); 24-hour ambulatory systolic BP decreased by 6.75 ± 15.11 mm Hg in the denervation group and by 4.79 ± 17.25 mm Hg in the sham-procedure group (P = .98 for superiority with a margin of 2 mm Hg). Both groups had significant reductions of BP compared with baseline (P < .001).14 RDN was an effective procedure by the original definition in 58% of those enrolled, compared with 84% in SYMPLICITY HTN-2.6
Future Directions
Although SYMPLICITY HTN-3 effectively addressed many factors that may have biased previous RDN studies, such as regression to mean and the Hawthorne effect, by adding a sham procedure, there are a few points that should be addressed before moving forward with this technology.
Was There a Problem With Trial Design ?
The study required patients to be on a stable antihypertensive regimen for 2 weeks prior to enrollment, which was the case in approximately 80% of the study population. Interestingly, 39% of the patients underwent medication changes after inclusion and randomization into the study group, with one-third of these patients having at least two medications changed, most often (70%) switching to maximally tolerated doses. This raises the question of whether 2 weeks is a sufficient duration to achieve maximal BP-lowering effects from a given antihypertensive regimen. It also highlights the importance of optimizing medical therapy in the treatment of resistant hypertension. In line with these findings, optimizing medical therapy was superior to RDN in a small randomized trial of 20 patients with true resistant hypertension.15 This should be kept in mind when designing future RDN trials.
Was This Due to Limited Operator's Experience?
Physicians involved in the SYMPLICITY HTN-3 trial were among the first practitioners to perform RDN in the United States. In comparison, physicians in Europe had performed more than 30 procedures in the SYMPLICITY Registry, and most had participated in previous SYMPLICITY studies. A subgroup analysis from SYMPLICITY HTN-3 showed that approximately 35 operators did only one procedure. With no available test to confirm procedural success, operator experience may have accounted for the discrepancy of results.
Was Denervation Adequate in This Trial?
Recent studies have delineated anatomic renal nervous systems in porcine models16 and in humans.17 As the nerves travel distally along the renal arteries, they are less abundant and closer to the lumen. There is also a difference in circumferential distribution, with the nerves more abundant in the ventral regions. Sakakura and colleagues17 found, in human autopsies, that the nerves are distributed as much as 10 mm from the vessel lumen. In the proximal regions, 75% of nerves are located within 4.67 mm of the lumen, whereas in the distal regions, they are located within 3.24 mm of the lumen. In the currently available RF ablation catheters, the average depth of ablations extends only to 3 to 4 mm; thus, these catheters will theoretically miss at least 30% of nerves, and even more if intimal hyperplasia or atherosclerotic plaques are present. These findings were confirmed when Vink and colleagues18 reported limited destruction of renal nerves in a postmortem study of a patient who underwent RDN with the Symplicity™ catheter (Medtronic, Minneapolis, MN).18 Although nerve bundles were seen at distances between 1 and 4 mm from the luminal surface, RF ablation did not penetrate deeper than 2 mm. The patient expired only 12 days after RDN due to aortic dissection, which limited the evaluation of effect of RDN on BP. Furthermore, when reduction in renal norepinephrine level was studied in a pig model to evaluate effectiveness of an RDN procedure, efficacy was seen in only one artery where ablation involved all four quadrants, reached a depth of 9.1 mm, and affected 50% of the nerves.19 In fact, in SYMPLICITY HTN-3, greater BP decreases occurred when more than eight ablations were performed, which was the case in only 163 (< 50%) patients.20
The Symplicity catheter requires the operator to perform the ablation while pulling and rotating the catheter to perform circumferential ablations in different quadrants of the renal artery to achieve at least four ablations. In SYMPLICITY HTN-3, only 25% of patients had four-quadrant ablations (inferior, superior, anterior, and posterior) in at least one renal artery with progressive reduction in SBP in patients who had four-quadrant ablations achieved neither one or both renal arteries.20 This supports the hypothesis that a circumferential ablation that is deep enough to knock out sufficient nerves is necessary to achieve effective denervation. Whether circumferential ablation can be achieved with RF catheters has been brought into question by demonstrations of thermal ablation limited to 20% circumferential extension.21 This circumferential limitation, when added to the limited penetration of RF ablation, results in a high rate of unaffected renal nerves, rendering the denervation incomplete and the procedure ineffective. In SYMPLICITY HTN-1, investigators measured norepinephrine spillover in 10 patients by a radiotracer methodology and found a reduction by 47% at 1 month after RDN.3 Applying this test to a large number of patients in a clinical trial would be hard to implement.
These factors taken together, notwithstanding the pain associated with the procedure that might limit the achievement of a sufficient number of ablations, raise the question of inadequate denervation as the main cause of the failure of SYMPLICITY HTN-3 and suggest the need for more preclinical data and a better understanding of the ablative procedure before moving forward with RDN.
Was the Study Population Inappropriate ?
The multifactorial pathophysiology of essential hypertension is now well known. Several factors affect the development and maintenance of hypertension in different patient populations. Blacks are known to have more severe and resistant hypertension and are more prone to have earlier onset of end-organ damage. Blacks more commonly have a repressed renin-aldosterone system22 and, therefore, are expected to have a lower response to therapies that directly act on the renin-angiotensin-aldosterone system for BP control. This explains the decreased response to enalapril in blacks (on either a low- or high-sodium diet) when compared with patients of European descent.23 Interestingly, the reduction in SBP in response to RDN was similar between African Americans and those who were not African American (15.5 mm Hg vs 15.2 mm Hg, respectively). Conversely, African Americans showed a marked reduction in SBP in response to sham procedure compared with those who were not African American (17.8 mm Hg vs 8.6 mm Hg, respectively), which could be due to unmeasured factors, such as socioeconomic status. Baseline medications were also associated with different responses to RDN. Being on an aldosterone antagonist was associated with a positive response whereas being on a direct vasodilator was associated with an increase in SBP at 6 months.20
Plasma renin and aldosterone activity also decline with age; thus, the renin-aldosterone system will have less implication in the pathogenesis of hypertension in elderly patients.24 A subgroup analysis of SYMPLICITY HTN-3 showed that RDN significantly decreased SBP in patients < 65 years old compared with sham procedure (16.7 mm Hg vs 10.9 mm Hg, respectively). Although SYMPLICITY HTN-3 was not powered to detect differences in each predefined subgroup, it does appear that RDN might be effective in certain populations, and we believe that further evaluation of these subgroups is warranted.
Current Technology
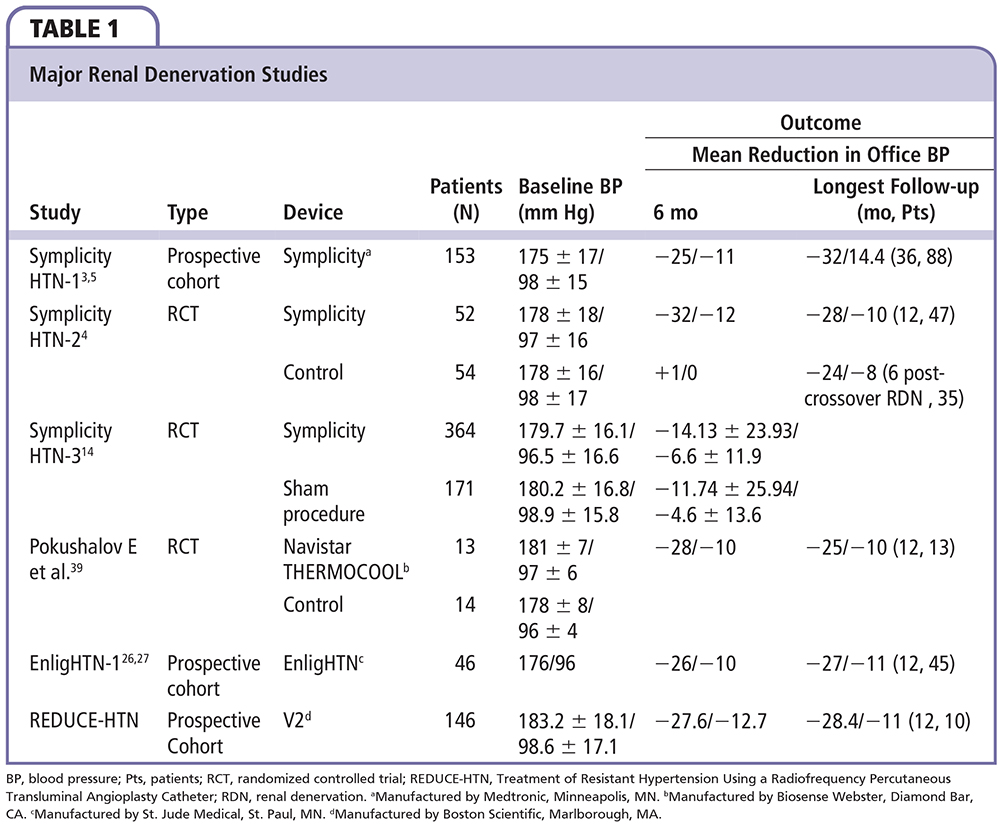
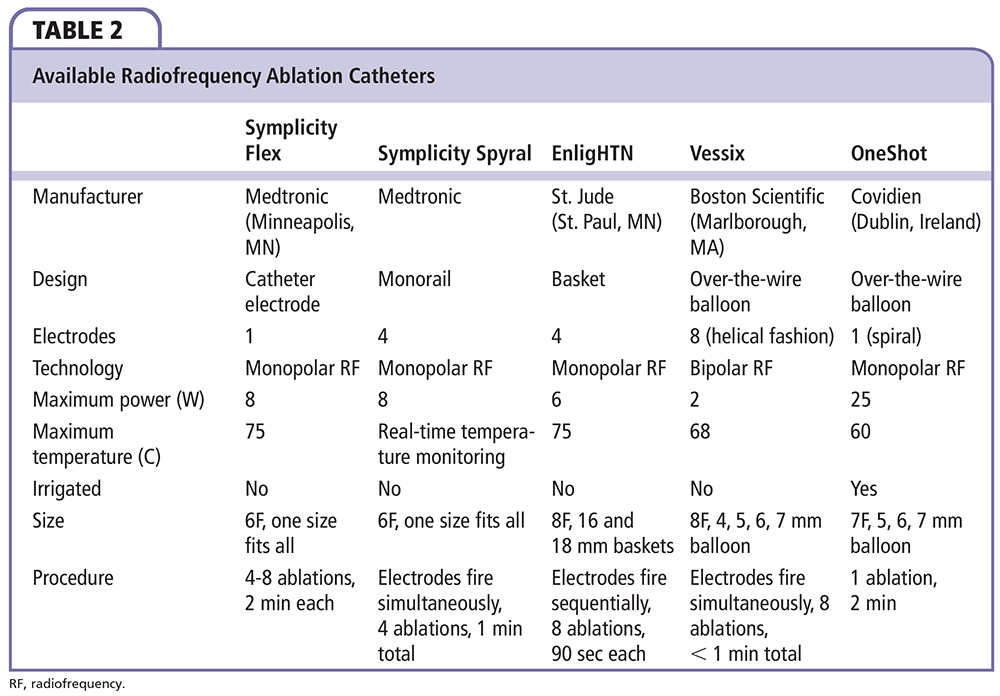
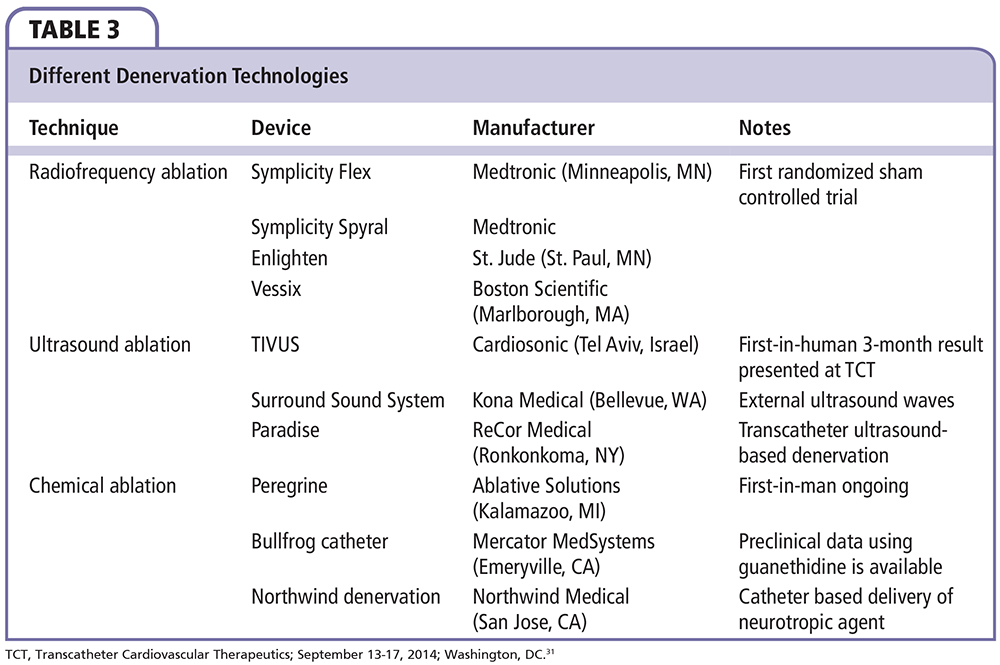
Different technologies have been developed to achieve RDN (Figure 1). Regardless of the specific technology being used, the device should be able to achieve a maximal and irreversible destruction of the renal nerves, in a predictable pattern, while minimizing injury to the renal artery. The two technologies that have been used in humans thus far are the RF ablation-based RDN and the ultrasound energy-based RDN. Table 1 summarizes the major published RDN studies. Table 2 summarizes the differences between the available RF catheters. Table 3 summarizes the available RDN technologies.
Radiofrequency Ablation Technology
Symplicity Flex™.
The Symplicity Flex Renal Denervation System (Medtronic) was the first and most commonly used system to utilize RF ablation to achieve RDN. After local anesthesia, the 6F Symplicity™ catheter (which has a single monopolar electrode attached to its tip) is delivered to the level of the renal artery. The catheter is attached to a generator that can provide a maximum energy of 8W to achieve a temperature between 40°C and 75°C, which is required to cause irreversible nerve damage. The generator shuts off automatically after 2 minutes or if either the impedance or temperature exceeds the programmed limits. The first RF ablation is applied distally in the renal artery, and the catheter is then retracted by 5 mm and rotated before the energy is reapplied. The process is repeated for four to six 2-minute treatments. This procedure is limited by achieving adequate arterial wall contact that is essential for effective denervation and is operator dependent.
Symplicity Spyral™.
The Symplicity Spyral (Medtronic) has four RF ablation electrodes mounted on a spiral catheter that delivers RF energy simultaneously to the electrodes at 60-second intervals for a 21-minute procedural time. The catheter has a self-expandable design that enables treating renal arteries ranging from 3 to 8 mm. Once the catheter is positioned inside the renal artery, the stiff wire is retracted, which allows the electrodes to shape like a corkscrew facing the luminal surface in a helical pattern. Its first-in-human study showed a decrease in office BP at 6 months by 19.9/7.3 mm Hg from a baseline of 181/95 mm Hg.25
EnligHTN™.
The EnligHTN Ablation Catheter (St. Jude Medical, St. Paul, MN) was designed with an expandable electrode basket with four ablation electrodes that allow the operator to deliver ablations for 90 seconds per electrode, sequentially. After the catheter is positioned distally, the basket is expanded and the first denervation is performed. The catheter is then pulled and rotated by 90° and the process is repeated. The basket comes in two sizes, small (16 mm) to accommodate renal arteries of 4 to 6 mm, and large (18 mm) for renal arteries of 5.5 to 8 mm. Compared with the Symplicity Flex, this catheter shortens the procedure period and delivers the RF in a more predictable way. The Safety and Efficacy Study of Renal Artery Ablation in Resistant Hypertension Patients (EnligHTN-1) trial, which recruited 46 patients with resistant hypertension, found a reduction of average office SBP/DBP at 6 months by 26/10 mm Hg,26 and remained consistent at 12 months with a reduction of 27/11 mm Hg from the baseline of 176/96 mm Hg.27 As with previous studies, ambulatory SBP/DBP were reduced by 10/6 mm Hg at 6 months. RDN was effective in 80% of patients, with up to 41% of patients achieving an office SBP < 140 mm Hg.
Vessix™ Vascular V2.
The Vessix Vascular V2 system (Boston Scientific, Marlborough, MA) consists of a low-pressure (3 atm) balloon catheter with four, six, or eight RF bipolar electrodes mounted on its surface in a helical fashion that deliver therapy of < 1W at 68°C, in addition to the V2 Bipolar RF Generator. The bal loon gives this system the advantage of occluding the renal artery and therefore minimizing energy loss into the blood stream. Once the balloon is inflated, all RF electrodes are placed in direct contact with the renal artery, allowing simultaneous and precise treatment in 30-second intervals per renal artery. This catheter is available in different sizes (4, 5, 6, and 7 mm) and can be used to treat renal arteries as small as 3 mm, which provides the potential for use in patients with accessory renal arteries. According to the Treatment of Resistant Hypertension Using a Radiofrequency Percutaneous Transluminal Angioplasty Catheter (REDUCE-HTN) post-market clinical study that included 146 patients treated with the Vessix system, RDN showed a change in BP by −27.6/−12.7 mm Hg at 6 months, and −28.4/−11.0 mm Hg at 12 months when compared with baseline (183.2/98.6 mm Hg).28
OneShot™.
OneShot (Covidien, Dublin, Ireland) is an over-the-wire balloon-based irrigated catheter that has one monopolar electrode arrayed in a 360° spiral fashion, capable of delivering 25W RF ablations in one 2-minute treatment per artery at a maximum temperature of 60°C. Irrigation with saline throughout the procedure is delivered through eight holes present along the electrode in order to create consistently deep lesions while minimizing endothelial tissue damage. The catheter is available in three sizes (5, 6, and 7 mm) for renal arteries from 4 to 7 mm. Interim results from the Rapid Renal Sympathetic Denervation for Resistant Hypertension Using the OneShot Ablation System Study (RAPID), a prospective cohort study that enrolled 50 patients with resistant hypertension showed a significant reduction in the office BP from a baseline of 181.6/95.5 mm Hg by -17/-7 mm Hg at 1 month.29 Of note, Covidien announced in January 2014 that they are eliminating the OneShot program due to slower- than-expected development in the RDN market.30
Ultrasound Ablation Technology
Ultrasound-based RDN was developed to achieve more predictable nerve ablations while minimizing injury to renal arteries. Ultrasound ablation technologies being tested in humans include the TIVUS™ System (Cardiosonic, Tel Aviv, Israel),31 the PARADISE™ Percutaneous Denervation System (ReCor Medical, Ronkonkoma, NY), and Kona Surround Sound system (Kona Medical, Bellevue, WA).
PARADISE™.
The PARADISE Percutaneous Denervation System (ReCor Medical) is an ultrasound-based system that consists of a 6F compatible balloon catheter with a cylindrical transducer that emits ultrasound energy circumferentially. The balloon catheter has a cooling fluid to allow simultaneous denervation and cooling. There is no need for direct contact between the probe and the endothelium, and this device causes minimal thermal damage to the renal artery. The balloon also positions the ultrasound in the center of the renal artery, delivering the energy uniformly and circumferentially. Results of the REDUCE-HTN trial using the PARADISE ablation catheter found a significant reduction in office BP by 30/15, 32/14, and 36/17 mm Hg from a baseline of 180/109 mm Hg at 1, 2 and 3 months, respectively.32 The more recent, multicenter, nonrandomized Transcatheter Intravascular Ultrasound Energy Delivery for Renal Denervation (ACHIEVE) study showed a change in office BP by −17/−6 mm Hg from a baseline of 177/95 mm Hg at 6 months. The study showed a 65% response rate to RDN based on original criteria.33
Kona Surround Sound System.
The Kona Surround Sound system (Kona Medical) is designed to deliver low-intensity, focused, ultrasound energy from an external probe with real-time monitoring of the treatment area using an external imaging modality. Because the technology is noninvasive, this allows for repeat sessions while monitoring patients’ BP response to therapy between sessions. In 24 patients with resistant hypertension, the Renal Denervation Therapy for Hpertension (WAVE I) study reported a significant decrease in SBP by 27 mm Hg at 6-month follow-up. The study involved making 18 focused lesions over 12.6 minutes on each renal artery.34
Pharmacologic Ablation Technology
Pharmacologic-based ablative catheters aim to directly affect sympathetic nerves by injecting ablative solutions into the adventitial space. Pharmacologic ablation has the potential advantage of achieving nearly complete nerve ablations with no injury to intima or media and essentially no pain.
Peregrine System™ Infusion Catheter.
The Peregrine System infusion catheter (Ablative Solutions, Kalamazoo, MI) is composed of a 7F endovascular delivery catheter that houses three evenly spaced microneedles designed to inject alcohol directly into the adventitial and periadventitial space. An animal study of eight cases showed successful injection of alcohol by the device without serious side effects. This was accompanied by a linear dose response between alcohol volume and norepinephrine reduction of up to 88% when using 0.60 mL of ethanol. Histologic examination showed profound, 360° circumferential nerve injuries as deep as 8 mm. There were no detectable stenoses at 45-day follow-up.35
RDN Safety
Procedural adverse effects that have been described in the literature were not directly related to the RDN procedure. These included renal artery dissection after placement of the RDN catheter before RF energy delivery, progression of preexisting renal artery stenosis,4 symptomatic hypotension requiring intravenous fluid and reduction in antihypertensive medications,7 and hypertensive renal disease progression.26 This safety profile was confirmed in the Global Symplicity Registry from preliminary results on the first 1000 patients showing < 1% experienced cardiovascular, renal, or postprocedural adverse events.36
Potential Applications of RDN Beyond Hypertension
Glucose Metabolism
In a subcohort of the SYMPLICITY HTN-2 trial, RDN decreased fasting glucose level, mean 2-hour glucose level during glucose tolerance test, insulin level, and C-peptide levels. RDN also decreased homeostasis model assessment-insulin resistance at 3-month follow-up.37
The Renal Sympathetic Denervation for Treatment of Metabolic Syndrome Associated Hypertension (Metabolic Syndrome Study) trial is going to study the effect of RDN on insulin resistance as determined by the homeostasis model assessment-insulin resistance method at 3 months as its primary outcome (ClinicalTrials.gov Identifier NCT01911078).
Left Ventricular Hypertrophy and Cardiac Function
In a study of 64 patients with resistant hypertension, 46 patients were treated with RDN and the other 18 patients served as a control group. Along with decreasing BP at 6 months from a baseline of 180.8/95.8 mm Hg to 152.9/88.6 mm Hg, RDN decreased left ventricular mass index, reduced interventricular septum thickness, decreased end-systolic volume, improved ejection fraction, and improved diastolic function.38
To better evaluate the effect of RDN on patients with heart failure, two randomized clinical trials are ongoing. The Denervation of the Renal Sympathetic Nerves in Heart Failure With Normal LV Ejection Fraction (DIASTOLE) trial will look at the effect of RDN on E/E’ ratio (ratio of mitral velocity to early diastolic velocity of the mitral annulus) as measured by echocardiography (ClinicalTrials.gov Identifier NCT01583881), and the Renal Denervation in Patients with Heart Failure and Severe Left Ventricular Dysfunction will assess a decrease in NT-proBNP levels at 6 and 12 months after RDN as an indicator of improvement in heart failure (ClinicalTrials.gov Identifier NCT01870310).
Atrial Fibrillation
Pokushalov and colleagues39 studied the effect of RDN on 27 patients with a history of drug-resistant hypertension and paroxysmal or persistent atrial fibrillation refractory to ≥ 2 antiarrhythmic medications. Patients were randomized to receive pulmonary vein isolation either alone or with RDN. At 12 months, patients who underwent RDN had significant reduction in BP by 25/10 mm Hg and a lower rate of atrial fibrillation recurrence (69% vs 29%; P = .033).39
Anxiety, Depression, and Quality of Life
Dysfunction of the sympathetic nervous system might be one of the contributors to psychologic impairment in patients with hypertension.37,40 To further investigate this relationship, Lenski and associates41 studied the effect of RDN on anxiety, depression, headache intensity, quality of life, and stress level in patients with resistant hypertension. Anxiety and depression were assessed by the self-assessment hospital anxiety and depression scale, with scores > 11 to indicate clinically relevant depression and anxiety. Quality of life was assessed by the SF-12® health status questionnaire (Medical Outcomes Trust, Hanover, NH). Headache intensity was measured by the visual analogous scale, with scores > 4 to indicate moderate to severe headache. RDN decreased BP at 6 months by 20/9.7 mm Hg from a baseline of 165/91 mm Hg. Furthermore, RDN decreased anxious and depressive mood, improved self-assessed physical and mental status, and reduced headache intensity. The improvement of psychologic factors was independent from the extent of BP reduction.41
Conclusions
Although RDN is a safe procedure, efficacy to control BP in patients with resistant hypertension is still controversial. Although it seems to have pleiotropic effects, the data are still scant. The concept of incomplete denervation has emerged as a possible explanation for inadequate response to RDN. RDN might be beneficial for only a subgroup of patients in whom hyperactivity of the sympathetic system is contributing to the development of hypertension. More preclinical data and development of the denervation technique are warranted.
The authors report no real or apparent conflicts of interest.
References
- Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217-223.
- Persell SD. Prevalence of resistant hypertension in the United States, 2003-2008. Hypertension. 2011;57: 1076-1080.
- Krum H, Schlaich M, Whitbourn R, et al. Catheterbased renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275-1281.
- Symplicity HTN Investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911-917.
- Krum H, Schlaich MP, Bohm M, et al. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet. 2014;383:622-629.
- Symplicity HTN Investigators, Esler MD, Krum H, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903-1909.
- Esler MD, Krum H, Schlaich M, et al; Symplicity HTN-2 Investigators.. Renal sympathetic denervation for treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126:2976-2982.
- Davis MI, Filion KB, Zhang D, et al. Effectiveness of renal denervation therapy for resistant hypertension: a systematic review and meta-analysis. J Am Coll Cardiol. 2013;62:231-241.
- Pickering TG, James GD, Boddie C, et al. How common is white coat hypertension? JAMA. 1988;259: 225-228.
- Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193-2198.
- Pierdomenico SD, Cuccurullo F. Prognostic value of white-coat and masked hypertension diagnosed by ambulatory monitoring in initially untreated subjects: an updated meta analysis. Am J Hypertens. 2011;24:52-58.
- Mahfoud F, Ukena C, Schmieder RE, et al. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation. 2013;128:132-140.
- Howard JP, Nowbar AN, Francis DP. Size of blood pressure reduction from renal denervation: insights from meta-analysis of antihypertensive drug trials of 4,121 patients with focus on trial design: the CONVERGE report. Heart. 2013;99:1579-1587.
- Bhatt DL, Kandzari DE, O’Neill WW, et al; SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393-1401.
- Fadl Elmula FE, Hoffmann P, Larstorp AC, et al. Adjusted drug treatment is superior to renal sympathetic denervation in patients with true treatment-resistant hypertension. Hypertension. 2014;63:991-999.
- Tellez A, Rousselle S, Palmieri T, et al. Renal artery nerve distribution and density in the porcine model: biologic implications for the development of radiofrequency ablation therapies. Transl Res. 2013;162:381-389.
- Sakakura K, Ladich E, Cheng Q, et al. Anatomic assessment of sympathetic peri-arterial renal nerves in man. J Am Coll Cardiol. 2014;64:635-643.
- Vink EE, Goldschmeding R, Vink A, et al. Limited destruction of renal nerves after catheter-based renal denervation: results of a human case study. Nephrol Dial Transplant. 2014;29:1608-1610.
- Tzafriri AR, Mahfoud F, Keating JH, et al. Innervation patterns may limit response to endovascular renal denervation. J Am Coll Cardiol. 2014;64:1079-1087.
- Kandzari D. Symplicity HTN-3: insights from the subgroup analysis. Paper presented at: EuroPCR 2014; May 20-23, 2014; Paris, France.
- Steigerwald K, Titova A, Malle C, et al. Morphological assessment of renal arteries after radiofrequency catheter-based sympathetic denervation in a porcine model. J Hypertens. 2012;30:2230-2239.
- He J, Klag MJ, Appel LJ, et al. The renin-angiotensin system and blood pressure: differences between blacks and whites. Am J Hypertens. 1999;12:555-562.
- Weir MR, Chrysant SG, McCarron DA, et al. Influence of race and dietary salt on the antihypertensive efficacy of an angiotensin-converting enzyme inhibitor or a calcium channel antagonist in salt-sensitive hypertensives. Hypertension. 1998;31:1088-1096.
- Bauer JH. Age-related changes in the renin-aldosterone system. Physiological effects and clinical implications. Drugs Aging. 1993;3:238-245.
- Whitbourn RJ, Walton T, Harding S. TCT-408: Renal artery denervation with a new simultaneous multielectrode catheter for treatment of resistant hypertension: 12-month update from the SYMPLICITY Spyral first-in-man study. J Am Coll Cardiol. 2014;64(11_S). doi:10.1016/j.jacc.2014.07.458. http://content.onlinejacc.org/issue.aspx?journalid=101&issueID=930843&direction=
P&page=9. Accessed April 1, 2015. - Worthley SG, Tsioufis CP, Worthley MI, et al. Safety and efficacy of a multi-electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur Heart J. 2013;34:2132-2140.
- Papademetriou V, Tsioufis CP, Sinhal A, et al. Catheterbased renal denervation for resistant hypertension: 12-month results of the EnligHTN I first-in-human study using a multielectrode ablation system. Hypertension. 2014 ;64:565-572.
- New data continue to show significant and sustained blood pressure reduction with Boston Scientific Vessix™ Renal Denervation System [press release]. Marlborough, MA; Boston Scientific: October 28, 2013.
- Verheye S. TCT-62: Preliminary Result of the Rapid Renal Sympathetic Denervation for Resistant Hypertension Using the Maya Medical OneShot™ Ablation System (RAPID) Study. J Am Coll Cardiol. 2013; 62(18 suppl 1):B20.
- Parmar A. Covidien pulls out of OneShot Renal Denervation program citing slow market development. MDDI: Medical Device and Diagnostic Industry website. http://www.mddionline.com/article/covidienpulls-out-oneshot-renal-denervation-program-citingslow-market-development. Published January 21, 2014. Accessed April 1, 2015.
- Renal denervation using the novel therapeutic intra- vascular ultrasound (TIVUSTM) catheter system: preliminary report of first-in-man safety and performance study. Paper presented at: EuroPCR 2014; May 20-23, 2014; Paris, France. OP206
- Mabin T, Sapoval M, Cabane V, et al. First experience with endovascular ultrasound renal denervation for the treatment of resistant hypertension. EuroIntervention. 2012;8:57-61.
- Daemen J. Ultrasound renal denervation in resistant hypertension: an ACHIEVE study analysis. Paper presented at: EuroPCR 2014; May 20-23, 2014; Paris, France.
- Neuzil P, Whitbourn RJ, Starek Z, et al. TCT-61: optimized external focused ultrasound for renal sympathetic denervation - Wave II trial. J Am Coll Cardiol. 2013;62(18_S1):B20.
- Fischell TA, Vega F, Raju N, et al. Ethanol-mediated perivascular renal sympathetic denervation: preclinical validation of safety and efficacy in a porcine model. EuroIntervention. 2013;9:140-147.
- Böhm M. The Global SYMPLICITY Registry: safety and effectiveness of renal artery denervation in real world patients with uncontrolled hypertension. Presented at: American College of Cardiology Scientific Sessions (ACC.14); March 29-31, 2014; Washington, DC.
- Mahfoud F, Schlaich M, Kindermann I, et al. Effect of renal sympathetic denervation on glucose metabolism in patients with resistant hypertension: a pilot study. Circulation. 2011;123:1940-1946.
- Brandt MC, Mahfoud F, Reda S, et al. Renal sympathetic denervation reduces left ventricular hypertrophy and improves cardiac function in patients with resistant hypertension. J Am Coll Cardiol. 2012;59:901-909.
- Pokushalov E, Romanov A, Corbucci G, et al. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol. 2012;60:1163-1170.
- Bajkó Z, Szekeres CC, Kovács KR, et al. Anxiety, depression and autonomic nervous system dysfunction in hypertension. J Neurol Sci. 2012;317:112-116.
- Lenski D, Kindermann I, Lenski M, et al. Anxiety, depression, quality of life and stress in patients with resistant hypertension before and after catheter-based renal sympathetic denervation. EuroIntervention. 2013;9:700-708.