The Effects of Type and Loading of Radiopaque Fillers on the Properties of Polyether Block Amide Compounds
By Breanna G. Boyden, Amar Nilajkar, and Charles J. O’Neil Foster Corporation, Putnam, Connecticut, USA

Catheter tubing extruded from a pigmented, radiopaque Pebax® compound (photo courtesy of Foster Corp.).

Catheter tubing extruded from a pigmented, radiopaque Pebax® compound (photo courtesy of Foster Corp.).
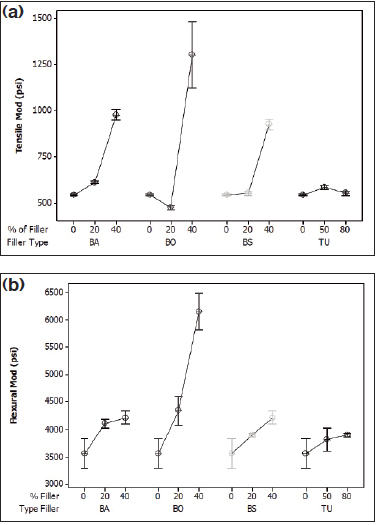
Figure 1: (a) Tensile modulus of Pebax 2533 compounds and (b) flexural modulus of Pebax 2533 compounds.
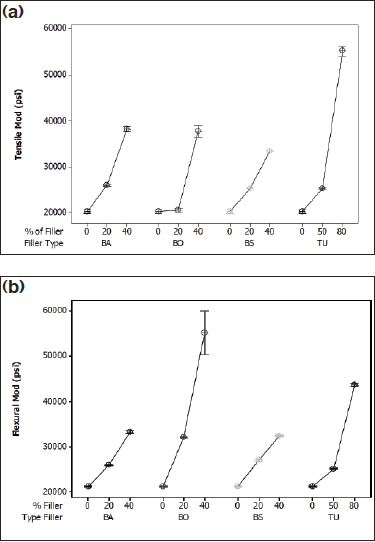
Figure 2: (a) Tensile modulus of Pebax 5533 and (b) flexural modulus of Pebax 5533 compounds.
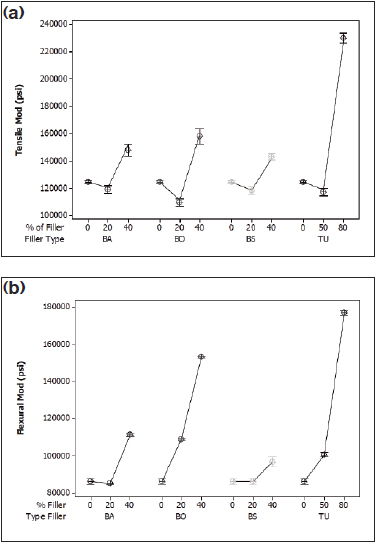
Figure 3: (a) Tensile modulus of Pebax 7233 and (b) flexural modulus of Pebax 7233 compounds.
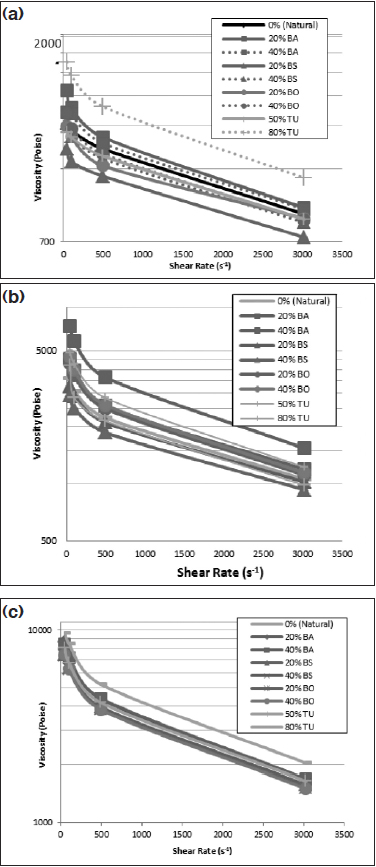
Figure 4: (a) Viscosity results of Pebax 2533, (b) viscosity results of Pebax 5533, and (c) viscosity results of Pebax 7233.
Radiopaque fillers are typically dense metal or ceramic powders that affect the energy attenuation of photons in an x-ray beam as it passes through matter, reducing the intensity of the photons by absorbing or deflecting them. The radiopaque compound will attenuate x-ray radiation energy differently than soft tissues and bones.1 This will then provide the visual contrast yielding an image of the device in the body. A clear picture facilitates accurate placement of the medical device inside the body. The manipulation of the device is monitored using fluoroscopy or x-ray imaging.2
The use of polyether block polyamide polymers has grown for components used in minimally invasive vascular surgical techniques, where the material has become the dominant polymer of choice.3 The selection of the radiopaque filler used will depend on where in the body the material is being used and the energy level of the fluoroscope or x-ray equipment being used.4
Vascular catheters used in minimally invasive surgery face a number of design challenges. The proximal end must be rigid enough to allow for “pushability” and maneuvering or steering through the vascular system to reach the site to be repaired.5 The distal end must be flexible and soft in order to avoid any trauma to the blood vessels themselves. Polyether block polyamides are unique in that they offer a wide range of durometers and flexibilities to ensure that catheters can be designed with progressive stiffness along the shaft length. They are also conformable enough to be used at the distal end and not cause trauma to the blood vessels.
Materials
The polyether block polyamide (PEBA) copolymer resins used for this study were Pebax® 7233 SA01 MED, Pebax 5533 SA01 MED, and Pebax 2533 SA01 MED. These grades of Pebax are biomedical grades manufactured by Arkema Inc. Pebax is widely used in the manufacture of vascular catheters because of its wide range of hardness and flexibility and low loss hysteresis properties.
The dense metal fillers typically used in medical device compounds are based on barium, bismuth, and tungsten. The radiopaque fillers chosen for this experiment were barium sulfate, bismuth subcarbonate, bismuth oxychloride, and tungsten. These four radiopaque fillers, along with bismuth trioxide, dominate the catheter medical device market. The bismuth trioxide has some limitations due to its yellow color. Other metals that would act as good radiopaque fillers are tantalum, platinum, gold, and lead. The first three of these other radiopaque fillers are too expensive, and the last is obviously too toxic.
Pebax is based on nylon 12. Nylons are known for fair-to-poor heat and light stability. Therefore, we used a heat and light stabilizer package that we typically use for medical compounds made from nylon. The type of heat and light stabilizer, as well as the loading, was the same for all compound evaluations. The grades of Pebax used were selected to cover the range of durometer and stiffness of the product line. We chose Pebax 2533 SA01 MED, Pebax 5533 SA01 MED, and Pebax 7233 SA01 MED.
Experimental
Compounding
Compounding was performed on a co-rotating twin-screw extruder. The filler loadings chosen for the barium sulfate, bismuth subcarbonate, and bismuth oxychloride were 20% and 40%. The filler loadings for the tungsten were 50% and 80%. The loading levels chosen represent the typical levels used to manufacture various sections and types of vascular catheters. All formulations processed well under the chosen processing parameters.
Rheology
Rheological analysis (ASTM D-3835) of the various blends was performed on a Dynisco Dual Capillary Rheometer model no. LCR 7000. This rheometer system operates with a single barrel and piston assembly.
Injection molding
Tensile and flexural specimens for all blends were manufactured using an Arburg Allrounder 221M injection molding machine. It was fitted with the appropriate tensile and flexural specimen mold for subsequent ASTM testing.
Mechanical analysis
The tensile properties of the blends were determined according to ASTM D638 and performed on a MTS QTest/25. The flexural properties were determined according to ASTM D790 and performed on a MTS QTest/25.
Discussion
Mechanical properties
Figures 1, 2, and 3 show the effects of the range of fillers on the tensile and flexural modulus of the Pebax compounds. Barium sulfate (BA) and bismuth subcarbonate (BS) perform nearly identically due to similar particle size and shape and chemical similarities. Both fillers increase the tensile modulus by nearly two times in the 2533 and 5533 SA01 MED, and by 30% in the hardest grade, 7233 SA01 MED. The flexural modulus increased most dramatically with the middle grade, with a 50% increase from the raw resin, although all grades showed a marked increase.
Bismuth oxychloride (BO) provided the most dramatic increase for the softest grade of resin, with an increase in tensile and flexural properties of 140% and 73%, respectively. Bismuth oxychloride compounds also showed the most variation of the compounds tested. This could be attributed to poor bonding at the filler/polymer interface, causing the polymer chains to “slip” past each other. This phenomenon can cause inconsistencies in molded samples for mechanical testing.
The data indicate that tungsten reinforces the harder nylon segment of Pebax. There was no statistical change in the tensile or flexural modulus for the 2533, but with the increase of nylon segments in 5533 and 7533, the mechanical properties improved dramatically. In the middle grade, the tensile and flexural modulus increased 173% and 107%, respectively. The hardest grade of resin had a tensile modulus that increased by 85%, with a 110% increase in flexural modulus.
Viscosity
Figure 4 shows the viscosity results of all the filled compounds. In all grades, BA slightly increases the viscosity, indicating there was enough reinforcement of the polymer to reinforce the compound from the effect of shear forces. Bismuth oxychloride-filled compounds showed no change in viscosity for all three grades of resin.
Conclusions
Results indicate that the chosen radiopaque fillers have a positive impact on the physical properties of Pebax. The ratio of soft to hard segments in Pebax determines the extent of impact on the physical properties, like tensile and flexural modulus. No processing issues were seen for all formulations. The fillers chosen vary widely in cost. Their intensity level under x-rays is different as well. Keeping the above in mind, the ultimate choice of radiopaque filler for a particular application will depend on the economics and the intensity of radiopacity needed.
Future work will focus on looking at various particle sizes for each filler and their effects on physical properties, melt viscosity, cost, and radiopacity.
References
- T. Shah, MDDI Medical Device and Diagnostic Industry News Products and Suppliers (2000).
- X. Guo, Journal of Applied Polymer Science, 4015-4024 (2008).
- C. O’Neil, Medical Device and Diagnostic Industry Magazine, 33-39 (2010).
- L. Acquarulo, Medical Plastics and Biomaterials (2006).
- L. Acquarulo and C. O’Neil, ANTEC Technical Papers (1999).