An Example of the Deleterious Effects of Right Ventricular Apical Pacing
Andrés M. Pineda, MD,1 Omar Issa, DO,2 Mery Cortes-Bergoderi, MD,2 Christos G. Mihos, MD,1 Juan Carlos Brenes, MD,1 Alfonso Tolentino, MD1
1Columbia University Division of Cardiology, Mount Sinai Medical Center, Miami Beach, FL; 2Department of Internal Medicine, Mount Sinai Medical Center, Miami Beach, FL
Pacemaker implantation remains the mainstay of treatment in patients with symptomatic sinus node disease or severe heart block. Despite the dramatic benefits of this therapy, a high burden of ventricular pacing is known to have its disadvantages. Reported is the case of an 85-year-old woman with a history of sick sinus syndrome who presented with congestive heart failure after her atrioventricular sequential pacemaker defaulted to ventricular pacing mode as a result of battery depletion. After replacement of her generator and reinstitution of atrial pacing, dramatic improvements in her symptoms and echocardiographic findings were observed. Although it is difficult to predict which patients will ultimately develop cardiac decompensation as a result of ventricular pacing, closer follow-up and early recognition of these complications is essential to prevent adverse outcomes.
[Rev Cardiovasc Med. 2015;16(1):84-89 doi: 10.3909/ricm0752]
© 2015 MedReviews®, LLC
An Example of the Deleterious Effects of Right Ventricular Apical Pacing
Andrés M. Pineda, MD,1 Omar Issa, DO,2 Mery Cortes-Bergoderi, MD,2 Christos G. Mihos, MD,1 Juan Carlos Brenes, MD,1 Alfonso Tolentino, MD1
1Columbia University Division of Cardiology, Mount Sinai Medical Center, Miami Beach, FL; 2Department of Internal Medicine, Mount Sinai Medical Center, Miami Beach, FL
Pacemaker implantation remains the mainstay of treatment in patients with symptomatic sinus node disease or severe heart block. Despite the dramatic benefits of this therapy, a high burden of ventricular pacing is known to have its disadvantages. Reported is the case of an 85-year-old woman with a history of sick sinus syndrome who presented with congestive heart failure after her atrioventricular sequential pacemaker defaulted to ventricular pacing mode as a result of battery depletion. After replacement of her generator and reinstitution of atrial pacing, dramatic improvements in her symptoms and echocardiographic findings were observed. Although it is difficult to predict which patients will ultimately develop cardiac decompensation as a result of ventricular pacing, closer follow-up and early recognition of these complications is essential to prevent adverse outcomes.
[Rev Cardiovasc Med. 2015;16(1):84-89 doi: 10.3909/ricm0752]
© 2015 MedReviews®, LLC
An Example of the Deleterious Effects of Right Ventricular Apical Pacing
Andrés M. Pineda, MD,1 Omar Issa, DO,2 Mery Cortes-Bergoderi, MD,2 Christos G. Mihos, MD,1 Juan Carlos Brenes, MD,1 Alfonso Tolentino, MD1
1Columbia University Division of Cardiology, Mount Sinai Medical Center, Miami Beach, FL; 2Department of Internal Medicine, Mount Sinai Medical Center, Miami Beach, FL
Pacemaker implantation remains the mainstay of treatment in patients with symptomatic sinus node disease or severe heart block. Despite the dramatic benefits of this therapy, a high burden of ventricular pacing is known to have its disadvantages. Reported is the case of an 85-year-old woman with a history of sick sinus syndrome who presented with congestive heart failure after her atrioventricular sequential pacemaker defaulted to ventricular pacing mode as a result of battery depletion. After replacement of her generator and reinstitution of atrial pacing, dramatic improvements in her symptoms and echocardiographic findings were observed. Although it is difficult to predict which patients will ultimately develop cardiac decompensation as a result of ventricular pacing, closer follow-up and early recognition of these complications is essential to prevent adverse outcomes.
[Rev Cardiovasc Med. 2015;16(1):84-89 doi: 10.3909/ricm0752]
© 2015 MedReviews®, LLC
KEY WORDS
Pacemaker • Complications • Heart failure
KEY WORDS
Pacemaker • Complications • Heart failure
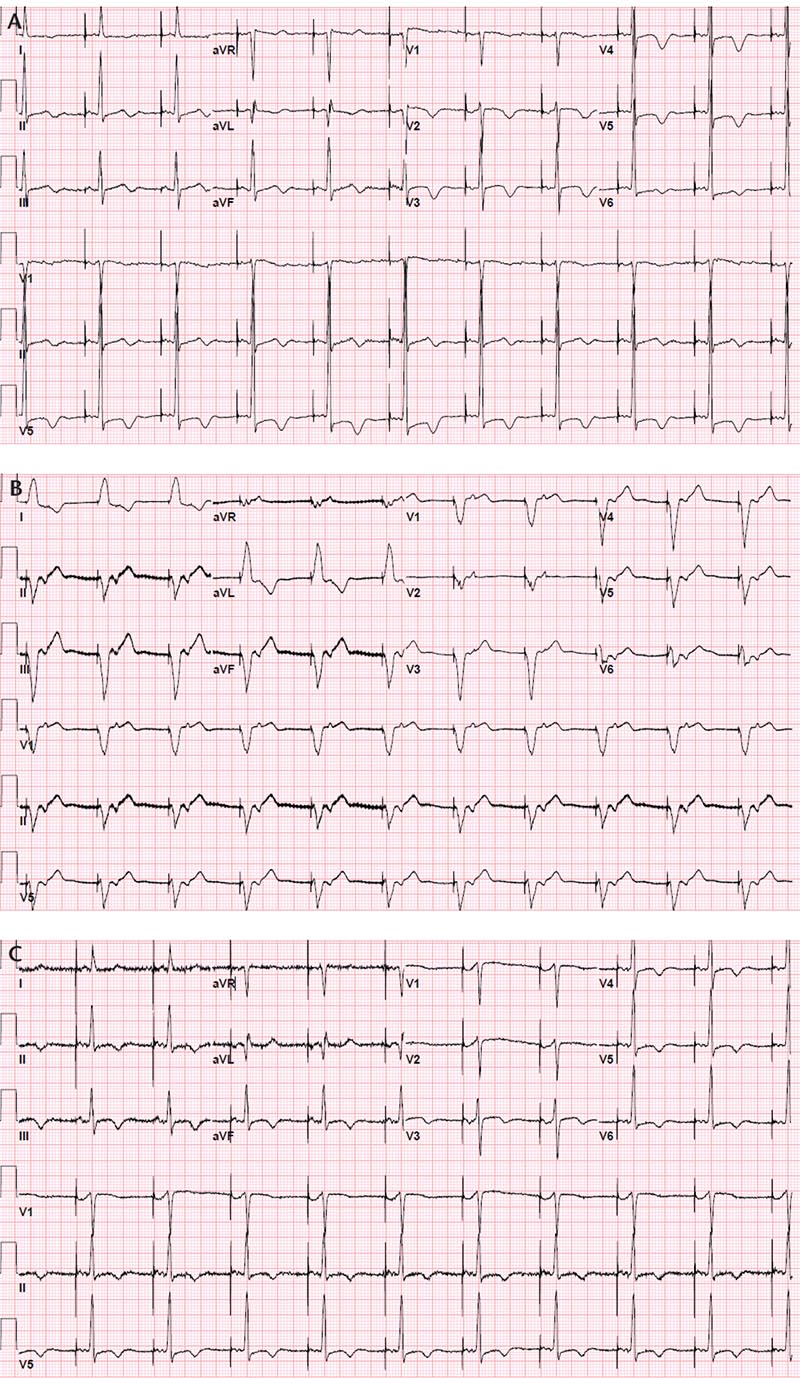
Figure 1. Atrial pacing noted in an electrocardiogram 10 months before presentation (A). Electrocardiogram at presentation demonstrated ventricular pacing with retrograde ventriculoatrial conduction (B). After the generator was changed, atrial pacing was restored (C).
Interrogation of the pacemaker revealed that the device had reached its elective replacement interval 1 month prior to admission, presumably at the time the device inherently switched from atrial pacing-ventricular sensing (DDD mode) to ventricular pacing (VVI mode).
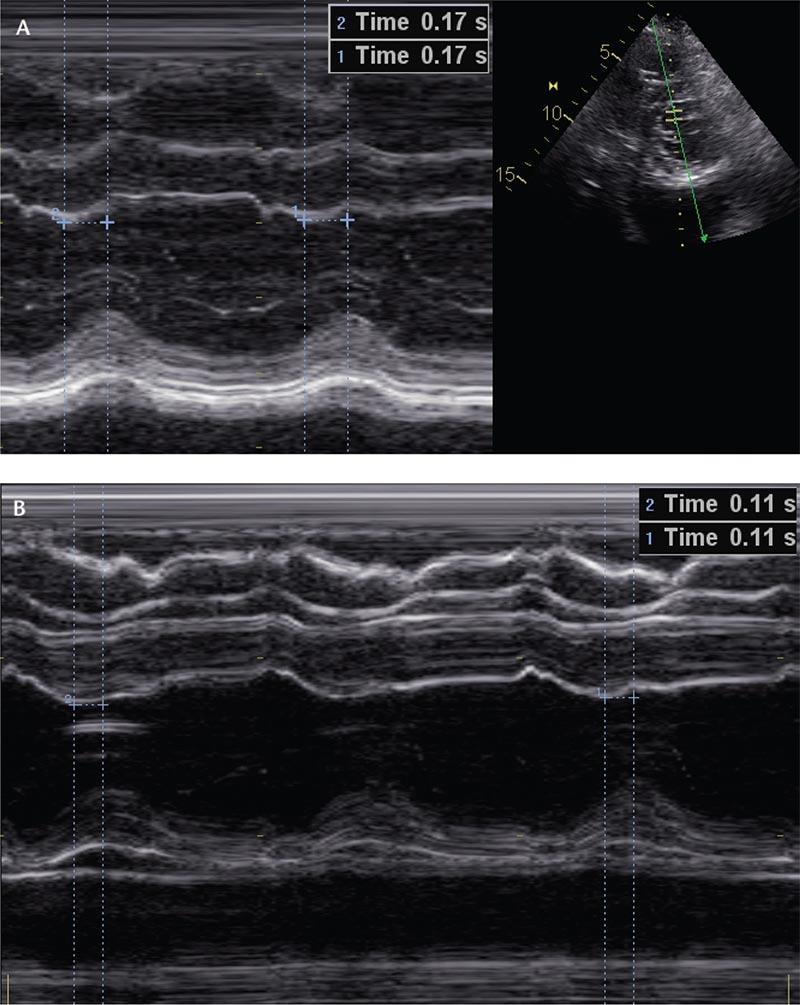
Figure 2. M-Mode echocardiography assessment of the septal to posterior wall motion delay prior to (A) and after (B) pacemaker generator replacement.
Long-term ventricular pacing can cause further reduction in systolic function, abnormal ventricular remodeling, and deterioration of functional capacity.
When compared with an LBBB, RV pacing generates an even greater conduction delay, which, in heart failure patients, can be further worsened by underlying electrical abnormalities.
Main Points
• Although pacemaker implantation can often be a life-saving intervention, there is evidence demonstrating the adverse effects of right ventricular apical pacing on cardiac function, attributed mostly to abnormal electrical activation resulting in mechanical dyssynchrony of the ventricles.
• Ventricular pacing behaves similarly to a left bundle branch block, with propagation of the cardiac impulse occurring from myocyte to myocyte, which results in a sluggish conduction that produces a heterogeneous pattern of ventricular activation and, ultimately, a delay in contraction between the interventricular septum and the posterior or lateral wall.
• Long-term ventricular pacing can cause further reduction in systolic function, abnormal ventricular remodeling, and deterioration of functional capacity.
• Selecting the appropriate device for pacing/defibrillation can be challenging, and multiple factors must be considered. Abnormalities of cardiac function, automaticity, and atrioventricular conduction, both at the time of implantation and those anticipated in the future, should affect this decision.
Main Points
• Although pacemaker implantation can often be a life-saving intervention, there is evidence demonstrating the adverse effects of right ventricular apical pacing on cardiac function, attributed mostly to abnormal electrical activation resulting in mechanical dyssynchrony of the ventricles.
• Ventricular pacing behaves similarly to a left bundle branch block, with propagation of the cardiac impulse occurring from myocyte to myocyte, which results in a sluggish conduction that produces a heterogeneous pattern of ventricular activation and, ultimately, a delay in contraction between the interventricular septum and the posterior or lateral wall.
• Long-term ventricular pacing can cause further reduction in systolic function, abnormal ventricular remodeling, and deterioration of functional capacity.
• Selecting the appropriate device for pacing/defibrillation can be challenging, and multiple factors must be considered. Abnormalities of cardiac function, automaticity, and atrioventricular conduction, both at the time of implantation and those anticipated in the future, should affect this decision.
A steady increase in pacemaker implantation is expected to continue, mostly related to an aging population. Despite the benefits provided by these devices, namely, clinical improvement in patients with symptomatic sinus node disease or severe heart block, a high burden of ventricular pacing has its disadvantages. We present a case of an 85-year-old woman who developed acute decompensated heart failure as a result of right ventricular (RV) apical pacing.
Presentation
An 85-year-old white woman presented to the hospital with a 2-week history of progressive dyspnea, which progressed to New York Heart Association class IV heart failure, associated with dry cough and bilateral lower extremity edema. She had otherwise been asymptomatic until 1 month prior to her presentation. Her past medical history is significant for hypertension, a prior transient ischemic attack, and implantation of a dual-chamber pacemaker (Sigma® SDR303; Medtronic, Minneapolis, MN) 12 years prior for symptomatic sinus node disease. Her last pacemaker interrogation had been more than 1 year before presentation, and had shown normal pacemaker function. She did not have a history of heart failure, and her left ventricular ejection fraction (LVEF) 2 years prior to this admission was 55% to 60%. Her outpatient medications included aspirin, 81 mg/d, lisinopril, 10 mg/d, simvastatin, 20 mg/d, and diltiazem, 120 mg/d.
On presentation to the emergency room, the patient had a heart rate of 65 beats/min, a blood pressure of 152/85 mm Hg, and an oxygen saturation of 90% on room air. She was noted to have crackles at both lung bases, a 1/6 apical systolic murmur, and 1+ bilateral lower extremity pitting edema. Initial laboratory studies were significant for normal cardiac enzymes and an elevated prohormone brain natriuretic peptide level of 2569 pg/mL. Her chest radiograph demonstrated mild cardiomegaly, perihilar congestion, and small bilateral pleural effusions. The initial electrocardiogram (ECG) revealed ventricular pacing at a rate of 65 beats/min with left bundle branch block (LBBB) morphology, retrograde ventriculoatrial conduction, and a QRS axis of -56 degrees. This was different from an ECG done 10 months prior, which showed an atrial paced rhythm at a rate of 60 beats/min with normal PR interval and intrinsically conducted QRS complexes (Figure 1A, B). Transthoracic echo-cardiogram demonstrated a mildly reduced LVEF, calculated at 44% by the modified Simpson method, an abnormal septal motion consistent with her conduction abnormality, mild left ventricular hypertrophy, and mild mitral regurgitation. The stroke volume and RV systolic pressure were estimated to be 61 mL per beat and 43 mm Hg, respectively.
The patient received treatment for acutely decompensated heart failure, including intravenous diuretics (furosemide 20 mg every 12 hours) and her home medications were continued. She did not require intravenous inotropic agents. Interrogation of the pacemaker revealed that the device had reached its elective replacement interval 1 month prior to admission, presumably at the time the device inherently switched from atrial pacing-ventricular sensing (DDD mode) to ventricular pacing (VVI mode). On the third day of her hospitalization, she underwent generator replacement and the pacemaker was programmed back to DDD mode with lower rate of 60 beats/min, upper rate of 120 beats/min, and an atrioventricular (AV) delay of 250 ms. A repeat echocardiogram 6 days after the battery change showed drastic improvements in her hemodynamics, mostly notable for an improved ejection fraction, calculated at 61% by the modified Simpson method, and a stroke volume of 77 mL per beat. The patient's symptoms improved significantly after her medical therapy was optimized and the pacemaker was programmed to a predominantly atrial paced rhythm (Figure 1C). She was discharged home shortly thereafter.
Discussion
Although pacemaker implantation can often be a life-saving intervention, there is evidence demonstrating the adverse effects of RV apical pacing on cardiac function. This has been attributed mostly to abnormal electrical activation resulting in mechanical dyssynchrony of the ventricles.1,2 Electrically, ventricular pacing behaves similarly to LBBB, with propagation of the cardiac impulse occurring from myocyte to myocyte, rather than the Purkinje system.1,2 This results in a sluggish conduction that produces a heterogeneous pattern of ventricular activation, and ultimately a delay in contraction between the inter ventricular septum and the posterior or lateral wall.2 The resulting dyssynchrony can be evaluated with conventional M-mode Doppler echocardiography by measuring the septal to posterior wall motion delay. A value > 130 ms is considered a marker of left ventricular dyssynchrony.3 Our patient had 170 ms (Figure 2A) during ventricular pacing.
RV apical pacing can also have adverse effects on the metabolism, perfusion, hemodynamics, and mechanical function of the myocardium.1,2 Although these derangements occur globally throughout the ventricle, studies have demonstrated that the effect is most prominent near the pacing site.1 Small observational studies have demonstrated significant reductions in ejection fraction and stroke volume, which can become evident as early as 2 hours after the initiation of ventricular pacing.1,2 Our patient, with no prior history of cardiomyopathy, developed an acute reduction in her ejection fraction to 45% to 50% within 1 month of ventricular pacing.
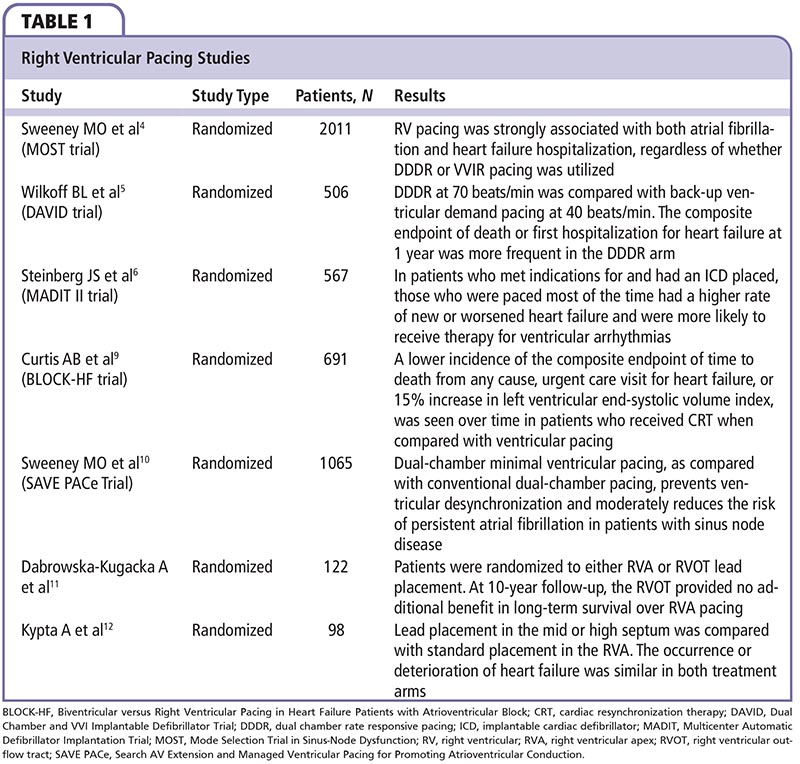
Long-term ventricular pacing can cause further reduction in systolic function, abnormal ventricular remodeling, and deterioration of functional capacity.1,2 Large clinical trials have confirmed these hemodynamic alterations but have been somewhat inconsistent with regard to outcomes in patients with chronic RV pacing (Table 1). A sub-study of the Mode Selection Trial in Sinus-Node Dysfunction (MOST) demonstrated that, in patients with sick sinus syndrome, RV pacing was strongly associated with both atrial fibrillation and heart failure hospitalization, regardless of whether DDDR or WIR pacing was utilized.4 Another pivotal study, the Dual Chamber and VVI Implantable Defibrillator (DAVID) trial, compared dual-chamber rate-responsive pacing (DDDR) at 70 beats/min to back-up ventricular demand pacing at 40 beats/ min in patients with an ejection fraction < 40% and an indication for an intracardiac device.5 The composite endpoint of death or first hospitalization for heart failure at 1 year was more frequent in the DDDR arm (83.9% vs 73.3%), which, surprisingly, had a much higher percentage of paced ventricular beats.5 Results from this and similar studies, such as a sub-study of the Multicenter Automatic Defibrillator Implantation Trial II (MADIT II), suggest that patients with preexistent systolic dysfunction may be even more susceptible to the deleterious effects of RV pacing.5,6
When compared with an LBBB, RV pacing generates an even greater conduction delay, which, in heart failure patients, can be further worsened by underlying electrical abnormalities.7 The success of cardiac resynchronization therapy (CRT) in patients with heart failure and conduction delay led to clinical trials evaluating its utility in patients with standard indications for pacing. Studies have demonstrated improvements in left ventricular hemodynamics and mechanical function, as well as reverse remodeling in these patients.7,8 The recently published Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK-HF) trial enrolled patients with pacing indications for AV block, an ejection fraction < 50%, and New York Heart Association class I to III heart failure.9 A lower incidence of the primary outcome, the composite of death from any cause, urgent care visit for heart failure, or 15% increase in left ventricular end-systolic volume index, was seen over time in patients who received CRT, which appeared to be independent of the initial ejection fraction.9
RV pacing in patients with complete heart block, both intrinsic and postablation, is unavoidable; even with CRT therapy, ventricular conduction remains from myocyte to myocyte. In patients with pacemakers implanted for sinus node disease, options for decreasing ventricular pacing are less limited. Managed ventricular pacing algorithms have been developed to minimize ventricular pacing, particularly AV search hysteresis, and have yielded variable results in clinical trials.10 Additionally, alternative pacing sites, such as the RV outflow tract, ventricular septum, and His bundle, have been studied with inconclusive results.7
In our patient, the problem was easily addressed with pulse generator replacement. Most major brands of dual-chamber devices default to ventricular pacing to save battery life once they reach their elective replacement interval. After battery replacement and reinstitution of atrial pacing and intrinsic AV conduction, a more synchronous pattern of ventricular activation was observed. Interestingly, the decrease in the septal to posterior wall motion delay from 170 ms to 110 ms (Figure 2A, B) corresponded with the marked improvement in her hemodynamic parameters.
Selecting the appropriate device for pacing/defibrillation can be challenging, and multiple factors must be considered. Abnormalities of cardiac function, automaticity, and AV conduction, both at the time of implantation and those anticipated in the future, should affect this decision. With this in mind, it is still difficult to predict which patients will tolerate the different pacing modalities. In the case discussed, it is important to note that the patient had not seen a physician for more than 1 year for pacemaker evaluation. Ideally, with the appropriate follow-up, this acute change could have been recognized early and addressed appropriately before the patient presented with heart failure. Perhaps the most effective method to minimize complications associated with RV apical pacing, or any form of cardiac pacing for that matter, is regular evaluation by a physician who is thoroughly familiar with the various complexities of implanted cardiac pacemakers. ![]()
References
- Bank AJ, Gage RM, Burns KV. Right ventricular pacing, mechanical dyssynchrony, and heart failure. J Cardiovasc Transl Res. 2012;5:219-231.
- Tops LF, Martin JS, Bax JJ. The effects of right ventricular apical pacing on ventricular function and dyssynchrony, implications for therapy. J Am Coll Card. 2009;54:764-776.
- Gorcsan J 3rd, Abraham T, Agler DA, et al; American Society of Echocardiography Dyssynchrony Writing Group. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting—a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008;21:191-213.
- Sweeney MO, Hellkamp AS, Ellenbogen KA, et al; Mode Selection Trial Investigators. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;23:2932-2937.
- Wilkoff BL, Cook JR, Epstein AE, et al; Dual Chamber and VVI Implantable Defibrillator Trial Investigators. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA. 2002;288: 3115-3123.
- Steinberg JS, Fischer A, Wang P, et al; MADIT II Investigators. The clinical implications of cumulative right ventricular pacing in the Multicenter Automatic Defibrillator Implantation Trial II. J Cardiovasc Electrophysiol. 2005;16:359-365.
- De Sisti A, Marquez MF, Tonet J, et al. Adverse effects of long-term right ventricular apical pacing and identification of patients at risk of atrial fibrillation and heart failure. Pacing Clin Electrophysiol. 2012;35:1035-1043.
- Rahmouni HW, Kirkpatrick JN, St. John Sutton MG. Effects of cardiac resynchronization therapy on ventricular remodeling. Curr Heart Fail Rep. 2008;5:25-30.
- Curtis AB, Worley SJ, Adamson PB, et al; Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK HF) Trial Investigators. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368:1585-1593.
- Sweeney MO, Bank AJ, Nsah E, et al; Search AV Extension and Managed Ventricular Pacing for Promoting Atrioventricular Conduction (SAVE PACe) Trial. Minimizing ventricular pacing to reduce atrial fibrillation in sinus node disease. N Engl J Med. 2007;357:1000-1008.
- Dabrowska-Kugacka A, Lewicka-Nowak E, Tybura S, et al. Survival analysis in patients with preserved left ventricular function and standard indications for permanent cardiac pacing randomized to right ventricular apical or septal outflow tract pacing. Cric J. 2009;73:1812-1819.
- Kypta A, Steinwender C, Kammler J, et al. Long-term outcomes in patients with atrioventricular block undergoing septal ventricular lead implantation compared with standard apical pacing. Europace. 2008;10:574-579.