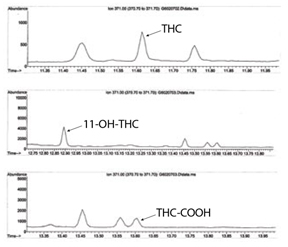
Solid phase extraction of THC, THC-COOH and 11-OH-THC from whole blood
Robert M. Sears, DFTCB
Forensic Toxicologist
South Carolina Law Enforcement Division
Application Note
Forensics & Toxicology
Introduction
Marijuana, one of the most widely abused drugs, after alcohol, is derived from Cannabis sativa. There are more than 400 chemicals in the cannabis plant. The Δ9-tetrahydrocannabinol (THC) is the most psychoactive of the various forms of THC. Marijuana is most often used in cigarette form, the user inhaling the marijuana smoke. THC and other forms of cannabinoids are lipid soluble and can enter body tissues rapidly. THC is rapidly metabolized to
11-hydroxy-Δ9-tetrahydocannabinol (11-0H-THC), which is then converted to 11-nor-Δ9 -THC-9-carboxylic acid (THC-COOH).
Detection of THC metabolites in urine, primarily THC-COOH, can indicate prior THC exposure but provides no indication of impairment. Testing for THC and its metabolites in blood can give a better indication of recent drug usage and can be of merit when testing for impairment.
This application note shows an effective SPE method for the extraction of THC and key metabolites from human blood and the GC/MS analysis of these compounds.
Instrumentation
GC with single quad mass spectrometer
Materials and Reagents
High–flow Agilent Bond Elut Certify II SPE cartridge, 200 mg (p/n 14113051). Bond Elut Certify II is a mix of C8 and a quanternary amine, a strong anion-exchange bonded silica. The two functionalities are effective in retaining the polar and nonpolar functionalities of the THC compounds
The column was a 5% phenyl substituted, low bleed GC/MS column, 30 m x 0.25 mm, 0.25µm.
Standards were d3-THC, d3-11-0H-THC and d9-carboxy-THC from Cerilliant.
Sample Preparation
Pipette 2 mL blood into a clean tube with ISTD equivalent to 10 - 11g/L (ng/mL).
Add 4 mL cold acetonitrile drop-wise while vortexing.
Centrifuge sample 5 min minimum at
2500 rpm.
Transfer supernatant to a clean
labeled tube.
Evaporate sample to about 3 mL with nitrogen at 35 - 40 °C.
Add 7 mL 0.1 M sodium acetate buffer,
pH 6.0 to each sample.
SPE Method
Conditioning
Condition Certify II cartridge with 2 mL MeOH. (All steps, except where noted, use low vacuum of approximately 2 - 5 in Hg).
Condition cartridge next with 2 mL 0.1 M sodium acetate buffer, pH 6.0 with
5% MeOH.
Cartridges should not be allowed to dry prior to sample addition.
Pour sample into column reservoir and
draw sample through the column slowly,
1-2 mL/min.
Washes
2 mL sodium acetate buffer, pH 6.0.
Dry column under maximum vacuum for approximately 5 minutes.
Wash with 1 mL hexane.
Elution
Elute THC with 2 mL 95:5 hexane:ethyl acetate.
Wash column with 5 mL 1:1 MeOH:DI water.
Dry column under maximum vacuum for approximately 5 minutes.
Wash with 1 mL hexane.
Elute (in a separate tube) THC-COOH and 11-O-THC with 2 mL 1% acetic acid in 75:25 hexane:ethyl acetate.
For best results, do not combine fractions. Run as two samples. Evaporate elution fractions under nitrogen no higher than
40 °C.
Derivatization
Add 500 µL elution solvent to sample, vortex and transfer to a clean, high recovery GC vial. Evaporate to dryness with nitrogen no higher than 40 °C.
Add 35 µL BSTFA with 1% TMCS and 35 µL ethyl acetate. Overlay samples with nitrogen, cap and heat 20 minutes at 70 °C.
Conditions
Inlet temperature:
250 °C
Mode:
Pulsed pressure injection
Injection volume:
2 µL
Initial oven temperature:
20 °C hold 1 min
5 °C/min to 300 °C old 0
0 °C/min to 310 °C hold 5.57 min
Target Ions
d3-THC
374, 389, 346 (dwell time 50 ms)
THC
371, 386, 343
Linear range
1 - 50µg/L
d3-11-0H-THC
374, 462, 477
11-0H-THC
371, 459, 474
Linear Range
1 - 50 µg/L
d9-THC-COOH
380, 479, 497
THC-COOH
371, 473, 488
Linear Range
1 - 100 µg/L
Conclusion
The figures show the effective use of mixed-mode SPE with GC/MS detection for the extraction and quantification of THC and key metabolites from whole blood at low levels.
©Agilent Technologies, Inc., 2011
October 31, 2011