By Jeffrey A. Jansen
Senior Managing Engineer & Partner, The Madison Group
By Jeffrey A. Jansen
Senior Managing Engineer & Partner, The Madison Group
By Jeffrey A. Jansen
Senior Managing Engineer & Partner, The Madison Group
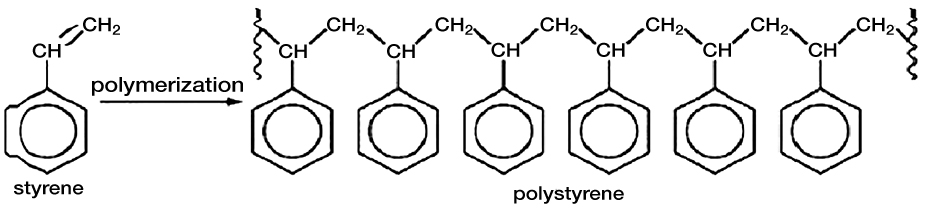
Figure 1. Addition reaction mechanism showing styrene monomer polymerizing into polystyrene.

Figure 1. Addition reaction mechanism showing styrene monomer polymerizing into polystyrene.

Figure 1. Addition reaction mechanism showing styrene monomer polymerizing into polystyrene.

Figure 2. Condensation reaction mechanism showing the polymerization of a polyamide from a diacid and a diamine.

Figure 2. Condensation reaction mechanism showing the polymerization of a polyamide from a diacid and a diamine.

Figure 2. Condensation reaction mechanism showing the polymerization of a polyamide from a diacid and a diamine.

Figure 3. Polymers contain a wide variety of functional groups, responsible for the diversity in physical properties.

Figure 4. Polymer chains consist of a high number of repeating units, and are entangled to form a spaghetti-like structure.

Figure 5. The repeating unit of polyethylene consists of two carbon atoms with pendant hydrogen atoms.

Figure 6. Structural representation of semicrystalline and amorphous polymers.

Figure 7. DSC thermogram six showing a melting endotherm for a semicrystalline polymer and a glass transition for an amorphous material.

Figure 8. Graphical representation of the changes in modulus characteristic of semicrystalline and amorphous polymers.
Semicrystalline
- Distinct and sharp melting point
- Opaque or translucent
- Better organic chemical resistance
- Higher tensile strength and modulus
- Better creep and fatigue resistance
- Higher density
- Higher mold shrinkage
Amorphous
- Soften over a wider range of temperature
- Transparent
- Lower organic chemical resistance
- Higher ductility
- Better toughness
- Lower density
Table 1.
Semicrystalline
- Distinct and sharp melting point
- Opaque or translucent
- Better organic chemical resistance
- Higher tensile strength and modulus
- Better creep and fatigue resistance
- Higher density
- Higher mold shrinkage
Amorphous
- Soften over a wider range of temperature
- Transparent
- Lower organic chemical resistance
- Higher ductility
- Better toughness
- Lower density
Table 1.
The characteristic properties exhibited by plastics are the direct result of the unique molecular structure of these materials. Taking that a step further, the variation within the properties demonstrated by different plastics arises from diversity in their structure. Plastics are polymers of very high molecular mass. To enhance their properties, they often contain additives, such as fillers and reinforcements, anti-degradants and stabilizers, flame retardants and plasticizers. However, the underlying attributes of a plastic material are determined by the polymer.
Polymerization
Polymers are macromolecules that are based on a structure built up, chiefly or completely, from a large number of similar structural units bonded together. Often called chains, the polymer consists of repeating units, similar to links. Polymers are formed through a process known as polymerization, in which monomer molecules are bonded together through a chemical reaction that results in a three-dimensional network of long individual polymer chains consisting of smaller repeated units.
There are two basic types of polymerization reactions — addition and condensation. Addition polymerization is the formation of polymers from monomers containing a carbon-carbon double bond through an exothermic addition reaction. Significantly, this reaction proceeds without the loss of any atoms or molecules from the reacting monomers. Common materials produced through addition polymerization include polyethylene, polypropylene, poly(vinyl chloride), and polystyrene as represented in Figure 1.
In contrast, condensation polymers are formed by a stepwise reaction of molecules with different functional groups. The reaction is endothermic and produces water, or other small molecules such as methanol, as a byproduct. Common polymers produced through condensation reactions include thermoplastic polyesters, polyacetal, polycarbonate and polyamides as represented in Figure 2.
Addition polymers form high-molecular-weight chains rapidly, and tend to be higher in molecular weight than condensation polymers. Comparing polymers produced via the two different mechanisms, addition polymers are generally chemically inert due to the relatively strong carbon-carbon bonds that are formed. Condensation polymers tend to be susceptible to hydrolytic molecular degradation through exposure to water at elevated temperatures, through a mechanism that resembles the reversion of the initial liberalization reaction.
By using different starting materials and polymerization processes and techniques, polymers having different molecular structures can be produced (see Fig. 3).
The fundamental differences between the properties of these different types of polymers are attributable to the varying functional groups within the molecular structure. These differences include mechanical, thermal and chemical resistance properties. As such, it is important to select the correct type of plastic based upon the requirements of the application.
Intermolecular Bonding
As indicated, polymerization results in the formation of multiple individual polymer chains made up of repeating units. A key aspect of polymeric materials is that the chains are entangled within each other. The individual chains are not covalently bonded to each other, but instead rely on intermolecular forces, such as Van der Waals forces, hydrogen bonding, and dipole interactions, to keep the chains from disentangling. This results in a structure that is similar to a bowl of spaghetti noodles (Fig. 4).
Molecular Weight
Through the polymerization process, materials of relatively high molecular weight, macromolecules, are produced. A key parameter of a polymer is its molecular weight. Molecular weight is the sum of the atomic weights of the atoms comprising a molecule. For example, the molecular weight of polyethylene is calculated by multiplying the molecular weight of the repeating ethylene functional group times the number of units comprising the chain. Thus, for polyethylene (Fig. 5), where the repeating unit contains two carbon atoms and four hydrogen atoms, the molecular weight is 28n, where n represents the number of repeating segments. Most commercial polymers have an average molecular weight between 10,000 and 500,000.
Higher molecular weights are associated with longer molecular chains, and this results in a greater level of entanglement. This has important implications, as higher-molecular-weight grades of plastics will have superior mechanical, thermal and chemical resistance properties compared with lower-molecular-weight grades of the same material.
It is important to remember that the polymerization process is a chemical reaction, and while carefully controlled, there is some inherent variation. This results in polydispersity, or polymer chains of unequal length. Because of this, commercial plastics have polymers with a molecular weight distribution. Simply put, molecular weight distribution represents the relative amounts of polymers of different molecular weights to comprise a given specimen of that material. Unlike molecular weight, the relationship between molecular weight distribution and end properties is not uniform. For example, in comparing two similar materials with different molecular weight distributions, in general the material with a wider distribution will exhibit better ductility and impact resistance, but will demonstrate reduced strength and stiffness.
Because of the structure of the molecules, polymeric materials have different properties compared to other materials, like metals. Specifically, the relatively high molecular weight and long polymer chain length result in entanglement, and the lack of covalent intermolecular bonds facilitates polymer chain mobility. This combination of entangled mobile chains results in viscoelasticity.
Viscoelasticity is the property of materials that exhibit both viscous and elastic characteristics when undergoing deformation. Viscous materials, like honey, resist shear flow and strain linearly with time when a stress is applied. Elastic materials, such as a steel rod, strain when stressed and quickly return to their original state once the stress is removed. Viscoelastic materials have elements of both of these properties and, as such, exhibit time-dependent strain.
There are three main factors that will affect the viscoelasticity of a plastic part — temperature, strain rate, and time. Because of this, plastics are temperature, strain rate and time sensitive. Temperature is the most obvious of these factors. Polymers exhibit a comparatively high level of change in physical properties over a relatively small temperature range. As the temperature is increased, the polymer chains are positioned further apart. This results in greater free volume and kinetic energy, and the chains can slide past one another and disentangle more easily.
As strain rate — the speed at which load is applied — is increased, the polymer chains do not have enough time to undergo ductile yielding, and they will disentangle through an increasingly brittle mechanism. This is why plastics are much more susceptible to impact failures than they are overload failures, which occur at more moderate strain rates.
The inherent viscoelastic nature of polymeric materials produces movement within the polymer chains under conditions of applied stress. This results in time dependency within polymeric materials. Because of this molecular mobility, plastic materials will exhibit differences in their long-term and short-term properties due to the application of stress over time. This means that the properties of a plastic material, such as strength and ductility, are not static, but will decrease over time. This often leads to creep and stress relaxation within plastic materials.
Crystalline/Amorphous Structure
Another fundamental characteristic of polymeric materials is the organization of their molecular structure. Broadly, plastics can be categorized as being semicrystalline or amorphous. Understanding the implications of the structure, and specifically, the crystallinity, is important as it affects material selection, part design, processing and the ultimate anticipated service properties.
Most non-polymeric materials form crystals when they are cooled from elevated temperatures to the point of solidification. This is well demonstrated with water. As water is cooled, crystals begin to form at 0°C as it transitions from liquid to solid. Crystals represent the regular, ordered arrangements of molecules, and produce a distinctive geometric pattern within the material. With small molecules, such as water, this order repeats itself and consumes a relatively large area relative to the size of the molecules, and the crystals organize over a relatively short time period.
However, because of the rather large size of polymer molecules and the corresponding elevated viscosity, crystallization is inherently limited, and in some cases, not possible. Polymers in which crystallization does occur still contain a relatively high proportion of non-crystallized structure. For this reason, those polymers are commonly referred to as semicrystalline. Polymers, which because of their structure, cannot crystallize substantially are designated as amorphous (Fig. 6).
Amorphous polymers have an unorganized, loose structure. Semicrystalline polymers have locations of regular patterned structure bounded by unorganized amorphous regions. While some modification can be made through the use of additives, the extent to which polymers are semicrystalline or amorphous is determined by their chemical structure, including polymer chain length and functional groups.
The ordered arrangement of the molecular structure associated with crystallinity results in melting when a sufficient temperature is reached. Because of this, semicrystalline polymers such as polyethylene, polyacetal and nylon will undergo a distinct melting transition, and have a melting point (Tm). Amorphous polymers, including polystyrene, polycarbonate and poly(phenyl sulfone), will not truly melt, but will soften as they are heated above their glass transition temperature (Tg). This is represented by the differential scanning calorimetry (DSC) thermograms (Fig. 7).
The difference between semicrystalline and amorphous molecular arrangement also has an implication on the mechanical properties of the material, particularly as they relate to temperature dependency. In general, amorphous plastics exhibit a relatively consistent modulus over a temperature range. However, as the temperature approaches the glass transition temperature of the material, a sharp decline occurs. In contrast, semicrystalline plastics exhibit modulus stability below the glass transition temperature, which is often subambient, but show a steady decline between the glass transition temperature and the melting point (Fig. 8).
Due to their viscoelastic nature, time and temperature act in the same way on polymeric materials. Because of this, the changes within the material as a function of time can be inferred from the stability of the material versus temperature.
Aside from the time and temperature dependence, other key properties of polymeric materials are determined by their semicrystalline/amorphous structure. Some generalizations of characteristic properties are listed in Table 1.
Plastics continue to be used in increasingly diverse and demanding applications. Given the cost of product failure, it is very important that the right material be chosen specifically for each situation. Because the base polymer determines many of the critical performance characteristics of the plastic resin, it is essential that the correlation between molecular structure and performance be understood. The difference between success and failure can hinge on the implications of molecular weight, molecular weight distribution, and crystalline/amorphous structure.
ABOUT THE AUTHOR
 Jeffrey A. Jansen is senior managing engineer and a partner with The Madison Group, a Madison, Wis.-based provider of consulting services to the plastics industry. He is an expert in failure analysis; material analysis, identification and selection; and aging studies for plastic and rubber components. A senior member of SPE, Jansen also is a past chairman of SPE’s Failure Analysis & Prevention Special Interest Group.
Jeffrey A. Jansen is senior managing engineer and a partner with The Madison Group, a Madison, Wis.-based provider of consulting services to the plastics industry. He is an expert in failure analysis; material analysis, identification and selection; and aging studies for plastic and rubber components. A senior member of SPE, Jansen also is a past chairman of SPE’s Failure Analysis & Prevention Special Interest Group.


