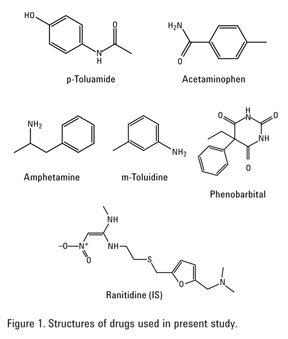
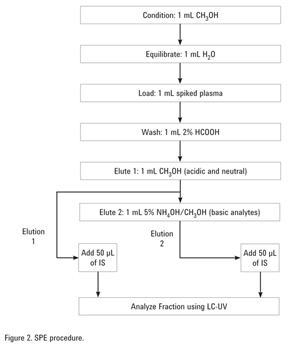
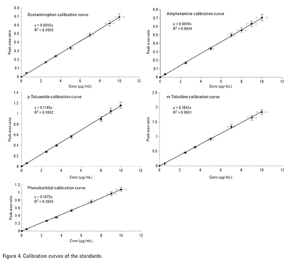
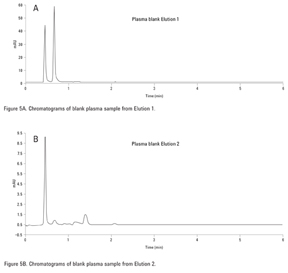
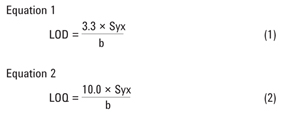Fractionation of Acidic, Basic, and Neutral Drugs from Plasma with an SPE Mixed Mode Strong Cation Exchange Polymeric Resin (Agilent SampliQ SCX)
Bellah O. Pule, Lesego C. Mmualefe,
and Nelson Torto
Department of Chemistry, Rhodes
South Africa
Application Note
Forensic Toxicology
Abstract
A method for the simultaneous extraction of drugs (amphetamine, acetaminophen, p-toluamide, m-toluidine, and phenobarbital) from spiked human plasma sample was developed. This procedure employed solid phase extraction with a mixed mode strong cation exchange resin, Agilent SampliQ SCX. The chromatographic separation and analysis of solid phase extraction extracts were achieved using 30% methanol and 70% potassium dihydrogen phosphate as a mobile phase under isocratic conditions on an Agilent ZORBAX Eclipse Plus C18
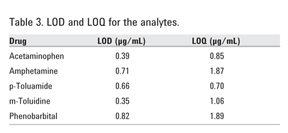
4.6 mm × 75 mm, 3.5 µm column at 1 mL/min flow rate and a diode array detector (DAD) set at 210 nm. High and reproducible recoveries (> 80%) for all the analytes were obtained. The limits of detection (LOD) and quantification (LOQ) were 0.39 and 0.71 µg/mL for acetaminophen, 0.84 and 1.87 µg/mL for amphetamine, 0.36 and 1.06 µg/mL for m-toluidine, 0.66 and 0.70 µg/mL for p-toluamide, as well as 0.80 and 1.89 µg/mL for phenobarbital, respectively.
Introduction
Sample preparation prior to chromatographic analysis presents a major challenge for the determination of drugs and their metabolites in complex matrices, such as biological fluids (for example, blood, plasma, urine, serum, saliva). Drugs normally exhibit a diverse polarity with acidic, basic, or neutral functionalities depending on the pH of the matrix. Liquid-liquid extraction (LLE) and solid phase extraction (SPE) have traditionally been employed for the extraction of drugs, their metabolites, and endogenous compounds from plasma [1-2]. Their quantification at a low concentration has proven to be a difficulty in pharmaceutical and forensic toxicology analyses. SPE is, however, the preferred method as it is not only employed for class fractionation, but it is also for trace enrichment and purification. Available commercial sorbents, such as chemically modified silica gel, polymer, and graphitized or porous carbon, are used [3]. These offer separations based on normal phase, reversed phase, ion exchange, and mixed mode ion exchange (combination of reversed phase and ion exchange) sorbents. The mixed mode sorbents have proven to give cleaner extracts and better separations than standard reversed phase or ion exchange sorbents as they take advantage of both the ion exchange and hydrophobic interactions [4,5]. For the extraction of compounds with a wide variety of polarity, polymeric sorbents have proven to be superior to other sorbents (for example, alkylated silica) and are, therefore, the choice of sorbent for this study [6–8].
In this application note, a method employing solid phase extraction was developed for the fractionation of acidic, basic, and neutral drugs from plasma with Agilent SampliQ SCX, a mixed mode strong cation exchange polymer. The resin is a sulfonic acid modified divinyl benzene polymer that exhibits both cation exchange and reversed phase behavior. In addition, it provides excellent reproducibility and enables a simple extraction protocol. Specific drugs (Figure 1) have been used as representatives of the three classes of drugs, for example, p-toluamide (acidic), amphetamine (basic), and acetaminophen (neutral).
Experimental
Chemicals
Acetaminophen, phenobarbital, p-toluamide, amphetamine, m-toluidine, and ranitidine (IS) were purchased from Sigma-Aldrich Chemicals (St. Louis, MO, USA). Phosphoric acid, formic acid, and potassium hydroxide were purchased from Merck Chemicals (Gauteng, South Africa) while the HPLC grade methanol was from Merck KGaA (Darmstadt, Germany), dipotassium hydrogen phosphate and potassium dihydrogen phosphate were purchased from Saarchem Analytical (Krugersdorp, South Africa).
The mobile phase was prepared with ultrapure water (18.2 M Ωcm) from a MilliQ system by Millipore (Milford, Mass, USA) and filtered through a Whatman membrane filter (47 mm diameter and 0.2 µm pore size). The stock solutions of the drugs and the internal standard were prepared in methanol (1,000 µg/mL) and kept at 4 °C while the working solutions were prepared daily by diluting the stock solutions, to appropriate concentrations, also in methanol. The plasma (ECZ HQ Donation 5497780, O+) was from SANBS (Port Elizabeth, South Africa).
Instrumental
The analysis was performed on an Agilent 1200 Series LC composed of a binary pump and a DAD set at λ = 210 nm. Separation of the compounds was achieved on an Agilent ZORBAX Eclipse Plus C18 column (4.6 mm × 75 mm, 3.5 µm, p/n 959933-902, Agilent Technologies, Santa Clara, CA). The data was processed by Agilent LC 2D ChemStation software. The SPE cartridges were Agilent SampliQ SCX, 1 mL/30 mg,
p/n 5982-3213, a polymeric strong cation exchanger with 25–35 µm average particle sizes. A Jenway 3510 pH meter (London, UK) was employed for pH adjustments.
Sample pretreatment: SPE procedure
The plasma sample (1 mL) was hydrolyzed with 1% formic acid (3 mL) for 30 minutes. The sample, spiked with drugs, was then loaded onto the SampliQ SCX cartridges as described in Figure 2. An internal standard, 50 µL of the ranitidine stock solution, was added to each SPE fraction. A blank plasma sample (1 mL) was carried through the procedure also.
Separation and analysis
Table 1 shows the reversed-phase chromatographic conditions. All drugs were separated within 5 minutes.
Results and Discussion
Separation
The standard mixture of the analytes was separated with the set chromatographic conditions (Table 1), and the chromatogram is reported in Figure 3.
Analysis of standard solutions
Calibration curves were processed at
0–10 µg/mL concentration ranges for all the analytes. They were linear with coefficient of regression (R2) greater than 0.999 as shown in Figure 4.
SPE procedure
A mixed mode strong cation exchange sorbent was used to extract basic drugs from the acidic and neutral drugs in plasma. The acidic and the neutral drugs, which exhibit a similar retention mechanism to that of the undissociated acidic compounds, were adsorbed in the hydrophobic portion of the sorbent and eluted in the neutral fraction while the basic drugs were retained by the cation exchange interactions with the sorbent and eluted in the ammoniated fraction.
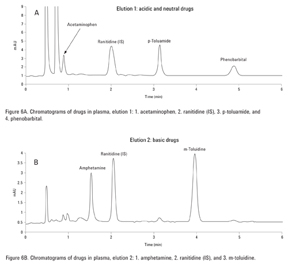
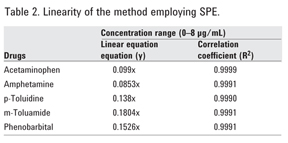
First, blank urine containing no drugs was treated using the SPE method. Figure 5A, for the acidic and neutral elution conditions (Elution 1), showed nothing eluting in the region of the acidic and neutral drugs in the standards. Figure 5B, which depicts a blank urine using the basic elution conditions (Elution 2), also showed nothing eluting in the region of the acidic drugs. For the spiked urine samples, the neutral and acidic drugs were eluted in the neutral fraction (Figure 6A, Elution 1) because they were retained through hydrophobic interactions while the basic drugs, retained by the strong anion exchange functionalities of the sorbent, eluted separately in the basic fraction as shown in Figure 6B, Elution 2. A small amount (< 10%) of the neutral/acidic drugs were also found in the basic fraction. A larger volume of methanol in the prior step could have been used to improve extraction efficiency.
Linearity, limits of detection, and limits of quantification
The blank plasma was spiked with the analytes at five different concentrations and subjected to the SPE procedure in Figure 2. The internal standard, 50 µL, was added. Each spiked plasma sample was prepared in triplicate. The analyte/IS peak area ratios were plotted against the corresponding concentrations. All the analytes were linear in the chosen concentration range (0–8 µg/mL) with R2 > 0.999 as shown in Table 2.
Equations 1 and 2 were used to calculate LOD and LOQ, where Syx is the standard error of the regression line and b is the gradient.
Table 3 shows the LOD and LOQ determined for each analyte.
Recovery and reproducibility studies
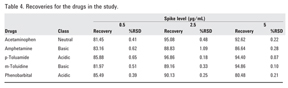
Spiked plasma samples at three concentration levels: 0.5, 2.5, and 5 µg/mL, corresponding to the lower, middle, and upper limit of the linearity curve, were subjected to SPE cleanup. The analyte chromatographic peak areas obtained were compared to those obtained from standard solutions at the same concentration, and the percentage extraction yield was calculated. To demonstrate reproducibility, the samples were analyzed at the three mentioned concentration levels (n = 6). Good recoveries were obtained as indicated in Table 4.
Conclusions
The SPE procedure was successfully carried out on Agilent SampliQ SCX sorbents for the simultaneous extraction of acidic, basic, and neutral drugs from plasma with high and reproducible recoveries
(> 80%). The LOD ranged from 0.39 to
0.84 µg/mL for the drugs studied. The LOQ ranged from 0.71 to 1.89 µg/mL. This method can be applied to compounds that exhibit a diverse polarity and acidic, basic, or neutral functionalities.
References
- A. Zwir-Ference, M. Bizziuk, J. Env 15 (2006) 677-690.
- K. B. Borges, Talanta 78 (2009) 233-241.
- K. A. Shaikh, S. D. Patil, A. B. Devkhile, J. Pharm. Biomed. Anal 48 (2008) 1481-1484.
- M. S. Landis J. Pharm. Biomed. Anal. 44 (2007) 1029‑1039.
- L. Mercolini, G. Gerra, M Consorti, L. Somaini, M. A. Raggi, Talanta 78 (2009) 150-155.
- M. A. Allabdalla, J. Clinical Forensic Medicine. 12 (2005) 310-315.
- N. H Yu, E. N. M Ho, F. P. W Tang, T. S. M Wan, A. S. Y. Wong, J. Chromatogr. A 1189 (2008) 426-434.
- S. Weigel, R Kallenborn, H. Huhnerfuss J. Chromatogr. A 1023.
For More Information
For more information on our products and services, visit our Web site at www.agilent.com/chem.
©Agilent Technologies, Inc., 2013
January 11, 2013
View this Application Note in its entirety: 5990-5001EN