Current Advances in Endovascular Therapy for Infrapopliteal Artery Disease
Ibrahim Sidiqi, MD, Patrick Alexander, MD
Providence Hospital and Medical Center, Southfield, MI
Peripheral arterial disease (PAD) is a systemic disease with significant morbidity and mortality. A substantial number of patients with PAD have infrapopliteal disease; however, diagnosis based solely on symptoms and ankle-brachial index can lead to delayed or missed opportunities to provide improved quality of life and limb salvage, and potentially reduce mortality. Advances in techniques and devices, and modification of classification systems have shown that an endovascular approach should be the primary therapeutic option for critical limb ischemia resulting from infrapopliteal disease.
[Rev Cardiovasc Med. 2015;16(1):36-50 doi: 10.3909/ricm0734]
© 2015 MedReviews®, LLC
Current Advances in Endovascular Therapy for Infrapopliteal Artery Disease
Ibrahim Sidiqi, MD, Patrick Alexander, MD
Providence Hospital and Medical Center, Southfield, MI
Peripheral arterial disease (PAD) is a systemic disease with significant morbidity and mortality. A substantial number of patients with PAD have infrapopliteal disease; however, diagnosis based solely on symptoms and ankle-brachial index can lead to delayed or missed opportunities to provide improved quality of life and limb salvage, and potentially reduce mortality. Advances in techniques and devices, and modification of classification systems have shown that an endovascular approach should be the primary therapeutic option for critical limb ischemia resulting from infrapopliteal disease.
[Rev Cardiovasc Med. 2015;16(1):36-50 doi: 10.3909/ricm0734]
© 2015 MedReviews®, LLC
Current Advances in Endovascular Therapy for Infrapopliteal Artery Disease
Ibrahim Sidiqi, MD, Patrick Alexander, MD
Providence Hospital and Medical Center, Southfield, MI
Peripheral arterial disease (PAD) is a systemic disease with significant morbidity and mortality. A substantial number of patients with PAD have infrapopliteal disease; however, diagnosis based solely on symptoms and ankle-brachial index can lead to delayed or missed opportunities to provide improved quality of life and limb salvage, and potentially reduce mortality. Advances in techniques and devices, and modification of classification systems have shown that an endovascular approach should be the primary therapeutic option for critical limb ischemia resulting from infrapopliteal disease.
[Rev Cardiovasc Med. 2015;16(1):36-50 doi: 10.3909/ricm0734]
© 2015 MedReviews®, LLC
KEY WORDS
Infrapopliteal artery disease • Endovascular therapy • Critical limb ischemia • Ischemic tissue loss
KEY WORDS
Infrapopliteal artery disease • Endovascular therapy • Critical limb ischemia • Ischemic tissue loss
Diabetes increases risk of PAD two- to fourfold, with greater risk in patients with longer duration of disease and poor glycemic control.
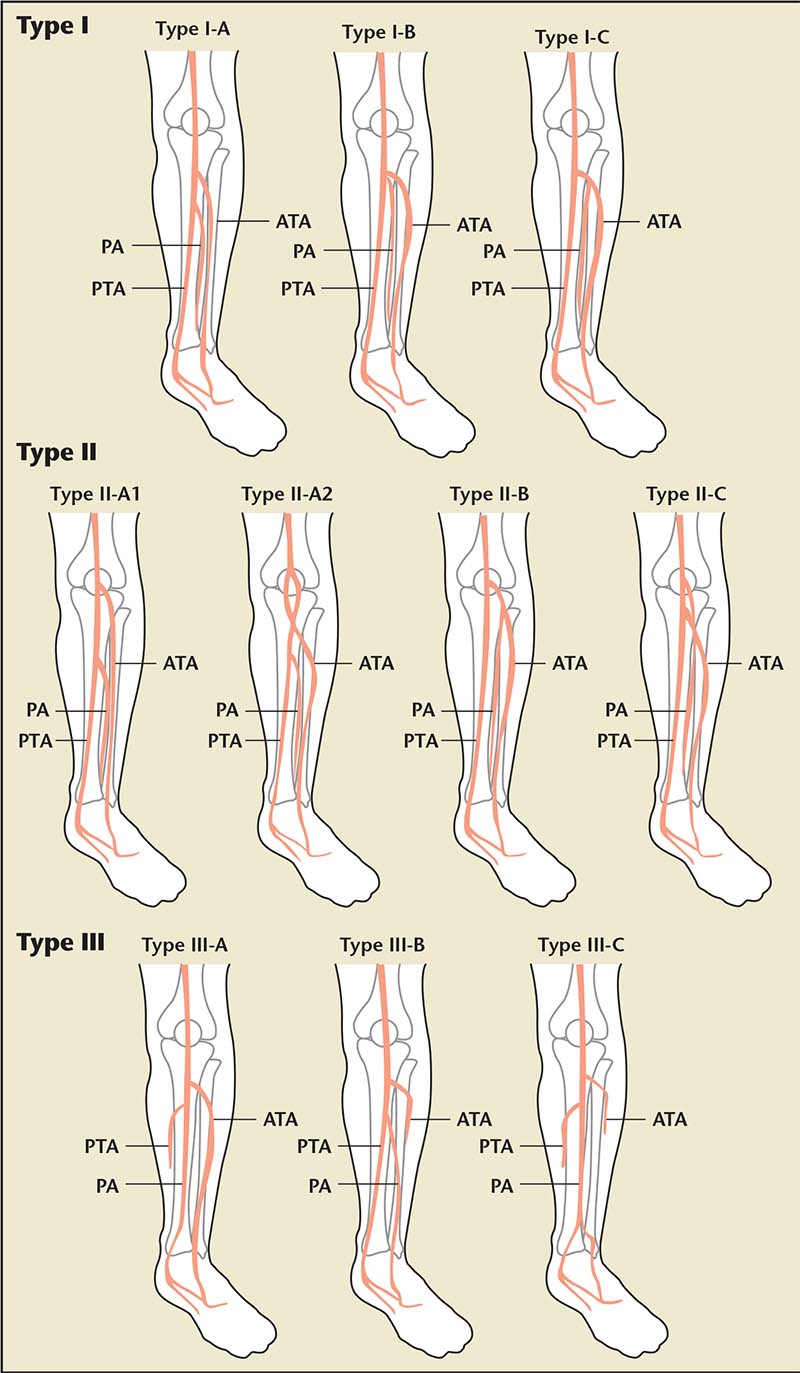
Figure 1. Anatomic infrapopliteal artery system variants. Reprinted with permission from Kawarada O et al.17
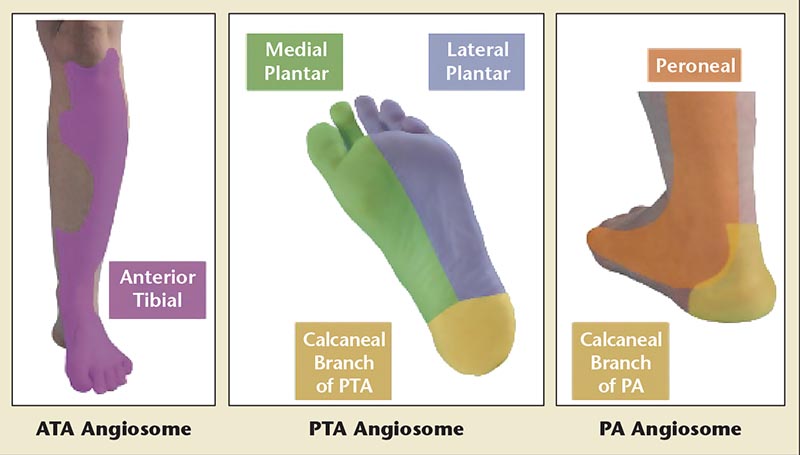
Figure 2. Angiosome identification of tibial artery distribution. ATA, anterior tibial artery; PA, peroneal artery; PTA, posterior tibial artery.
Currently, angiography is the gold standard in the evaluation of lower extremity CLI.
The ultimate goal of infrapopliteal intervention is to restore straight-line blood flow to the distal foot for limb salvage…
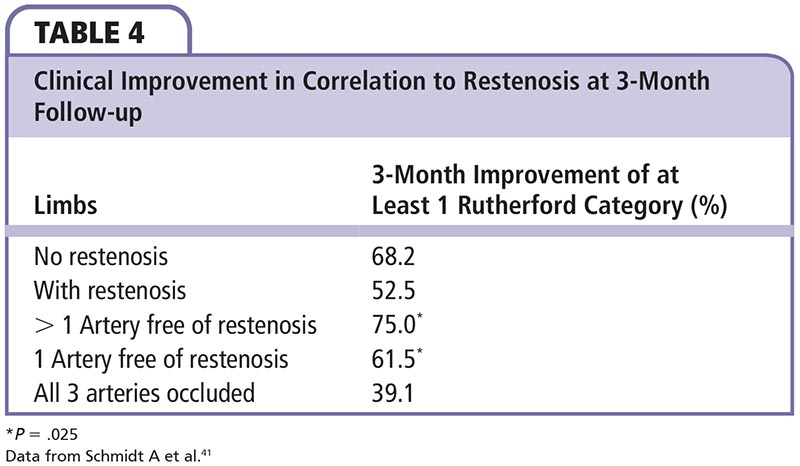

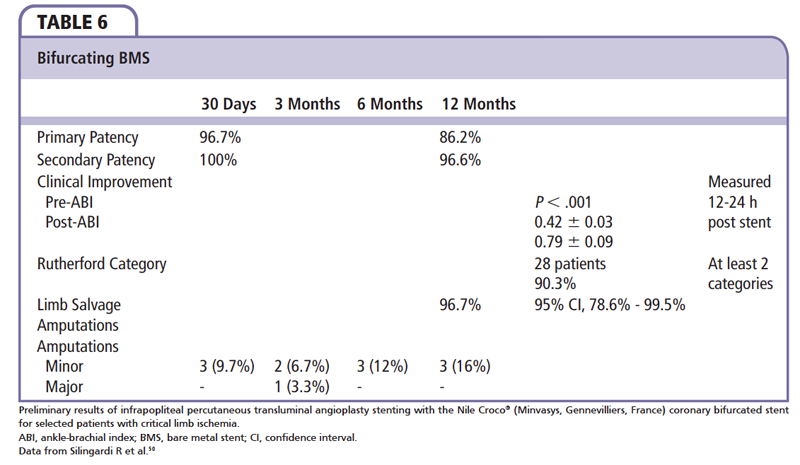
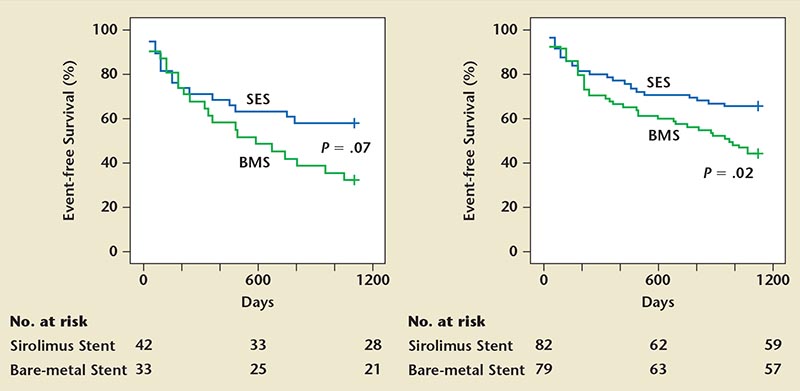
Figure 3. Sirolimus-eluting stent (SES) and bare metal stent (BMS) with clinical event rates.
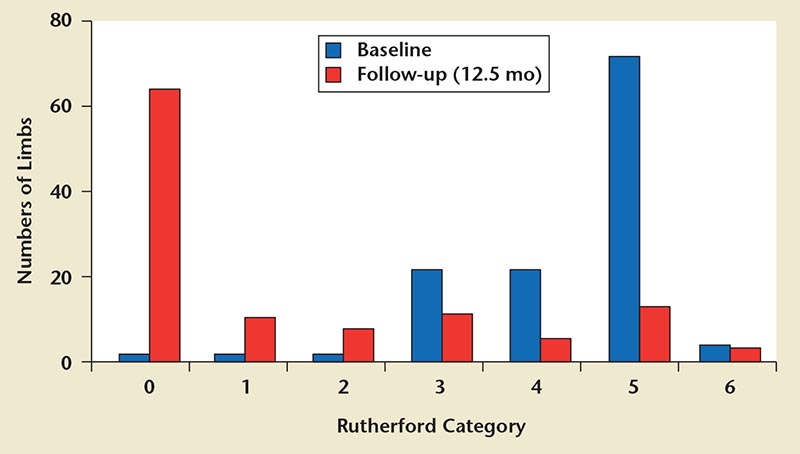
Figure 4. Midterm clinical outcomes with drug-eluting balloons. Reprinted with permission from Schmidt A et al.60
Main Points
• Peripheral arterial disease (PAD) is a systemic disease with significant morbidity and mortality. A substantial number of patients with PAD have infrapopliteal disease. Critical limb ischemia (CLI), defined as . 2 weeks of rest pain, ulcers, or tissue loss attributed to arterial occlusive disease, is associated with great loss of both limb and life.
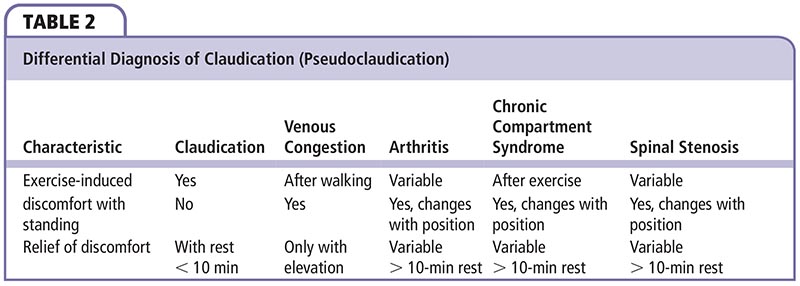
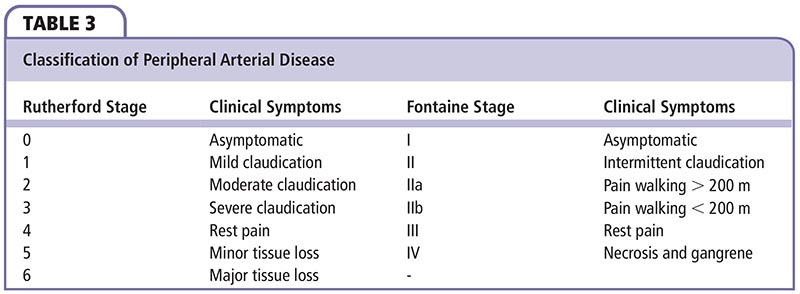
• Claudication is the classic presentation of suspected or presumed PAD. It is reproducible leg pain with exertion that is relieved with rest; however, few patients complain of intermittent claudication. Most are either asymptomatic or have atypical leg pain. Currently, there are two classifications for categorizing lower extremity PAD: the Fontaine system and the Rutherford system.
• The primary goal of medical therapy is prevention of disease progression. Endovascular treatment of infrapopliteal disease is rapidly evolving. Advances in medical therapy, devices, techniques, and technology have resulted in mid- to long-term success in infrapopliteal disease treatment when compared with open surgery, with reduced complications, morbidities, amputation rates, and increased rates of limb salvage. Moreover, functional status and quality of life outcomes are significantly improved with endovascular interventions..
Main Points
• Peripheral arterial disease (PAD) is a systemic disease with significant morbidity and mortality. A substantial number of patients with PAD have infrapopliteal disease. Critical limb ischemia (CLI), defined as . 2 weeks of rest pain, ulcers, or tissue loss attributed to arterial occlusive disease, is associated with great loss of both limb and life.
• Claudication is the classic presentation of suspected or presumed PAD. It is reproducible leg pain with exertion that is relieved with rest; however, few patients complain of intermittent claudication. Most are either asymptomatic or have atypical leg pain. Currently, there are two classifications for categorizing lower extremity PAD: the Fontaine system and the Rutherford system.
• The primary goal of medical therapy is prevention of disease progression. Endovascular treatment of infrapopliteal disease is rapidly evolving. Advances in medical therapy, devices, techniques, and technology have resulted in mid- to long-term success in infrapopliteal disease treatment when compared with open surgery, with reduced complications, morbidities, amputation rates, and increased rates of limb salvage. Moreover, functional status and quality of life outcomes are significantly improved with endovascular interventions..
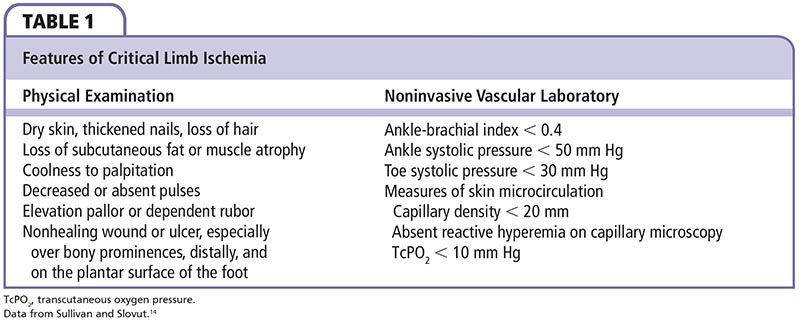
By definition, peripheral arterial disease (PAD) denotes progressive arterial stenosis, dilatation, or aneurysm of the aorta and its branches, exclusive of coronary arteries. Historically, the term peripheral vascular disease was understood to refer to the entire pathophysiologic syndrome. However, PAD is a more specific and appropriate terminology when referring exclusively to the arterial system. The classic, herald symptom of PAD is intermittent claudication, often described as pain in the calf, thigh, or buttock muscles with ambulation that is relieved with rest. With progression of the disease, patients experience rest pain that is relieved with dependency. Final stages of the disease result in tissue loss and gangrene, requiring amputation due to significant or absent blood flow to the lower extremity. Critical limb ischemia (CLI), defined as > 2 weeks of rest pain, ulcers, or tissue loss attributed to arterial occlusive disease, is associated with great loss of both limb and life (Table 1).1-3 Ischemic tissue loss is defined as tissue loss associated with an ankle pressure < 70 mm Hg or a toe pressure < 50 mm Hg according to the Transatlantic Inter-Society Consensus (TASC).
Epidemiology
Traditional risk factors of atherosclerosis, such as diabetes, hypertension, hypercholesterolemia, and smoking, also contribute to PAD. Smoking plays a more significant role for development of lower extremity PAD (2-3 times) than coronary artery disease (CAD).4 The risk of PAD in a patient with a history of smoking is directly proportional to the cumulative dose and duration of smoking over the course of his or her lifetime.5
Diabetes increases risk of PAD two- to fourfold, with greater risk in patients with longer duration of disease and poor glycemic control. Diabetic women demonstrated a threefold higher risk of developing PAD than men in the Framingham Heart Study (FHS).6 Importantly, diabetic patients with lower extremity PAD have a five- to sevenfold higher risk of amputation than patients without diabetes.7
Unlike in CAD, hypertension causes a modest increase in risk of lower extremity PAD. In the FHS, intermittent claudication was increased by 2.5- to 4-fold in men and women, respectively.6 Additional risk factors include elevated C-reactive protein and homocysteine levels.
Prevalence
Symptoms of claudication are present in a subset of patients with lower extremity PAD. The FHS surveyed patients with the standardized Rose questionnaire starting in 1948. The study demonstrated an increase in prevalence of lower extremity PAD with increased age.8 Despite the two- to fourfold increase in PAD, the Rose questionnaire underestimated the true prevalence in the population. Studies have shown asymptomatic lower extremity PAD is two to five times more common than symptomatic lower extremity PAD. Several recent studies utilizing ankle-brachial index (ABI) instead of a Rose questionnaire or symptoms of claudication demonstrated an almost four- to sevenfold increase in lower extremity PAD in the general population. Similarly, prevalence increased with age and in patients with higher risk factors.9 In the PAD Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) study,10 patients age 50 to 69 years with risk factors and those over age 70 years were screened by primary care physicians in a prospective study with ABI. PAD was detected in a high percentage of patients (29%), with 55% of the population diagnosed with new PAD only.
The circulatory system is a single unit. In general, disease in one segment indicates possible disease in other sites. For example, patients with carotid artery disease or PAD are presumed to have coronary artery disease (CAD).11 Consequently, there is a significant increase in risk of CAD-related mortality in the setting of PAD, with one-third to two-thirds of PAD patients having a myocardial infarction (MI). There is a high risk of transient ischemic attack/cerebrovascular accident (CVA) in patients with PAD, with an incidence of up to 40%.12,13 Cardiovascular (CV) events are even higher in those with CLI and is the most common cause of mortality. At 1 year following treatment for CLI, 25% of patients have resolved CLI, 20% have ongoing CLI, 30% are alive with amputations, and 25% are dead.14
In the United States, it is estimated that over 250,000 amputations are performed annually. Therefore, a major goal in the treatment of lower extremity PAD is limb salvage. Without revascularization, either surgical or endovascular, patients with CLI or nonhealing ulcers often progress to amputation despite optimal medical management. Marston and colleagues15 evaluated 142 patients with arterial insufficiency and ulcerations who did not undergo revascularization. At 12-month follow-up, rates of amputations ranged from 15% (ABI < 0.7) to 43% (ABI < 0.4). Wolfe and colleagues evaluated over 6000 patients with CLI. Patients without revascularization progressed to amputation in 73% with rest pain and in 95% if there was evidence of tissue loss at 1 year. Conversely, revascularization achieved a 75% 1-year limb salvage rate.16
Unfortunately, morbidity and mortality are increased with amputations. Additionally, quality of life is significantly reduced. If amputation is the treatment plan, then the goal should be to preserve the knee joint. Energy expenditure is more with above the knee as compared with below the knee amputation (BKA). The 30-day mortality rate is 5% with BKA and 16% with above the knee amputation.
Anatomy
There is minimal anatomic variation of the aortoiliac and femoral popliteal system. However, a unique characteristic of the infrapopliteal artery system is the wide range of anatomic variations, unlike the iliac and femoropopliteal arteries. Clinically, failure to recognize the anatomic variants can result in serious complications, suboptimal interventional outcomes, and limb salvage.17 Incidence of infrapopliteal arterial variation ranges from 7.8% to 10.8%; variations have been divided into three classifications. Type I indicates a normal level of popliteal arterial branching, including the usual trifurcation pattern and the tibioperitoneal trunk. Type II indicates a high division of popliteal artery branching, including the anterior tibial (AT), posterior tibial (PT), and peroneal artery all arising at or above the knee joint. Type III indicates hypoplastic or aplastic branching with an altered distal supply including a hypoplastic-aplastic PT, AT, or both. Type III is most common (l%-7.6%), followed by Type II (1.6%-7.5%) (Figure 1).17
Dermatomal representation of the infrapopliteal arteries is described by angiosomes. The three main arteries and their branches to the foot and ankle give off six angiosomes (Figure 2). The PT and its branches feed three angiosomes: the medial calcaneal branch, the medial plantar artery, and the lateral plantar artery (lateral midfoot and forefoot). The peroneal artery feeds two angiosomes: the anterior perforating branch and the lateral calcaneal branch. Finally, the AT supplies the anterior ankle via the dorsalis pedis artery. Choke vessels link adjacent angiosomes and provide blood flow if a source artery is occluded.18
Pathophysiology
The underlying factor for infrapopliteal arterial disease is presumed to be different from disease of the coronary, carotid, renal, and iliac arteries. Calcium phosphate deposition in the arterial walls is of two types: intimal calcification and medial calcification, also termed Monckeberg medial calcific sclerosis, after the German physician Johann Georg Monckeberg.19 Patients with chronic kidney disease or those on dialysis clearly have increased levels of calcification deposition in all arteries, but variability exists according to cell composition of the different arterial beds. In diabetic patients, the predominant deposition is hydroxyapatite in the media of arterial walls. Clinically, intimal calcification appears to contribute to plaque vulnerability, whereas media calcification results in rigid calcified vessels with patent lumen until late in the disease stage, which contributes to vascular stiffness. In general, infrapopliteal disease is a combination of both disease processes.
Clinical Evaluation
Claudication is the classic presentation of suspected or presumed PAD (Table 2). It is reproducible leg pain with exertion that is relieved with rest. However, only a minority of patients complain of intermittent claudication. The majority of patients are either asymptomatic or have atypical leg pain. CLI is present in 1% to 2% of patients.20 Currently, there are two classifications for categorizing lower extremity PAD: the Fontaine system and the Rutherford system (Table 3).21 Calf and foot pain indicate infrapopliteal disease. Of note, calf pain in the lower two-thirds is due to popliteal disease, whereas calf pain in the upper two-thirds is related to the superficial femoral artery (SFA). Rest pain is suggestive of severe ischemic disease. Patients relieve pain by hanging their feet over the edge of a bed or paradoxically by walking because of the gravitational effect of dependence on limb blood pressure.
Multiple studies using various questionnaires to assess for PAD failed to demonstrate a strong correlation. The Edinburgh Artery Study, the World Health Organization Rose questionnaire, and San Luis Valley Diabetes Study evaluated more than 10,000 patients combined. These studies found 1.5% to 5% of patients to be symptomatic, but when objective testing was performed, 25% to 35% of patients met the criteria for PAD. Overall, these studies concluded that a majority of patients do not have the classic symptoms of PAD. 22,23
Noninvasive evaluation of the infrapopliteal system involves ABI, segmental Doppler pressures and volume plethysmography, duplex imaging, computed tomography angiography, and magnetic resonance angiography. Currently, angiography is the gold standard in the evaluation of lower extremity CLI.
Medical Therapy
The primary goal of medical therapy is prevention of disease progression. The major risk factors in CLI are smoking, diabetes, hypertension, and hyper-lipidemia. Despite being considered a CAD equivalent by the National Cholesterol Education Program expert panel, a significant percentage of patients are not on appropriate therapy unless there is evidence of concomitant CAD, CKD, or diabetes.10 Complete medical management of PAD can be found in the recent American College of Cardiology (ACC)/American Heart Association (AHA) guidelines.20
Smoking
Smoking cessation and discussion with the patient on each visit is a class IB indication. A simple reminder of smoking cessation during office visits increased the annual cessation rate from 0.1% to 5% compared with no physician intervention; the further benefit of smoking cessation at 1 year was 15% to 30% with the addition of pharmacologic therapy.
Diabetes
Patients with both type 1 and 2 diabetes have been evaluated for risk of mortality with PAD disease. The Diabetes Control and Complication Trial24 looked at over 1400 patients with type 1 diabetes and found no significant improvement in risk of PAD. The United Kindom Prospective Diabetes Study25 assessed 3862 newly diagnosed patients with type 2 diabetes and concluded similar findings—reduction in microvascular but not macrovascular benefit with tight glycemic control. The current recommendation by the American Diabetes Association and the AHA is a target hemoglobin A1C of less than 7% (class lla). In addition, it is a class IB recommendation to maintain proper foot care with appropriate footwear and daily inspection.
Hypertension
Despite lack of evidence that blood pressure control alters progression of PAD, improved blood pressure control reduced mortality and morbidity from CV and cerebrovascular events. β-blockers improved CV events but did not worsen claudication.26 The Heart Outcomes Prevention Evaluation study randomized over 4000 patients into ramapril and placebo groups. At the end of 5 years, there was a 25% reduction in MI, CVA, and vascular mortality in patients with PAD.27
Hyperlipidemia
Use of 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase inhibitors is a class IB recommendation by the ACC/AHA guidelines. Both older and more current studies have shown significant benefits of HMG-CoA use for cardiovascular benefit as well as functional improvement in exercise capacity. The Heart Protection Study Collaborative Group looked at 6748 patients with multiple risk factors and total cholesterol of over 135 mg/dL treated with simvastatin over a 5-year period.28 There was a 25% reduction in cardiovascular mortality in the treated group. Current ACC/AHA recommendation is treatment of low-density lipoprotein cholesterol > 100 mg/dL (or < 70 mg/dL in those with high risk) with HMG-CoA inhibitors.
Antiplatelet and Antithrombotic Therapy
The Antithrombotic Trialists’ Collaboration29 was a meta-analysis of over 287 studies involving 135,000 patients on antiplatelet therapy compared with a control group. The trial also included 77,000 high-risk patients who were on antiplatelet therapy and compared one antiplatelet with another. There was a reduction in acute MI, CVA, vascular-related mortality, and any death in the treatment group. There was a 23% reduction in mortality in patients who had revascularization. In each of these high-risk categories, the absolute benefits substantially outweighed the absolute risks of major extracranial bleeding. Aspirin was the most widely studied antiplatelet drug; doses of 75 to 150 mg/d were at least as effective as higher daily doses. The effects of doses < 75 mg/d were less certain. When compared with other antiplatelets, clopidogrel reduced vascular events more than aspirin or ticlopidine (10% vs 4% and 12% vs 7%, respectively). The addition of dipyridamole to aspirin produced no significant further reduction in vascular events when compared with aspirin alone.
Both the Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE) and Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management and Avoidance (CHARISMA) trials also showed that aspirin alone or in combination with clopidogrel reduced the event rates of MI, stroke, or death from CV events. Thus, TASC II guidelines recommend antiplatelet (75-162 mg) therapy initiated prior to endovascular therapy and continued indefinitely if not contraindicated.30,31
Cilostazol has an ACC/AHA class la recommendation in symptomatic claudication. Cilostazol is a phosphodiesterase type 3 inhibitor that increases cyclic adenosine monophosphate. Cilostazol has vasodilator and platelet inhibitory properties but is contraindicated in patients with left ventricular systolic dysfunction or heart failure.
Numerous studies have evaluated warfarin with high (International Normalized Ratio 2.5-4.8) and moderate (International Normalized Ratio 2-3) range. Although there was, on average, 25% to 40% reduction in CV mortality, there was also a two- to fourfold increase in the risk of major bleeding. At the current time, there is insufficient evidence to support the use of anticoagulation therapy for PAD without additional indications for its use.32,33
Endovascular Therapy
Endovascular treatment of infrapopliteal disease is rapidly evolving. In the past decade, significant advances in medical therapy, devices, techniques, and technology have resulted in mid- to long-term success in infrapopliteal disease treatment when compared with open surgery, with reduced complications, morbidities, amputation rates, and increased limb salvage. Moreover, functional status and quality of life outcomes are significantly improved with endovascular interventions.34 The ultimate goal of infrapopliteal intervention is to restore straight-line blood flow to the distal foot for limb salvage, with secondary objectives of wound healing and symptom resolution.
Angioplasty Versus Surgery
Traditionally, open surgical revascularization has been considered the standard of care for infrapopliteal CLI disease. Unfortunately, patients undergoing open revascularization have multiple comorbidities leading to significant surgical complications. To date, there is a paucity of randomized, prospective studies comparing the two methods. However, with improvement in medical therapy, revascularization techniques, and devices, general expert consensus supports percutaneous transluminal angioplasty (PTA) as the primary approach.35-37
The initial study to compare surgery with PTA of infrainguinal disease in randomized fashion was the Bypass Versus Angioplasty in Severe Ischemia of the Leg (BASIL) study.38 Patients were randomized into surgery first (n = 228) or PTA first (n = 224) strategy with primary endpoint of amputation-free survival. At the end of follow-up of 3 to 7 years, 248 (55%) patients were alive without amputation (of trial leg), 38 (8%) were alive with amputation, 36 (8%) were dead after amputation, and 130 (29%) were dead without amputation. After 6 months, the two strategies did not differ significantly in amputation-free survival (48 vs 60 patients) and the trend continued at 2-year follow-up. After 2 years, the surgical group offered better long-term amputation-free survival. There was no difference in quality of life among the two strategies. Patients in the surgical group had higher initial morbidity, longer hospital stays, and greater use of the intensive care unit. Conversely, the PTA-treated group had a higher revascularization rate.39
More recently, Casella and colleagues39 retrospectively compared bypass graft surgery (BGS) with PTA for CLI of infrapopliteal lesions. Primary endpoints were primary patency, assisted primary patency, secondary patency, and limb salvage (defined as absence of major amputation, healed lesions, and/or the absence of rest pain). Mean follow-up time was 16.5 months and no patients were lost to follow-up. Primary patency, assisted primary patency, secondary patency, and limb salvage at 24 months for the BGS group were 50.2%, 59.7%, 64.7%, and 73.2%, respectively. For the PTA group, rates were 42.0%, 52.7%, 63.7%, and 68.2%, respectively. No statistical significance was noted between the two groups. The BGS group had a significantly higher mortality rate at 30 days (4.08% vs 0%; P = .049). At 24 months there was no difference statistically. In patients with TASC D lesions, the BGS group presented better secondary patency results at 12 months.
Angioplasty Alone
Historically, PTA of infrapopliteal arteries was avoided due to high restenosis rates, in large part due to the progressive nature of the disease that is further complicated by such comorbidities as atherosclerosis, diabetes, and renal dysfunction. In addition, older studies showed that proximal disease in the femoropopliteal arteries with reduced distal flow contributes to the progression of infrapopliteal disease. Willenberg and colleagues40 analyzed atherosclerotic progression in BTK arteries after femoropop-liteal angioplasty in claudicants. They performed angiograms on 58 consecutive claudicants with endovascular treatment of femoropopliteal arteries with a mean follow-up of 3.6 ± 1.2 years. There was a statistically significant increase in angiographically confirmed atherosclerotic burden for the composite of each limb as well as individual BTK arteries. Diabetes was found to be an independent risk factor. Intervention of the proximal femoropopliteal artery did not slow or regress distal disease progression.
Unlike femoropopliteal diseases, BTK lesions commonly respond with less than optimal results to PTA intervention. Unfortunately, most patients with CLI have multiple comorbidities such as diabetes and renal disease, with long diffuse lesions and high surgical risk.
Schmidt and colleagues41 investigated restenosis rates after balloon angioplasty of long segments in patients with CLI of the infrapopliteal artery. Inclusion criteria included lesions > 70% or occlusion with a lesion length of > 80 mm. A total of 62 legs and 77 treated arteries with CLI were successfully treated with balloon angioplasty. Patients had Rutherford categories of 4 (16/62; 25.8%) and 5 (46/62; 74.6%). Prior to study, 35.1% had target artery stenosis and 64.9% were occluded. Average lesion length was 18.3 cm, with 16.9 cm in the stenosed vessels and 19.2 cm in the occluded ones. Clinical improvement in correlation to restenosis at 3-month follow-up is shown in Table 4. At the time of initial PTA, those with inflow PTA compared with exclusively infrapopliteal PTA showed no improvement in Rutherford category (57.1% vs 54.2%; P = .62) or clinically (87.6% vs 75%; P = .57). At long-term follow-up of 15.4 ± 6.2 months, six patients had died of other causes. Similar to results at 3 months, 76.5% of patients showed further clinical improvement and 51% improved at least in Rutherford category. Improvement was seen irrespective of whether patients had intervention at 3-month follow-up. No major amputation or bypass surgery was required at 3-month or 15-month follow-up, resulting in a limb salvage rate of 100% in patients with long infrapopliteal lesions.
Fernandez and colleagues42 assessed predictors of failure and success in CLI patients. There were a total of 123 limbs in 111 patients with Rutherford category 4 to 6 with 83% exhibiting tissue loss; the remaining patients had rest pain. All patients had PTA of the tibial arteries, and 20% had multiple tibial vessel intervention. Fifty (41%) patients had isolated infrapopliteal interventions, and 73 (60%) patients had ipsilateral SFA or popliteal artery intervention, concurrently. At 1-year follow-up, the primary patency rate was 33%, the assisted primary patency rate was 50%, and the secondary patency rate as maintained by additional endovascular interventions was 56%. Single-level tibial intervention provided a significantly shorter wound-healing time as compared with multiple-level tibial intervention. Overall, limb salvage at 1 year was 75%. There was no significant difference in limb salvage between patients who were on dialysis and those who were not. Reintervention was performed in 33 patients (58% due to poor wound healing). The lower primary patency rates were attributed to poor runoff scores and with limited target vessel options.
Romiti and associates43 performed a meta-analysis of 30 articles from 1990 to 2006 (63% published from 2000-2006) comparing infrapopliteal PTA with comparison of popliteal to distal vein bypass graft. Inclusion criteria required (1) > 15 infrapopliteal PTAs, (2) rest pain or tissue loss, (3) survival analysis to describe outcomes, and (4) minimum 12-month follow-up (Table 5). Although primary patency was significantly better in the bypass group, there was no difference in limb salvage during short- or long-term follow-up. This and more recent studies of infrapopliteal PTA continue to raise questions with regard to the clinical importance of restenosis as the endpoint in CLI. Once healing has completed, additional blood flow may not be required to maintain skin integrity, as evidenced by the absence of any limb salvage difference between the PTA and bypass groups.
Atherectomy for Infrapopliteal Disease
Due to increased lesion length with calcification, the use of atherectomy has been applied in BTK arteries with the hope of improving patency and stent deployment. By shaving, cutting, and pulverizing the atherosclerotic lesion is removed or debulked with subsequent change in vessel compliance. The Orbital Atherectomy System for the Treatment of Peripheral Vascular Stenosis (OASIS)44 trial is a multicenter, prospective, nonran-domized trial looking at short- and mid-term efficacy of orbital atherectomy (OA) for chronic infrapopliteal disease. The study involved 124 patients with 201 stenoses; 84 patients (68%) had Rutherford category 1 to 3, 40 patients (32%) had Rutherford category 4 to 5. OA was successful in 201 lesions; 68 patients (54.8%) had a single lesion and 56 patients (45.2%) had multiple lesions. Angiographically, 24 lesions (12%) were chronic total occlusions and 111 lesions (55%) had heavy calcification. Standalone OA was performed in 117 lesions (58.2%), adjunctive angioplasty was performed in 79 lesions (39.3%), and stenting occurred in 5 lesions. A total of 90.1% of patients achieved the primary outcome of < 30% final diameter stenosis and no major amputations at 6 months (2.4% minor amputations). Despite many other devices, there are no randomized trials comparing atherectomy with PTA for BKA disease.
The CALCIUM 360° pilot trial randomized 50 patients comparing OA Diamondback 360°®; Cardiovascular Systems, Inc., St. Paul, MN plus balloon angioplasty (ABA) with balloon angioplasty alone in patients with Rutherford category 4 to 6 and > 50% stenosis of the infrapopliteal arteries.45 Primary endpoint was defined as restoration of lumen with < 30% residual stenosis without bailout stenting or dissection types C through F. The patients were followed for 12 months. Although a greater percentage of patients in the ABA group achieved procedural success (93% vs 82%), less bailout stenting (6.9% vs 14.3%), and greater freedom from target vessel revascularization (TVR; 93% vs 80%), the results were not statistically significant (P = .27, P = .44, P = .14, respectively). Freedom from all-cause mortality was significantly greater in the balloon angioplasty alone group and was attributed to acute postprocedure residual stenosis of > 30% (P = .01).46
Definitive LE, the largest study to date regarding directional atherectomy using SilverHawk™ and TurboHawk™ Plaque Excision Systems (ev3 Endovascular, Inc., Plymouth, MN), was recently completed.47 It was a prospective, multi-center (US and Europe) randomized trial utilizing an independent core laboratory for angiographic and duplex analysis. The study enrolled 800 patients with PAD (claudication and CLI), of whom 18% had infrapopliteal disease. All patients with Rutherford category 1 to 6, > 50% stenosis, lesion length < 20 cm, and reference vessel size > 1.5 mm but < 7 mm were included in the study and followed for 12 months. Primary patency rate (patency of treated vessel) of infrapopliteal lesions for claudicants was 90% for a mean lesion length of 5.5 cm and 78% for a mean lesion length of 6.0 cm in CLI patients at 12 months. Overall, 95% of CLI patients were amputation free in the target limb at 12 months. More significantly, these results were no different in patients with or without; it was the first study to show such findings. Other unrelated findings from the study included 83% patency of SFA with mean lesions of 4 to 10 cm and 77% primary patency of popliteal lesions with mean lesion length of 6 cm. Thus, the DEFINITIVE LE trial demonstrated significant and robust 1-year primary patency of the SFA and popliteal and infrapopliteal arteries comparable with stents in patients with or without diabetes.
Bare Metal Stents
Despite improving outcomes with standalone angioplasty, long-term outcomes are still less than desirable. Excellent success with use of stents in the coronary arteries has encouraged their deployment in the infrapopliteal arteries, especially because tibial arteries are comparable in size with coronary arteries. However, significant debate remains regarding their use because of the risk of stent fracture, restenosis, and thrombosis. Moreover, unlike coronary arteries, there is more stress from motion in infrapopliteal arteries and stents are susceptible to being crushed. However, these concerns have been reduced with self-expanding stents that are more flexible and resistent to variable forces. In a comparative study, Randon and associates48 examined the patency rates of angioplasty compared with primary stenting. A total of 38 limbs in 35 patients with CLI were randomized to angioplasty (22 patients) or primary stenting (16 patients). Lesion size averaged between < 2 cm and > 15 cm in length. At 12-month follow-up, there was no statistical difference in limb salvage, survival, or in primary or secondary patency. The authors concluded from their results that stenting cannot be supported in all cases.
Biondi-Zoccai and colleagues49 performed a meta-analysis of 18 studies involving 640 patients, of whom 232 had been treated with bare metal stents (BMS) and 116 with self-expanding BMS. After an average of 12 months, binary restenosis rate was 25.7%, primary patency rate was 78.9% (95% confidence interval [CI], 71.8-86.0), secondary patency rate was 92.6% (95% CI, 87.7-97.5), and improvement in Rutherford category from baseline to follow-up was achieved in 91.3% (95% CI, 85.5-97.1), with 92.9% (95% CI, 87.6-98.3) of patients in Rutherford category 1 to 3 and 7.1% (95% CI, 1.7-12.4) in Rutherford category 4 to 6. Limb salvage rate was 96.4% (95% CI, 94.7-98.1).
Even with good initial success rates with PTA, restenosis rates from progressive residual stenosis, postdilatation dissections, elastic recoil, and early thrombosis of treated segments for long-term success are low. Further complicating long-term outcomes are complex bifurcating lesions and major side branch lesions that are ignored to achieve patency in the main vessel. Silingardi and colleagues50 examined preliminary results of infrapopliteal PTA stenting with Nile Croco (Minvasys, Gennevilliers, France) coronary bifurcating stents (chromium-cobalt BMS with a short scaffold at the side port to provide coverage of the side branch ostium and allow access to the side branch). The system has two independent balloons, one for the main branch and one for the side branch; the main is deployed prior to the side branch. A total of 31 patients with CLI were enrolled (popliteal, n = 17 [54.8%]; distal tibioperoneal trunk, n = 14 [45.2%]) from October 2006 to December 2010. Preliminary data from this study represent high success rates, both early and mid-term patency rates, and clinical improvement and limb salvage in selected patients with complex, bifurcating infrapopliteal lesions (Table 6).
Donas and coworkers51 perfomed one of the larger studies assessing BMS for high-risk patients with infrapopliteal CLI, with a 2-year follow-up. A total of 53 high-risk patients (multiple comorbidities, Rutherford category 4-5, American Society of Anesthesiologists score > 3, previous MI, coronary artery bypass graft, or peripheral intervention complicated with infection) had self-expanding nitinol stents implanted. Mean lesion length was 5.5 ±1.9 cm. Successful intervention was performed in 98.1% of patients. The cumulative primary and secondary patency at 24 months was 75.5% and 88.7%, respectively; two patients required repeat interventions at 5 and 9 months, respectively, due to significant stenosis (> 70%). Four patients had rest pain with poor runoff, which required bypass. Significant improvement was achieved immediately post-procedure with sustained benefits at 24-month re-evaluation. Limb salvage and amputation-free interval in this high-risk patient population at 24 months was 88.7%. Six patients required amputations due to stent occlusions and were not candidates for either endovascular or surgical revascularization. Higher patency rates were seen with proximal compared with distal (83.4% vs 62.2%; P = .04) lesions but morphology (complete occlusion vs stenosis) of the lesion did not influence patency. The authors concluded that infrapopliteal stent placement is a durable option in high-risk CLI patients with suboptimal angioplasty.
Current literature lacks a large, prospective, randomized trial evaluating the efficacy of BMS for CLI of infrapopliteal disease. The majority of studies have failed to sufficiently provide evidence that BMS is superior to PTA. As a result, there is no level I recommendation to support primary PTA over angioplasty and BMS has been reserved for bailout treatment of suboptimal results after angioplasty.
Drug-Eluting Stents
Despite technical adavances, durability of infrapopliteal patency with PTA or BMS has been less than desirable. In fact, the mean patency rate is approximately 50% with BMS and 70% with PTA alone at 1 year.52-54 Given the high success rates of drug-eluting stents (DES) in the coronary arteries, some experts have suggested their use in the treatment of infrapopliteal lesions. After dilatation, microdissections lead to platelet activation and aggregation, resulting in potential vessel thrombosis. Vessel expansion also leads to recoiling and vessel narrowing. Because the body considers the stent a foreign object, macrophages accumulate and lead to smooth muscle proliferation, in turn, leading to stent stenosis. Less common, neo-intimal proliferation can occur and lead to stent stenosis. DES are coated with antiproliferative drugs that are gradually released to prevent stenosis. One commonly used family of DES is the limus family (eg, evero-limus, sirolimus, and paclitaxel), which stops cell division from the G1 to the S phase in smooth muscle cells by stabilizing microtubules, thereby inhibiting cell division in the G0/G1 and G2/M phases.
Recently, multiple randomized and nonrandomized trials have shown improved patency rates and durability of DES for infra popliteal lesions. Bosiers and col leagues55 (Drug Eluting Stents in the Critically Ischemic Lower Leg [DESTINY] trial) prospectively compared everolimus-eluting (Xience V; Abbott Vascular, Abbott Park, IL) versus BMS (MULTI-LINK VISION; Abbott Vascular) in patients with CLI with infrapopliteal occlusions. A total of 140 patients were blindly randomized to BMS (66) and DES (74) groups at five European investigative sites between 2008 and 2009. All patients were symptomatic and presented with Rutherford category 4 (39% BMS vs 50% DES) or 5 (61% BMS vs 50% DES). At the end of 1 year, there were three major amputations (2 for BMS and 1 for DES) and 23 deaths due to significant comorbidities. At 12-month follow-up, primary patency rates were significantly different between the DES (85.2%) and BMS (54.4%; P = .0001) groups. The superiority of patency was higher with DES in the proximal (85% vs 58%; P = .0001) and distal (87% vs 43%; P = .08) segments. According to the authors, P value was not significant in the distal segment due to small sample size. Target lesion revascu-larization (TLR) was significantly reduced in the DES group (65% vs 92%; P = .005). Overall, clinical outcomes in the DESTINY trial showed no significance between the two groups. Sustained improvement in Rutherford category of 0 or 1 at 12 months (56% vs 60%; P = .68) or survival at 12 months (84% vs 82%; P = .96) in the BMS or DES treated arms was similar.
Similar patency rates were demonstrated in the Yukon Drug-Eluting Stent-Below the Knee (YUKON-BTK) trial56 by Rastan and colleagues. The study compared sirolimus-eluting stents (SES) with BMS in a prospective, randomized, double blind, multicenter trial of 161 patients (BMS = 79, SES = 82) with claudication or CLI (Rutherford category 3-6). Primary patency and clinical improvement were assessed at 12 months. Mean lesion length was 31 ± 9 mm. After 1 year, primary patency rates were 80.6% and 55.6% (P = .004) in the SES and BMS groups, respectively. The results were adjusted for diabetes, smoking, body mass index, and stage of disease (claudication or CLI). There was significant improvement in Rutherford classification in the SES compared with BMS (P = .004) groups. In this study there was no difference in TLR, limb salvage, wound healing, ABI, or major adverse events between the two groups after 1-year follow-up.
As an extension of the study, Rastan and associates57 investigated long-term clinical event rates of SES and BMS. The mean follow up time was 1005 days in the SES and 1027 in the BMS groups. Primary endpoints were event-free survival rate from TVR, major and minor amputation, MI, and death. Secondary end-points included amputation rate, TVR, and change in Rutherford category. Using Kaplan-Meier, event-free survival was significantly better in the SES compared with the BMS groups (65.8% vs 44.6%), with a BMS group hazard ratio of 1.8 (95% CI, 1.1-2.9; P = .02). Similar significance results were evident in CLI patients with SES as compared with BMS (Figure 3). Significantly more patients required amputation in the SES as compared with the BMS group (2 vs 7; P = .04). TVR and change in Rutherford category were also significant in the SES group. No significant difference was seen in rate of MI.
The Comparing Angioplasty and DES in the Treatment of Subjects with Ischemic Infrapopliteal Arterial Disease (ACHILLES) trial58 with 1-year results compared SES with PTA in patients with symptomatic infrapopliteal disease. The large study was based on findings of several small studies showing the benefit of SES for treatment of infrapopliteal lesions. It was a prospective, multicenter, randomized study evaluating binary restenosis rate in de-novo and restenotic infrapopliteal lesions at 1 year. A total of 200 patients were randomized in a 1:1 fashion to SES (n = 99) and PTA (n = 101) groups. Mean lesion size was 27 ± 21 mm for both groups with an immediate postprocedure success rate of 95% for SES and 93% for PTA. At 12 months, primary binary restenosis was significantly better with SES than PTA (22.4% vs 41.9%; P = .019). This difference was even more profound in diabetic patients (17.6% vs 53.2%; P < .001). Although TLR, death, bypass/ amputation, and Rutherford category improvement were all numerically better with SES, they were not statistically significant. The authors felt low power may have led to these numbers.
It is well known that patients with CLI have a significant 1-year mortality rate (25%) and higher amputation rates (30%).4 A majority of these patients also have substantial infrapopliteal disease and comorbidities, making surgical bypass highly complicated or prohibitive in this population. Endovascular treatment has become a therapeutic option with the goal of establishing straight-line blood flow to the foot. With improved patency rates with DES, the Preventing Amputations Using Drug-Eluting Stents (PARADISE) trial enrolled 106 patients (118 limbs) from May 2003 to March 2009 in a prospective, nonrandomized trial to investigate the safety and efficacy of DES in patients with infrapopliteal disease and CLI. There were 228 DES (83% Cypher® [Cordis, Bridgewater, NJ] and 17% Taxus® [Boston Scientific, Marlborough, MA]) implanted with an average of 1.9 stents per limb. All patients were in Rutherford category 4 or higher (> 60% were in category 5 and 6) with mean age of 74 years; mean follow-up time was 27.4 ± 18.6 months. Occlusion of all three tibial vessels was present in 74% of patients.
There were six major amputations in the first year and none thereafter, and 93% of patients had resolution of their rest pain. At 3 years, 96% of patients were without major amputation and 88% of those who died did not have major amputation. When the results of the study were compared with surgical and PTA groups from the BASIL trial, the PARADISE trial with DES had statistically better outcomes with regard to amputation-free survival until the third year. Additionally, primary DES for infrapopliteal CLI is safe, with minimal early procedural morbidity and mortality, good long-term durability, and low rates of repeat intervention.
With an increase in endovascular procedures, and advances in techniques and devices, therapeutic options for restoration of blood flow to the foot are expanding. Restenosis rates with simple PTA are still suboptimal. Although stent use has increased for the treatment of infrapopliteal CLI, their primary use is relegated to bailout after PTA for no flow, dissection, or recoil phenomenon.14
Lookstein and associates59 evaluated the efficacy of DES after failed PTA for infrapopliteal lesions in CLI. In this retrospective study, 67 consecutive patients who had undergone PTA with subsequent DES for inadequate patency between 2005 and 2010 were evaluated. All patients had CLI, and 63% had Rutherford category 5 lesions. A total of 123 DES (94 sirolimus, 27 everolimus, 2 paclitaxel) were deployed in 84 lesions after suboptimal PTA. Technical success, defined as < 30% stenosis, after DES deployment was 100%. Follow-up occurred at 6 (n = 100), 12 (n = 88), and 24 (n = 45) months. Primary patency rates were > 90% at 6 months, 86% at 12 months, and 72% at 24 months. There were no planned major amputations in the patients in Rutherford category 4 or 5, but six patients with Rutherford category 6 lesions had minor amputations that had been planned prior to the endovascular procedure.
Drug-Eluting Balloons
Suboptimal patency rates and repeat interventions has led to development of drug-eluting balloons to improve outcomes. Schmidt and colleagues60 published their initial experience with paclitaxel-eluting balloons. A total of 104 patients (109 limbs) were treated for Rutherford category 4/5 disease (82.6%) or Rutherford category 3 disease (17.4%). Prior to treatment, 77.1% had complete or functional occlusion of three infrapopliteal arteries with a mean lesion length of 176 mm. At 3-month follow-up, of the 94 patients seen, 75.8% had clinical improvement, 22.2% were unchanged, and 2% were clinically worse. Complete wound healing was noted in 41.9% of Rutherford category 5 limbs. Three toes were amputated. At 3-month follow-up, angiographically, 72.6% were free of significant restenosis, 27.4% had > 50% restenosis, and 8.3% (baseline 61.9%) had occlusion. When restenoses did occur, they were focal (< 20% of the length of the initial target) in 61% of the cases. At 1-year follow-up, 91 treated limbs were evaluated; 91.2% showed continued clinical improvement and 74.2% had complete wound healing in the Rutherford 5 category. TVR was performed in 17.3%, and limb salvage was achieved in 95.6% (Figure 4). The mortality rate was 16.3% at 1 year for unrelated reasons. Overall, as compared with uncoated balloon stents, the authors reported a lower 3-month restenosis rate (61%-27%) and fewer focal restenosis lesions (< 20% vs whole treated segments).
Conclusions
PAD is a systemic disease with significant morbidity and mortality. A substantial number of patients with PAD have infrapopliteal disease with similar prognostic implications; however, diagnosis based on symptoms and ABI alone can lead to delayed or missed opportunities to provide improved quality of life and limb salvage, and potentially reduce mortality. Advances in techniques and devices, and modification of classification systems have determined that an endovascular approach should be the primary therapeutic option for CLI due to infrapopliteal diseases.
Unfortunately, despite advances in percutaneous treatment of infrapopliteal disease, there are still limitations that bring about less than optimal results. Complications that are secondary to the disease process (eg, significant calcification, advanced age, multiple comorbidites, and long occlusions) can interefere with infrapopliteal interventions. Given the infancy of focus on infrapopliteal disease, both technical skills and technology are rapidly evolving to pave the way for continued improvement of endovascular strategies. Interest and research through randomized trials comparing different treatment options, such as new-generation stents (DES or BMS) with atherectomy/PTA, and drug-coated balloons, will further advance our knowledge, resulting in improved outcomes both technically and clinically. ![]()
The authors report no real or apparent conflicts of interest.
References
- Anderson JL, Halperin JL, Albert NM, et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA guideline recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:1425-1443.
- Creager MA, Libby P. Peripheral arterial disease. In: Bonow RO, Mann DL, Zipes DP, Libby P, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed. Philadelphia, PA: WB Saunders; 2011.
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45(suppl S): S5-S67.
- Price JF, Mowbray PI, Lee AJ, et al. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur Heart J. 1999;20:344-353.
- Cole CW, Hill GB, Farzad E, et al. Cigarette smoking and peripheral arterial occlusive disease. Surgery. 1993;114:753-756; discussion 756-757.
- Kannel WB, McGee DL. Update on some epidemiologic features of intermittent claudication: the Framingham Study. J Am Geriatr Soc. 1985;33:13-18.
- Most RS, Sinnock P. The epidemiology of lower extremity amputations in diabetic individuals. Diabetes Care. 1983;6:87-91.
- Kannel WB, Skinner JJ Jr, Schwartz MJ, Shurtleff D. Intermittent claudication: incidence in the Framingham Study. Circulation. 1970;41:875-883.
- Kannel WB. The demographics of claudication and the aging of the American population. Vasc Med. 1996;1:60-64.
- Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286: 1317-1324.
- Long TH, Criqui MH, Vasilevskis EE, et al. The correlation between the severity of peripheral arterial disease and carotid occlusive disease. Vasc Med. 1999;4:135-142.
- Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326:381-386.
- Zheng ZJ, Sharrett AR, Chambless LE, et al. Associations of ankle-brachial index with clinical coronary heart disease, stroke and preclinical carotid and popliteal atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 1997;131:115- 125.
- Sullivan TM, Slovut DP. Critical limb ischemia: medical and surgical management. Vasc Med. 2008;13:281-291.
- Marston WA, Davis SW, Armstrong B, et al. Natural history of limbs with arterial insufficiency and chronic ulcerations treated without revascularization. J Vasc Surg. 2006;44:108-114.
- Wolfe JHN, Wyatt MG. Critical and subcritical ischaemia. Eur J Vasc Endovasc Surg. 1997;13:578-582.
- Kawarada O, Yokoi Y, Honda Y, Fitzgerald PJ. Awareness of anatomical variations for infrapopliteal intervention. Catheter Cardiovasc Interv. 2010;76: 888-894.
- Bernstein O, Chalmers N. New treatments for infrapopliteal disease: devices, techniques, and outcomes so far. Cardiovasc Intervent Radiol. 2012;35: 715-724.
- Drüeke TB. Arterial intima and media calcification: distinct entities with different pathogenesis or all the same? Clin J Am Soc Nephrol. 2008;3:1583-1584.
- Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary: a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47:1239-1312.
- Dormandy JA, Rutherford RB. Management of peripheral arterial disease (PAD). TASC Working Group. TransAtlantic Inter-Society Consensus (TASC). J Vasc Surg. 2000;31(1 Pt 2):S1-S296.
- Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645-658.
- Hiatt WR, Marshall JA, Baxter J, et al. Diagnostic methods for peripheral arterial disease in the San Luis Valley Diabetes Study. J Clin Epidemiol. 1990;43:597-606.
- Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol. 1995;75: 894-903.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837-853.
- American Diabetes Association. Standards of medical care for patients with diabetes mellitus. Diabetes Care. 2003;26(suppl 1):S33-S50.
- Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study. N Engl J Med. 2000;342:145-153.
- Heart Protection Study Collaborative Group. MRC/ BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7-22.
- Antithrombotic Trialists’ Collaboration. Collaborative metaanalysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71-86.
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events. Lancet. 1996;348:1329-1339.
- Bhatt DL, Fox KA, Hacke W et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. JACC. 2007;49:1982-1988.
- Girolami B, Bernardi E, Prins MH, et al. Antithrombotic drugs in the primary medical management of intermittent claudication: a meta-analysis. Thromb Haemost. 1999;81:715-722.
- Anand SS, Yusuf S. Oral anticoagulant therapy in patients with coronary artery disease: a meta-analysis. JAMA. 1999;282:2058-2067.
- Kalbaugh CA, Taylor SM, Blackhurst DW, et al. One year prospective quality of life outcomes in patients treated with angioplasty for symptomatic peripheral arterial disease. J Vasc Surg. 2006;44:296-302.
- Bosiers M, Hart JP, Deloose K, et al. Endovascular therapy as the primary approach for limb salvage in patients with critical limb ischemia: experience with 443 infrapopliteal procedures. Vascular. 2006;14: 63-69.
- Kudo T, Chandra FA, Kwun WH, et al. Changing pattern of surgical revascularitzation for critical limb ischemia over 12 years: endovascular vs open bypass surgery. J Vasc Surg. 2006;44:304-313.
- Giles KA, Pomposelli FB, Spence TL, et al. Infrapopliteal angioplasty for critical limb ischemia: relation of TransAtlantic InterSociety Consensus class to outcome in 176 limbs. J Vasc Surg. 2008;48:128-136.
- Adam DJ, Beard JD, Cleveland T, et al; BASIL trial participants. Bypass versus angioplasty in severe ischemia of the leg (BASIL): multicenter, randomized controlled trial. Lancet. 2005;366:1925-1934.
- Casella IB, Brochado-Neto FC, Sandri Gde A, et al. Outcome analysis of infrapopliteal percutaneous transluminal angioplasty and bypass graft surgery with nonreversed saphenous vein for individuals with critical limb ischemia. Vasc Endovascular Surg. 2010;44:625-632.
- Willenberg T, Baumgartner I, Silvestro A, et al. An angiographic analysis of atherosclerosis progression in below-the-knee arteries after femoropopliteal angioplasty in claudicants. J Endovasc Ther. 2010; 17:39-45.
- Schmidt A, Ulrich M, Winkler B, et al. Angiographic patency and clinical outcome after balloon-angioplasty for extensive infrapopliteal arterial disease. Catheter Cardiovasc Interv. 2010;76:1047-1054.
- Fernandez N, McEnancy R, Marone LK, et al. Predictors of failure and success of tibial interventions for critical limb ischemia. J Vasc Surg. 2010;52:834-842.
- Romiti M, Albers M, Brochado-Neto FC, et al. Metaanalysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg. 2008;47:975-981.
- Safian RD, Niazi K, Runyon JP, et al; OASIS Investigators. Orbital atherectomy for infrapopliteal disease: device concept and outcome data for the OASIS trial. Catheter Cardiovasc Interv. 2009;73:406-412.
- Shammas NW, Lam R, Mustapha J, et al. Comparison of orbital atherectomy plus balloon angioplasty vs. balloon angioplasty alone in patients with critical limbischemia: results of the CALCIUM 360 randomized pilot trial. J Endovasc Ther. 2012;19: 480-488.
- Shammas NW, Lam R, Mustapha J, et al. Comparision of orbital atherectomy plus balloon angioplasty vs. balloon angioplasty alone in patients with critical limb ischemia: results of the CALCIUM 360 randomzied pilot trial. J Endovasc Ther. 2012;19:480-488.
- McKinsey J, Zeller T, Rocha-Singh KJ, et al. Lower extremity revascularization using directional atherectomy: 12-month prospective results of the DEFINITIVE LE study. JACC Cardiovasc Interv. 2014;7: 923-933.
- Randon C, Jacobs B, De Ryck F, Vermassen F. Angioplasty or primary stenting for infrapopliteal lesions: results of a prospective randomized trial. Cardiovasc Intervent Radiol. 2010;33:260-269.
- Biondi-Zoccai GG, Sangiorgi G, Lotrionte M, et al. Infragenicular stent implantation for below-the-knee atherosclerotic disease: clinical evidence from an international collaborative meta-analysis on 640 patients. J Endovasc Ther. 2009;16:251-260.
- Silingardi R, Tasselli S, Cataldi V, et al. Bifurcated coronary stents for infrapopliteal angioplasty in critical limb ischemia. J Vasc Surg. 2013;57: 1006-1013.
- Donas KP, Torsello G, Schwindt A, et al. Below knee bare nitinol stent placement in high risk patients with critical limb ischemia is still durable after 24 months of follow up. J Vasc Surg. 2010;52:356-361.
- Siablis D, Karnabatidis D, Katsanos K, et al. Sirolimuseluting versus bare metal stents after suboptimal infrapopliteal angioplasty for critical limb ischemia: enduring 1-year angiographic and clinical benefit. J Endovasc Ther. 2007;14:241-250.
- Romiti M, Albers M, Brochado-Neto FC, et al. Metaanalysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg. 2008;47:975-981.
- Balzer JO, Khan V, Thalhammer A, et al. Below the knee PTA in critical limb ischemia results after 12 months: single center experience. Eur J Radiol. 2010;75: 37-42.
- Bosiers M, Scheinert D, Peeters P, et al. Randomized comparison of everolimus-eluting versus bare metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive disease. J Vasc Surg. 2012;55:390-399.
- Rastan A, Tepe G, Krankenberg H, et al. Sirolimuseluting stents vs. bare-metal stents for treatment of focal lesions in infrapopliteal arteries: a double-blind, multi-centre, randomized clinical trial. Eur Heart J. 2011;32:2274-2281.
- Rastan A, Brechtel K, Krankenberg H, et al. Sirolimus eluting stents for treatment of infrapopliteal arteries reduce clinical event rate compared to bare metal stents: long term results from a randomized trial. J Am Coll Cardiol. 2012;60:587-591.
- Scheinert D, Katsanos K, Zeller T, et al; ACHILLES Investigators. A prospective randomized multicenter comparison of balloon angioplasty and infrapopliteal stenting with the sirolimus eluting stent in patient with ischemic peripheral arterial disease: 1-year results from the ACHILLES trial. J Am Coll Cardiol. 2012;60:2290-2295.
- Lookstein R, Ward T, Kim E, et al. Value of drugeluting stents after failed percutaneous transluminal angioplasty in the infrapopliteal vessels for the treatment of critical limb ischemia: favorable mid-term patency and limb salvage results. J Cardiovasc Surg (Torino). 2011;52:461-466.
- Schmidt A, Piorkowski M, Werner M, et al. First experience with drug-eluting balloons in infrapopliteal arteries: restenosis rate and clinical outcome. J Am Coll Cardiol. 2011;58:1105-1109.