Natriuretic Peptides and the Management of Heart Failure
Tyler Kern, MD,1 Ilan Kedan, MD, MPH,2 Asher Kimchi, MD1,2
1David Geffen School of Medicine at UCLA, Los Angeles, CA; 2Cedars-Sinai Heart Institute, Los Angeles, CA
Natriuretic peptide has been validated as a useful biomarker in the diagnosis of heart failure, although its role in guiding medical management of heart failure is not well established. We endeavored to determine if natriuretic peptide-guided therapy is associated with improved outcomes for heart failure patients. A total of 11 trials (2628 patients) comparing natriuretic peptide-guided therapy with standard therapy for heart failure patients were identified; follow-up times ranged from 3 months to 3 years. Our data indicate that natriuretic peptide levels and all-cause mortality rates do not appear to benefit from natriuretic peptide-guided therapy when compared with standard therapy. However, a decreased rate of cardiovascular events does appear to be associated with natriuretic peptide-guided therapy.
[Rev Cardiovasc Med. 2015;16(2):95-104 doi: 10.3909/ricm0736]
© 2015 MedReviews®, LLC
Natriuretic Peptides and the Management of Heart Failure
Tyler Kern, MD,1 Ilan Kedan, MD, MPH,2 Asher Kimchi, MD1,2
1David Geffen School of Medicine at UCLA, Los Angeles, CA; 2Cedars-Sinai Heart Institute, Los Angeles, CA
Natriuretic peptide has been validated as a useful biomarker in the diagnosis of heart failure, although its role in guiding medical management of heart failure is not well established. We endeavored to determine if natriuretic peptide-guided therapy is associated with improved outcomes for heart failure patients. A total of 11 trials (2628 patients) comparing natriuretic peptide-guided therapy with standard therapy for heart failure patients were identified; follow-up times ranged from 3 months to 3 years. Our data indicate that natriuretic peptide levels and all-cause mortality rates do not appear to benefit from natriuretic peptide-guided therapy when compared with standard therapy. However, a decreased rate of cardiovascular events does appear to be associated with natriuretic peptide-guided therapy.
[Rev Cardiovasc Med. 2015;16(2):95-104 doi: 10.3909/ricm0736]
© 2015 MedReviews®, LLC
Natriuretic Peptides and the Management of Heart Failure
Tyler Kern, MD,1 Ilan Kedan, MD, MPH,2 Asher Kimchi, MD1,2
1David Geffen School of Medicine at UCLA, Los Angeles, CA; 2Cedars-Sinai Heart Institute, Los Angeles, CA
Natriuretic peptide has been validated as a useful biomarker in the diagnosis of heart failure, although its role in guiding medical management of heart failure is not well established. We endeavored to determine if natriuretic peptide-guided therapy is associated with improved outcomes for heart failure patients. A total of 11 trials (2628 patients) comparing natriuretic peptide-guided therapy with standard therapy for heart failure patients were identified; follow-up times ranged from 3 months to 3 years. Our data indicate that natriuretic peptide levels and all-cause mortality rates do not appear to benefit from natriuretic peptide-guided therapy when compared with standard therapy. However, a decreased rate of cardiovascular events does appear to be associated with natriuretic peptide-guided therapy.
[Rev Cardiovasc Med. 2015;16(2):95-104 doi: 10.3909/ricm0736]
© 2015 MedReviews®, LLC
KEY WORDS
Heart failure • Natriuretic peptide • Guided therapy • BNP • NT-proBNP
KEY WORDS
Heart failure • Natriuretic peptide • Guided therapy • BNP • NT-proBNP
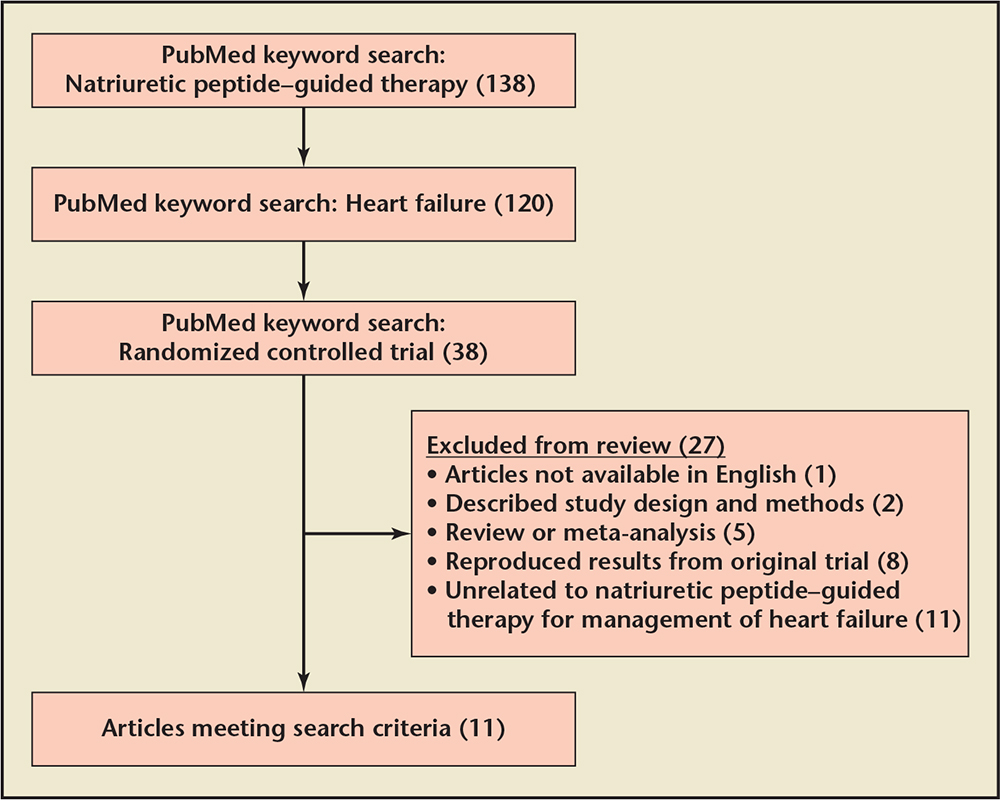
Figure 1. Study selection flowchart.
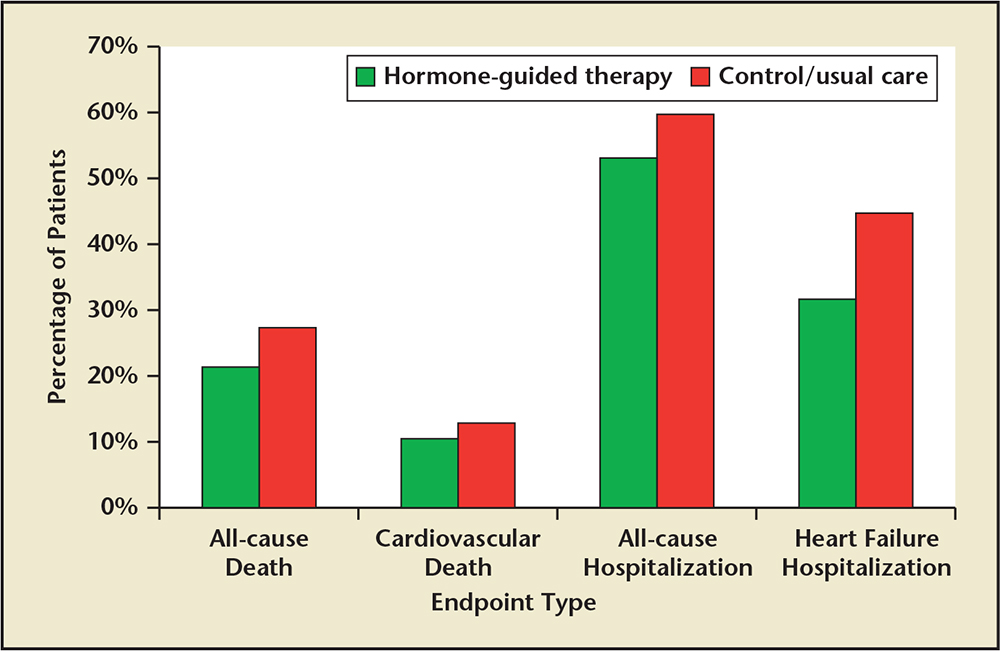
Figure 2. Percentage of patients in hormone-guided therapy and control/usual care groups that reached specific endpoints. Sample sizes: all-cause death (8 studies, combined N = 2076)a; cardiovascular death (7 studies, combined N = 1344)b; all-cause hospitalization (3 studies, combined N = 529)c; heart failure hospitalization (4 studies, combined N = 912).d
aData from Troughton RW et at,5 Jourdain P et al,7 Pfisterer M et al,8 Persson H et al,9 Lainchbury JG et al,10 Eurlings LW et al,11 Berger R et al,12 and Karlström P et al.14
bData from Troughton RW et at,5 Beck-da-Silva L et al,6 Jourdain P et al,7 Persson H et al,9 Eurlings LW et al,11 Karlström P et al,14 and Januzzi JL Jr et al.15
cData from Beck-da-Silva L et al,6 Jourdain P et al,7 and Karlström P et al.14
dData from Jourdain P et al,7 Lainchbury JG et al,10 Berger R et al,12 and Karlström P et al.14
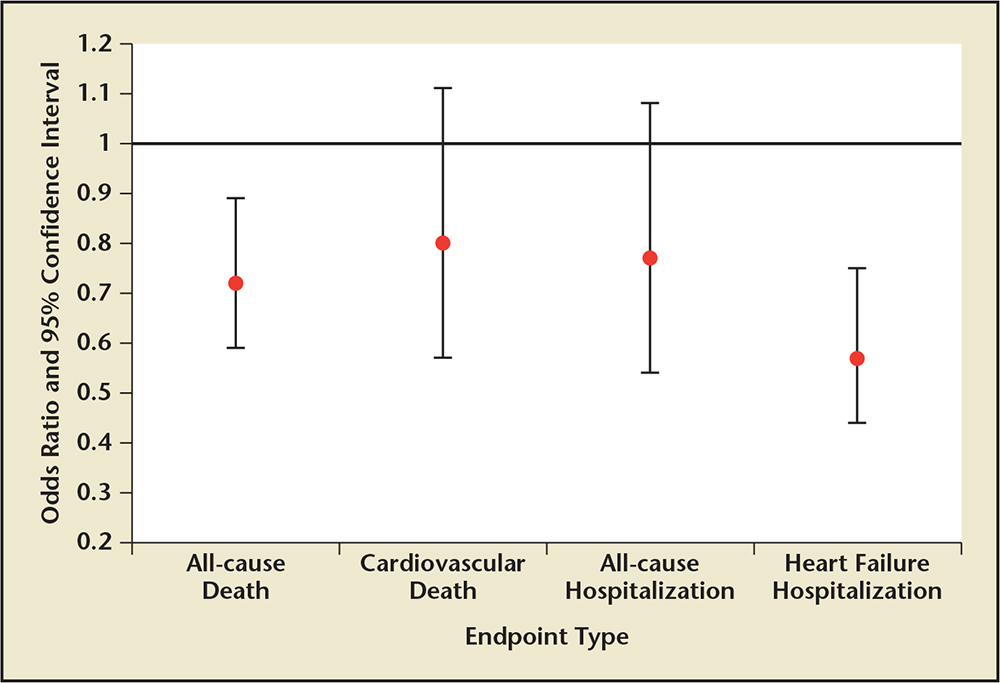
Figure 3. Unadjusted pooled odds ratios and 95% confidence intervals for the association between hormone-guided therapy and specific study outcomes compared with control/usual care therapy. Sample sizes: all-cause death (8 studies, combined N = 2076)a; cardiovascular death (7 studies, combined N = 1344)b; all-cause hospitalization (3 studies, combined N = 529)c; heart failure hospitalization (4 studies, combined N = 912).d
aData from Troughton RW et at,5 Jourdain P et al,7 Pfisterer M et al,8 Persson H et al,9 Lainchbury JG et al,10 Eurlings LW et al,11 Berger R et al,12 and Karlström P et al.14
bData from Troughton RW et at,5 Beck-da-Silva L et al,6 Jourdain P et al,7 Persson H et al,9 Eurlings LW et al,11 Karlström P et al,14 and Januzzi JL Jr et al.15
cData from Beck-da-Silva L et al,6 Jourdain P et al,7 and Karlström P et al.14
dData from Jourdain P et al,7 Lainchbury JG et al,10 Berger R et al,12 and Karlström P et al.14
Contrary to the authors' original hypothesis, peptide-guided therapy was more beneficial in the younger group than in the older group.
Unlike the TIME-CHF and BATTLESCARRED trials, UPSTEP revealed no significant difference in outcomes between the older and younger patients.
… the number of CV events per patient was significantly lower for elderly patients managed by peptide-guided therapy compared with standard of care therapy.
Judging from previous research investigating natriuretic peptides and hospital readmission rates for HF patients, it appears that a valuable predictor of patient outcomes is the percentage decrease in natriuretic peptide levels over a period of time.
Main Points
• Natriuretic peptide has been validated as a useful biomarker in the diagnosis of heart failure (HF). Because the diagnosis of HF carries a 5-year mortality rate of nearly 50%, research resources are being directed toward developing and validating strategies aimed at reducing HF readmissions and improving patient outcomes.
• It remains unclear which age group, if any, is likely to benefit from natriuretic peptide-guided therapy.
• There may be more value in establishing a prespecified percentage reduction goal than in establishing an absolute B-type natriuretic peptide (BNP) target when treating heart failure patients. Given the variable effects of patient age, ethnicity, comorbidities, body habitus, and variable manifestations of HF, BNP levels should be used as a tool for guiding medical therapy.
• Natriuretic peptide-guided therapy appears to be associated with reduced cardiovascular events compared with standard therapy.
Main Points
• Natriuretic peptide has been validated as a useful biomarker in the diagnosis of heart failure (HF). Because the diagnosis of HF carries a 5-year mortality rate of nearly 50%, research resources are being directed toward developing and validating strategies aimed at reducing HF readmissions and improving patient outcomes.
• It remains unclear which age group, if any, is likely to benefit from natriuretic peptide-guided therapy.
• There may be more value in establishing a prespecified percentage reduction goal than in establishing an absolute B-type natriuretic peptide (BNP) target when treating heart failure patients. Given the variable effects of patient age, ethnicity, comorbidities, body habitus, and variable manifestations of HF, BNP levels should be used as a tool for guiding medical therapy.
• Natriuretic peptide-guided therapy appears to be associated with reduced cardiovascular events compared with standard therapy.
In the United States, more than 670,000 new cases of heart failure (HF) are diagnosed annually.1 Despite improvements in management, the diagnosis of HF carries a 5-year mortality rate of nearly 50%.1,2 Recent data estimate the national cost of HF care to exceed $30 billion each year.1,3 With 30-day readmission rates averaging 25%, HF hospitalizations account for more than half of the total expenditure on HF care.1,3 HF prevalence increases with patient age. The lifetime risk of developing HF for Americans aged ≥ 40 years is 20%.1,4 Given these statistics, research resources are being directed toward developing and validating strategies aimed at reducing HF readmissions and improving patient outcomes.
B-type natriuretic peptide (BNP) and N-terminal prohormone of BNP (NT-proBNP) concentrations are increased in HF. Pressure-overloaded ventricular cardiomyocytes secrete these polypeptides, which act to induce natriuresis and decrease systemic vascular resistance. BNP and NT-proBNP are secreted in equal quantities; therefore both peptides can be measured for diagnostic purposes. The two different peptides have different serologic properties and are thus comparable in assessing ventricular pressure overload, but their values are not identical.
Natriuretic peptides have been validated as biomarkers capable of predicting outcomes in HF patients. Elevated and rising peptide levels have been associated with increased mortality and hospitalization rates.5 However, the role of natriuretic peptides in guiding medical management of HF is not well established. This study reviews and summarizes all published randomized controlled trials investigating natriuretic peptide-guided therapy. A thorough understanding of current trials will allow physicians and care providers to make more informed decisions regarding management strategies for patients with HF.
Methods
We conducted a PubMed keyword search with specific selection and exclusion criteria to identify prospective randomized controlled trials evaluating natriuretic peptide-guided therapy (Figure 1). Eligible studies were evaluated based on sample size, patient population, study design, follow-up time, and study endpoints. Study endpoints included natriuretic peptide levels, all-cause mortality rates, and cardiovascular (CV) events (rehospitalization and CV death).
Percentages and pooled unadjusted relative risk estimates (Figures 2 and 3) were calculated by combining the numbers of patients in each study publication. Risk estimates do not account for heterogeneity across studies. Only data from studies that reported the number or percentage of patients who had specific endpoints in each treatment group were included in this analysis. Some studies only reported hazard ratios, P values, or number of events for certain endpoints. These results are not included.
Results
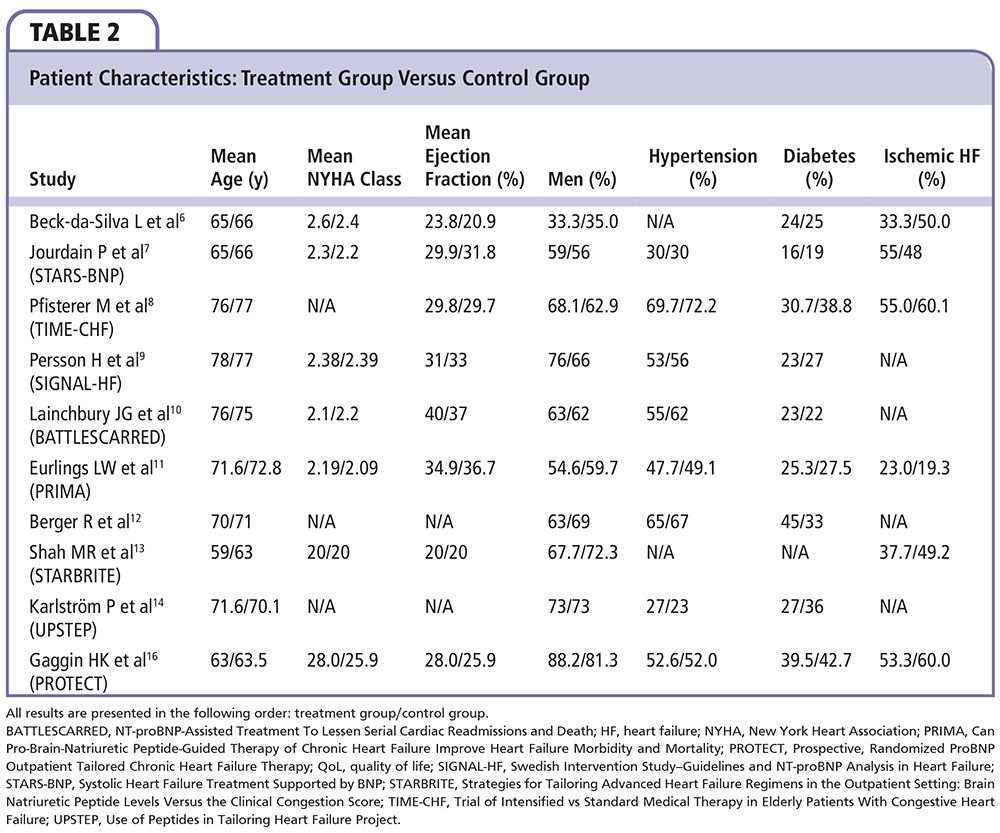
A total of 11 randomized controlled trials investigating natriuretic peptide-guided therapy were identified. A summary and analysis of each trial is provided in chronologic order, along with reference tables documenting study and patient characteristics (Tables 1 and 2). Table 3 compares the three major study endpoints evaluated: natriuretic peptide levels, all-cause mortality rates, and CV events. Figures 2 and 3 depict pooled percentages and unadjusted risk estimates for the following end-points: all-cause death, CV death, all-cause hospitalization, and HF hospitalization.
Treatment of Heart Failure Guided by Plasma Aminoterminal Brain Natriuretic Peptide Concentrations
Troughton and colleagues5 conducted the first randomized controlled trial evaluating natriuretic peptide-guided therapy; 69 patients with systolic HF (New York Heart Association [NYHA] class II-IV, left ventricular ejection fraction [LVEF] < 40%) were randomized to receive either peptide-guided therapy (target NT-proBNP < 1691 pg/mL) or symptom-guided standard of care therapy. During a median follow-up of 9.5 months, the peptide-guided therapy group experienced fewer CV events—a composite of CV deaths, hospitalizations, and new episodes of decompensated HF (19 events vs 54 events; P = .02). Within 6 months, 27% of patients in the peptide-guided arm had their first CV event, compared with 57% of patients in the standardized care arm (P = .034). In both groups, mean NT-proBNP levels fell below baseline at 6 months (668 pg/mL decrease in the peptide-guided group vs 25.4 pg/mL decrease in the standard care group; P = .16). These results prompted further investigation into the potential benefit and role of natriuretic peptide-guided therapy in the management of HF patients.
BNP-guided Therapy not Better Than Expert's Clinical Assessment for β-Blocker Titration in Patients With Heart Failure
Beck-da-Silva and colleagues6 randomized 41 patients with symptomatic systolic HF (NYHA class II-IV, LVEF < 40%) into one of two study arms. The treatment arm used plasma BNP levels and clinical signs to guide titration of β-blockers, whereas the control arm relied on clinical signs alone. BNP-guided dose titration of β-blockers did not result in higher doses at 3 months compared with the control group (5.9 ± 4.3 mg vs 4.4 ± 3.4 mg; P = .22). Additionally, there was no significant difference between arms with regard to mortality rates (P = .52) or hospitalizations (P = .34). Despite a small sample size, short follow-up, and use of single-drug titration, this trial cast doubt on the generally positive outcomes from the trial conducted by Troughton and colleagues.5
Systolic Heart Failure Treatment Supported by BNP Trial
The Systolic Heart Failure Treatment Supported by BNP (STARS-BNP) trial7 was a multicenter trial that randomized 220 patients with symptomatic systolic HF (NYHA class II-III, LVEF < 45%) into a BNP group or clinical group with a 15-month follow-up. In the BNP group, medical therapy was titrated based on measured plasma BNP levels with the goal to lower plasma BNP < 100 pg/mL. In the clinical group, medical therapy was titrated based solely upon physical examination and clinical signs. Fewer patients in the BNP group reached the combined primary endpoint of HF-related death or hospitalization (24% vs 52%; P < .001). Additionally, at 3 months, medications were more frequently titrated in the BNP group, resulting in significantly higher mean dosages of angiotensin-converting enzyme inhibitors and β-blockers (P < .05). These results suggest that natriuretic peptide-guided therapy may significantly improve both medical management and patient outcomes.
Trial of Intensified Versus Standard Medical Therapy in Elderly Patients With Congestive Heart Failure
With 499 patients (NYHA class II-IV, LVEF ≤ 45%), the Trial of Intensified Versus Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF)8 remains the largest prospective randomized controlled trial evaluating natriuretic peptide-guided therapy. Researchers subdivided the NT-proBNP-guided group and the symptom-guided group by age (60-74 y vs ≥ 75 y) to investigate any age-related effects and responses. Similar to the STARS-BNP trial, medication doses were more frequently titrated in the peptide-guided group. Despite more frequent dose titrations, however, rates of survival free from hospitalization and rates of overall survival were not significantly different between the peptide-guided group and the symptom-guided group (41% vs 40%; P = .39 and 84% vs 78%; P = .06). Rates of survival free from HF-related hospitalizations did significantly improve in the peptide-guided therapy compared with the symptom-guided therapy (72% vs 62%; P = .01). In both study arms, quality of life improved significantly but the magnitude of improvement was not significantly different between arms. Contrary to the authors’ original hypothesis, peptide-guided therapy was more beneficial in the younger group than in the older group. In fact, peptide-guided therapy in the older group was associated with significantly increased rates of adverse events (17.5% vs 12.6%; P = .01) and no significant difference in hospital-free survival (P = .54) when compared with symptom-guided therapy. Unlike previous trials, TIME-CHF included patients with more advanced HF; nearly 75% of patients had an NYHA classification ≥ III. Additionally, the mean patient age was 76.5 years, 10 years older than the mean age of patients in the STARS-BNP trial.
Swedish Intervention Study-Guidelines and NT-proBNP Analysis in Heart Failure
The Swedish Intervention Study-Guidelines and NT-proBNP Analysis in Heart Failure (SIGNAL-HF) trial9 randomized 252 patients (NYHA class II-IV, LVEF < 50%) to receive either NT-proBNP-guided therapy or symptom-guided therapy for 9 months. The primary endpoint was a composite of days alive, days out of hospital, and symptom score from the Kansas City Cardiomyopathy Questionnaire. There was no significant difference in the primary composite endpoint between the two groups (P = .28). Additionally, there was no difference between the individual components of the combined endpoint: CV death (P = .93), CV hospitalizations (P = .88), and symptom score (P = .28). The NT-proBNP concentrations decreased by approximately 10% in both groups, with only 19% of patients in the treatment group reaching the target of 50% reduction in baseline NT-proBNP, compared with 22% of patients in the control group. The authors concluded that natriuretic peptide-guided therapy was no more effective than education and structured treatment of HF. Similar to the TIME-CHF population, the SIGNAL-HF patients were older, with a mean age of 78.5 years. Given the neutral results from this trial, it appeared as if natriuretic peptide-guided therapy might not benefit patients ≥ 75 years old.
NT-proBNP-Assisted Treatment to Lessen Serial Cardiac Readmissions and Death
The NT-proBNP-Assisted Treatment To Lessen Serial Cardiac Readmissions and Death (BATTLESCARRED)10 trial randomized 364 patients with symptomatic HF and NT-proBNP > 400 pg/mL into three distinct treatment arms: NT-proBNP-guided therapy, intensive clinical management, and usual care. After 1 year, the peptide-guided therapy group and the clinical management group showed a mortality rate of 9.1%—significantly less than the 18.9% rate of the usual care group. This significant improvement was not present at 2- and 3-year follow-up. However, at 3 years, mortality rates were significantly reduced in patients ≤ 75 years old in the peptide-guided group relative to the usual care group (15.5% vs 31.3%; P = .021). The younger age group consistently benefited from peptide-guided therapy throughout the trial, whereas the older patients showed no significant benefit. These results further solidified the assumption that peptide-guided therapy was most beneficial to patients ≤ 75 years. The authors proposed that the lack of benefit observed in older patients may be partially attributed to their decreased tolerance to maximum medication dosages. Additionally, elderly patients are more likely to have diastolic HF, which is notoriously resistant to medical therapy.
Can Pro-Brain-Natriuretic Peptide-Guided Therapy of Chronic Heart Failure Improve Heart Failure Morbidity and Mortality?
The Can Pro-Brain-Natriuretic Peptide-Guided Therapy of Chronic Heart Failure Improve Heart Failure Morbidity and Mortality (PRIMA)11 trial randomized 345 patients recently hospitalized with decompensated HF (NT-proBNP > 1700 pg/mL at admission with > 10% reduction during hospitalization) to receive either NT-proBNP-guided therapy or clinically guided therapy. This was the first randomized controlled trial to provide individual target NT-proBNP levels for each patient. The target NT-proBNP level was defined as the lowest peptide level measured either at discharge or at 2-week follow-up. The authors argue that NT-proBNP levels do not necessarily return to baseline after an episode of HF decompensation. As such, it may be reasonable to guide treatment with the intention of lowering and maintaining NT-proBNP levels to an achievable level to avoid unnecessarily high doses of medications. However, despite individualized target NT-proBNP levels, the PRIMA trial did not show any significant improvement in the number of days alive outside the hospital in the peptide-guided group compared with the clinically guided group (685 d vs 664 d; P = .49). Secondary endpoints, including the number of CV-related hospitalizations, revealed no significant difference between groups (P = .552). In contrast to the most recent studies, the PRIMA trial did not demonstrate a significant benefit in patients < 75 years old (P = .114).
N-terminal Pro-B-type Natriuretic Peptide-guided, Intensive Patient Management in Addition to Multidisciplinary Care in Chronic Heart Failure: a Three-arm, Prospective, Randomized Pilot Study
In their three-arm study, Berger and colleagues12 randomized 278 patients with a recent HF-related hospitalization (NYHA class III-IV) to receive either NT-proBNP-guided intensive management, multidisciplinary care, or usual care. Multidisciplinary care involved two consultations with an HF specialist and four home visits from a nurse that specialized in HF care. The peptide-guided treatment group received similar treatment to the multidisciplinary care group, along with biweekly visits to an HF specialist for patients discharged with NT-proBNP levels > 2200 pg/mL until NT-proBNP levels fell < 2200 pg/mL. The NT-proBNP-guided group had a total of 488 days of hospitalization, significantly fewer than the 1254 days seen in the multidisciplinary care group and the 1588 days seen in the usual care group (P < .0001, significant differences among all groups). The combined rates of death and HF rehospitalizations were significantly lower in the NT-proBNP-guided group (37%) compared with the multi-disciplinary care group (50%; P < .05) and the usual care group (65%; P = .04). A greater proportion of patients in the NT-proBNP-guided group were treated with triple therapy (spironolactone, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker, and a β-blocker) and patients from this group had significantly more consultations with HF specialists than did the usual care group. These results suggest that an intensive approach to HF management involving peptide-guided therapy may improve patient outcomes.
Strategies for Tailoring Advanced Heart Failure Regimens in the Outpatient Setting: Brain Natriuretic Peptide Levels Versus the Clinical Congestion Score
The Strategies for Tailoring Advanced Heart Failure Regimens in the Outpatient Setting: Brain Natriuretic Peptide Levels Versus the Clinical Congestion Score (STARBRITE) trial13 randomized 130 patients (NYHA class III-IV, LVEF ≤ 35%) to receive either BNP-guided therapy or clinically guided therapy. Patients were followed for 90 days and the primary endpoint was defined as “the number of days alive and not hospitalized.” Medications for BNP-group patients were titrated to achieve a BNP level ≤ the level at discharge, whereas medications for the clinically guided therapy patients were titrated to achieve a congestion score ≤ the score at discharge. No significant difference was observed between treatment strategies with respect to the primary endpoint (85 d in the BNP-group vs 80.4 d in the clinical group; P = .25). Additionally, there was no significant difference between treatment strategies with regard to the number of diuretic adjustments (P = .84), days alive (P = .29), or days hospitalized (P = .45).
Brain Natriuretic Peptide-guided Treatment Does not Improve Morbidity and Mortality in Extensively Treated Patients With Chronic Heart Failure: Responders to Treatment Have a Significantly Better Outcome
The Use of Peptides in Tailoring Heart Failure Project (UPSTEP) trial14 randomized 279 patients (NYHA class II-III, LVEF < 40%) to receive either BNP-guided treatment or conventional treatment. BNP-guided therapy titrated medications to achieve BNP levels < 150 pg/mL in patients < 75 years and < 300 pg/mL in patients > 75 years. There was no significant difference between treatment strategies for the primary endpoint, a composite of all-cause mortality, all-cause hospitalization, and worsening of HF (P = .18). Unlike the TIME-CHF and BATTLESCARRED trials, UPSTEP revealed no significant difference in outcomes between the older and younger patients. Researchers performed a subgroup analysis comparing treatment responders (patients with > 30% decrease in baseline BNP level at 48 weeks) with nonresponders, which revealed a significant improvement among treatment responders for the primary endpoint (P < .0001) and all secondary endpoints. These results suggest that a certain degree of response to peptide-guided therapy is necessary to observe a clinically significant improvement in outcomes. As such, peptide-guided therapy may not be optimal for every patient. Currently, it is not known which patient factors can accurately predict a significant response to peptide-guided therapy. However, in the UPSTEP trial, treatment responders were significantly younger (69 ± 10 y vs 75 ± 8 y; P < .001) with superior renal function (estimated glomerular filtration rate: 68 ± 20 vs 52 ± 20) compared with nonresponders.
Use of Amino-terminal Pro-B-type Natriuretic Peptide to Guide Outpatient Therapy of Patients With Chronic Left Ventricular Systolic Dysfunction
The Pro-BNP Outpatient Tailored Chronic HF Therapy (PROTECT) trial15 randomized 151 patients to receive either NT-proBNP-guided therapy (target NT-proBNP level < 1000 pg/mL) or standard of care therapy. During a mean follow-up of 10 months, there was a significant reduction in the primary endpoint of total CV events (composite of HF exacerbations, CV hospitalizations, and CV deaths) in the peptide-guided group compared with the standard of care group (58 events vs 100 events; P = .009). NT-proBNP concentrations decreased significantly from baseline in the peptide-guided group (P = .01) but not in the standard of care group (P = .61). Researchers initially concluded that there was no clear interaction between age and response to therapy, as the frequency of the primary endpoint in elderly patients (≥ 75 y) was similar to that of the entire treatment group. However, post-hoc analysis of the elderly patients (n = 38) revealed that NT-proBNP levels significantly decreased in the peptide-guided group (2664-1418 mg/mL; P = .001) and significantly increased in the standard of care group (2570-3523 pg/mL; P = .01).16 Additionally, the number of CV events per patient was significantly lower for elderly patients managed by peptide-guided therapy compared with standard of care therapy (1.76 events vs 0.71 events; P = .03). Prior to this analysis, there was no evidence supporting the use of peptide-guided therapy in elderly HF patients.
Discussion
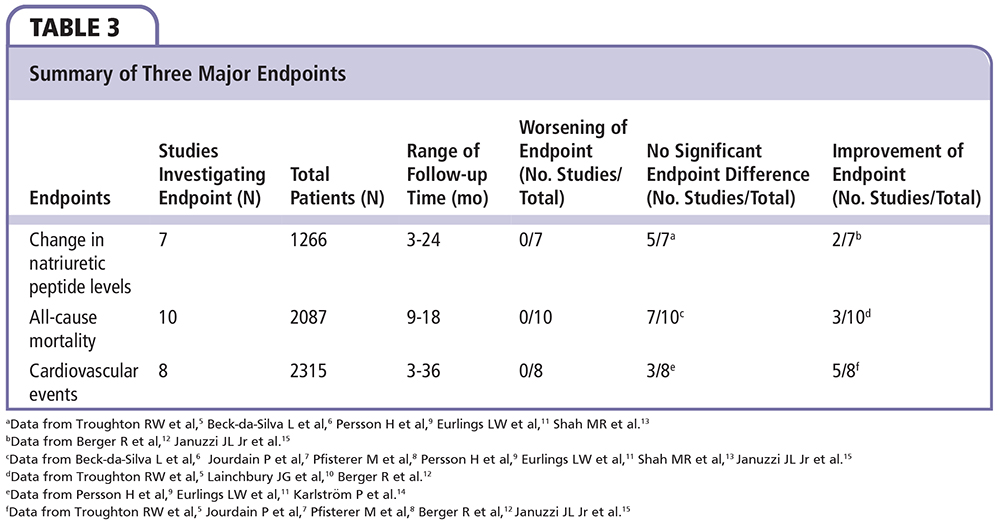
In total, 11 trials with 2628 patients were reviewed. Although all trials investigated natriuretic peptide-guided therapy, they differed in patient characteristics (eg, patient age, functional status, baseline natriuretic peptide levels) and study characteristics (eg, sample size, frequency of assessment, target peptide levels, endpoints).
Although these differences preclude performance of a statistical meta-analysis, the pooled data suggest improved outcomes in both all-cause and CV death, in addition to all-cause and HF hospitalization. Additionally, the unadjusted pooled odds ratios also depict favorable odds ratios for all-cause mortality and HF hospitalization.
It remains unclear which age group, if any, is likely to benefit from natriuretic peptide-guided therapy. Of the four trials that identified age-specific subgroups, the study designs and endpoints were too different to conclude statistical differences. BATTLESCARRED and TIME-CHF revealed clinically significant improvements in outcomes in younger patients (< 75 y) managed with natriuretic peptide-guided therapy.8,10 In contrast, UPSTEP revealed no significant difference in outcomes between age groups.14 A post-hoc analysis of the PROTECT trial identified a significant improvement in elderly patients in the natriuretic peptide group.16 As such, the subject of age-specific response to therapy deserves further investigation.
Each trial designated a unique treatment strategy with different prespecified natriuretic peptide goals, be it a 50% reduction from baseline NT-proBNP in the SIGNAL-HF trial or a BNP < 100 pg/mL in the STARS-BNP study.7,9 The PRIMA trial even set individual BNP targets for each patient.11 Despite multiple trials testing a variety of targets and strategies, it remains unclear what the ideal natriuretic peptide target should be (or if there should be one at all). An aggressively low target BNP level may lead to excessive use of diuretics and overmedication without additional therapeutic benefit. Conversely, a natriuretic peptide target that is not low enough may lead to suboptimal dosages of medications and inadequate medical intervention and undertreatment.
Judging from previous research investigating natriuretic peptides and hospital readmission rates for HF patients, it appears that a valuable predictor of patient outcomes is the percentage decrease in natriuretic peptide levels over a period of time.17-19 We believe there is more value in establishing a prespecified percentage reduction goal, such as a 25% reduction in BNP level within 30 days, than there is in establishing an absolute BNP target, such as BNP < 150 pg/mL within 3 months. Given the variable effects of patient age, ethnicity, comorbidities, body habitus, and variable manifestations of HF, BNP levels should be used as more of a tool for guiding medical therapy. In this vein, a percentage reduction goal for natriuretic peptide levels is more likely applicable to the diverse HF population than is a single numeric target.
There are two ongoing clinical trials investigating the role of natriuretic peptide-guided therapy in the management of HF. The Improvement of Patients With Chronic Heart Failure Using NT-proBNP (EXIMPROVECHF) trial20 is a randomized controlled trial intended to assess whether serial NT-proBNP measurements taken every 3 months for 1 year will result in improved outcomes for patients with chronic CHF. In the treatment group, natriuretic peptide levels will be made available to physicians, whereas in the control group, patient natriuretic peptide levels will be blinded. The primary outcome is HF hospitalization and death; the estimated enrollment is 400 subjects. The Guiding Evidence Based Therapy Using Biomarker Intensified Treatment (GUIDE-IT) trial21 is a randomized controlled trial investigating the efficacy of natriuretic peptide-guided therapy compared with usual care in high-risk HF patients with left ventricular systolic dysfunction. Specifically, NT-proBNP levels in the treatment group will be measured to assist physicians in HF medication management. The primary outcome is time to CV death or HF hospitalization; the estimated enrollment is 1100 subjects. Studies such as these will further help clarify the role of natriuretic peptide-guided therapy in the management of HF.
Conclusions
No adverse outcomes were noted in natriuretic peptide-guided therapy compared with standard therapy. The majority of current studies do not show a significant reduction in natriuretic peptide levels and all- cause mortality rates when comparing natriuretic peptide-guided therapy and standard therapy. However, natriuretic peptide-guided therapy appears to be associated with reduced CV events compared with standard therapy. There is a need for further randomized controlled trials to assess the use of natriuretic peptide-guided therapy. ![]()
Grant support provided, in part, by Ruth and Charles Gold.
References
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/ AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:1810-1852.
- Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344-350.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation. 2013;127:143-152.
- Djoussé L, Driver JA, Gaziano JM. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302:394-400.
- Troughton RW, Frampton CM, Yandle TG, et al. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet. 2000;355:1126-1130.
- Beck-da-Silva L, de Bold A, Fraser M, et al. BNP-guided therapy not better than expert’s clinical assessment for beta-blocker titration in patients with heart failure. Congest Heart Fail. 2005;11:248-253.
- Jourdain P, Jondeau G, Funck F, et al. Plasma brain natriuretic peptide-guided therapy to improve outcome in heart failure: the STARS-BNP Multicenter Study. J Am Coll Cardiol. 2007;49:1733-1739.
- Pfisterer M, Buser P, Rickli H, et al; TIME-CHF Investigators. BNP-guided vs symptom-guided heart failure therapy: the Trial of Intensified vs Standard Medical Therapy in Elderly Patients With Congestive Heart Failure (TIME-CHF) randomized trial. JAMA. 2009;301:383-392.
- Persson H, Erntell H, Eriksson B, et al. Improved pharmacological therapy of chronic heart failure in primary care: a randomized Study of NT-proBNP Guided Management of Heart Failure-SIGNAL-HF (Swedish Intervention study-Guidelines and NT-proBNP AnaLysis in Heart Failure). Eur J Heart Fail. 2010;12:1300-1308.
- Lainchbury JG, Troughton RW, Strangman KM, et al. N-terminal pro-B-type natriuretic peptideguided treatment for chronic heart failure: results from the BATTLESCARRED (NT-proBNP-Assisted Treatment To Lessen Serial Cardiac Readmissions and Death) trial. J Am Coll Cardiol. 2009;55:53-60.
- Eurlings LW, van Pol PE, Kok WE, et al. Management of chronic heart failure guided by individual N-terminal pro-B-type natriuretic peptide targets: results of the PRIMA (Can PRo-brain-natriuretic peptide guided therapy of chronic heart failure IMprove heart fAilure morbidity and mortality?) study. J Am Coll Cardiol. 2010;56:2090-2100.
- Berger R, Moertl D, Peter S, et al. N-terminal pro-B-type natriuretic peptide-guided, intensive patient management in addition to multidisciplinary care in chronic heart failure a 3-arm, prospective, randomized pilot study. J Am Coll Cardiol. 2010;55: 645-653.
- Shah MR, Califf RM, Nohria A, et al. The STARBRITE trial: a randomized, pilot study of B-type natriuretic peptide-guided therapy in patients with advanced heart failure. J Card Fail. 2011;17:613-621.
- Karlstrom P, Alehagen U, Boman K, Dahlstrom U; UPSTEP-study group. Brain natriuretic peptideguided treatment does not improve morbidity and mortality in extensively treated patients with chronic heart failure: responders to treatment have a significantly better outcome. Eur J Heart Fail. 2011;13:1096- 1103.
- Januzzi JL Jr, Rehman SU, Mohammed AA, et al. Use of amino-terminal pro-B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol. 2011;58:1881-1889.
- Gaggin HK, Mohammed AA, Bhardwaj A, et al. Heart failure outcomes and benefits of NT-proBNP-guided management in the elderly: results from the prospective, randomized ProBNP outpatient tailored chronic heart failure therapy (PROTECT) study. J Card Fail. 2012;18:626-634.
- Bayes-Genis A, Pascual-Figal D, Fabregat J, et al. Serial NT-proBNP monitoring and outcomes in outpatients with decompensation of heart failure. Int J Cardiol. 2007;120:338-343.
- Bettencourt P, Azevedo A, Pimenta J, et al. N-terminalpro- brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110:2168-2174.
- Michtalik HJ, Yeh HC, Campbell CY, et al. Acute changes in N-terminal pro-B-type natriuretic peptide during hospitalization and risk of readmission and mortality in patients with heart failure. Am J Cardiol. 2011;107:1191-1195.
- Improvement of Patients With Chronic Heart Failure Using NT-proBNP (EXIMPROVECHF). ClinicalTrials.gov website. https://clinicaltrials.gov/ct2/show/
NCT00601679. Accessed March 25, 2015. - Guiding Evidence Based Therapy Using Biomarker Intensified Treatment (GUIDE-IT). ClinicalTrials.gov website. https://clinicaltrials.gov/ct2/show/NCT01685
840?term=NCT01685840&rank=1. Accessed March 25, 2015.