Degradation of Microcellular PLGA-PEG Copolymer for Use in a Drug Delivery System for the Urinary Bladder
Previous Article Next Article
By Christian Hopmann1, Daniel Kaltbeitzel1, Theresa Kauth1, Barbara Dittrich2, Joachim Grosse3, Nadine Huppertz3, Ulrich Schwantes4, Claudia Neumeister4, and Matthias von Walter5
1Institute of Plastics Processing (IKV), RWTH Aachen University, Aachen, Germany
2DWI at RWTH Aachen, Aachen, Germany,
3Department of Urology, University Hospital Aachen, Aachen, Germany
4Dr. Pfleger GmbH, Bamberg, Germany
5Hemoteq AG, Würselen, Germany
Degradation of Microcellular PLGA-PEG Copolymer for Use in a Drug Delivery System for the Urinary Bladder
Previous Article Next Article
By Christian Hopmann1, Daniel Kaltbeitzel1, Theresa Kauth1, Barbara Dittrich2, Joachim Grosse3, Nadine Huppertz3, Ulrich Schwantes4, Claudia Neumeister4, and Matthias von Walter5
1Institute of Plastics Processing (IKV), RWTH Aachen University, Aachen, Germany
2DWI at RWTH Aachen, Aachen, Germany,
3Department of Urology, University Hospital Aachen, Aachen, Germany
4Dr. Pfleger GmbH, Bamberg, Germany
5Hemoteq AG, Würselen, Germany
Degradation of Microcellular PLGA-PEG Copolymer for Use in a Drug Delivery System for the Urinary Bladder
Previous Article Next Article
By Christian Hopmann1, Daniel Kaltbeitzel1, Theresa Kauth1, Barbara Dittrich2, Joachim Grosse3,
Nadine Huppertz3, Ulrich Schwantes4, Claudia Neumeister4, and Matthias von Walter5
1Institute of Plastics Processing (IKV), RWTH Aachen University, Aachen, Germany
2DWI at RWTH Aachen, Aachen, Germany,
3Department of Urology, University Hospital Aachen, Aachen, Germany
4Dr. Pfleger GmbH, Bamberg, Germany
5Hemoteq AG, Würselen, Germany
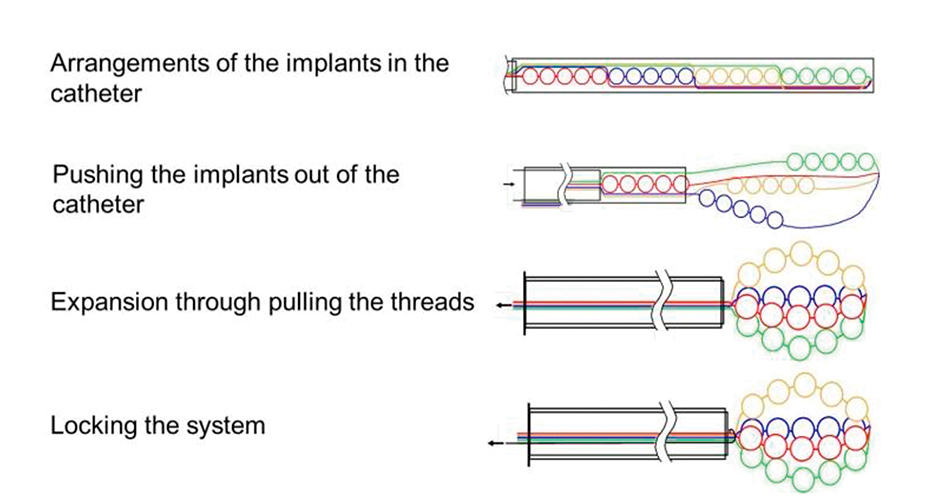
Figure 1: Functional principle of the drug delivery system composed of multiple implants which are connected flexibly with each other by means of an absorbable suture.
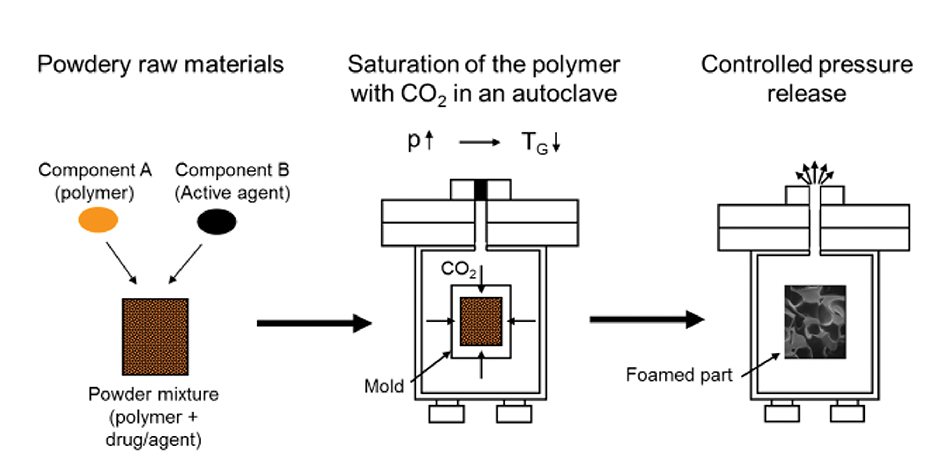
Figure 2: Schematic diagram of the CESP process.
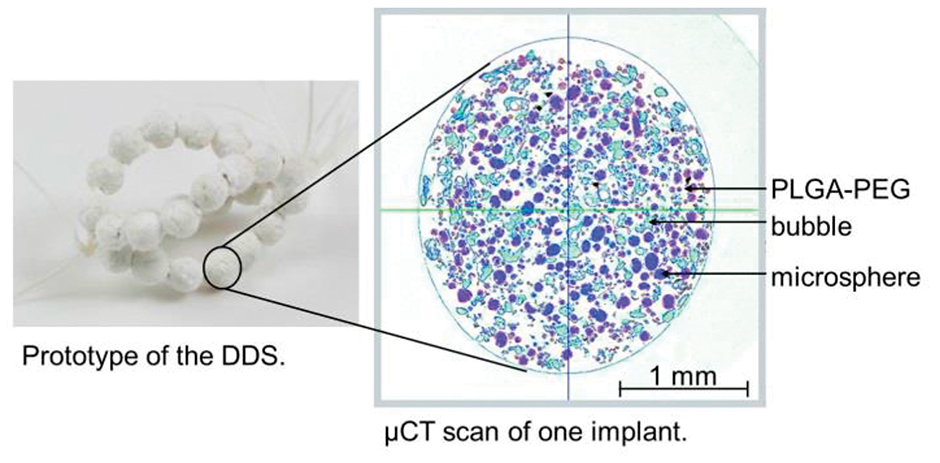
Figure 3: Prototype of the DDS and μCT scan of one implant that consists of non-absorbable, drug-carrying microspheres which are embedded in an absorbable matrix.
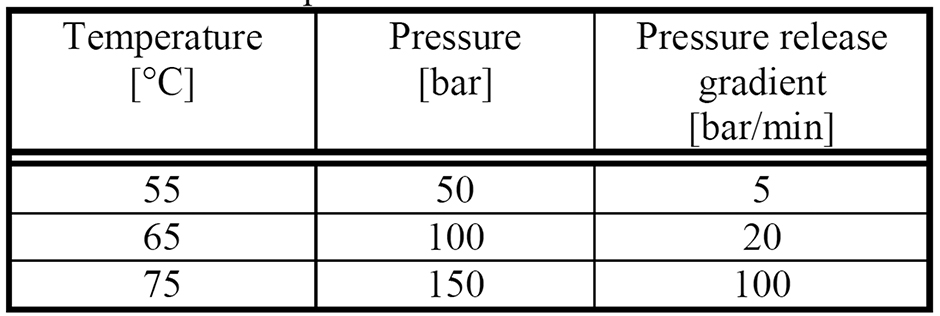
Table 1: Processing Parameters for Resomer RGP d 5055 Mixed with Microspheres

Table 1: Processing Parameters for Resomer RGP d 5055 Mixed with Microspheres
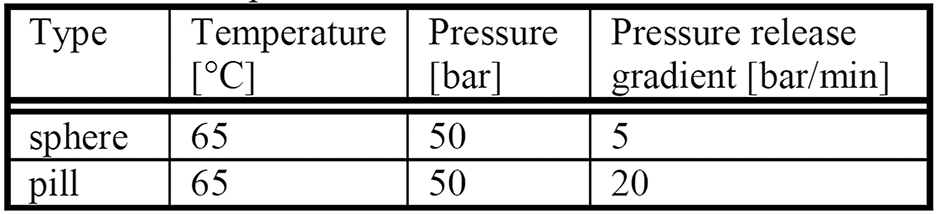
Table 2: CESP Parameter Sets of Total Dissolution

Table 2: CESP Parameter Sets of Total Dissolution
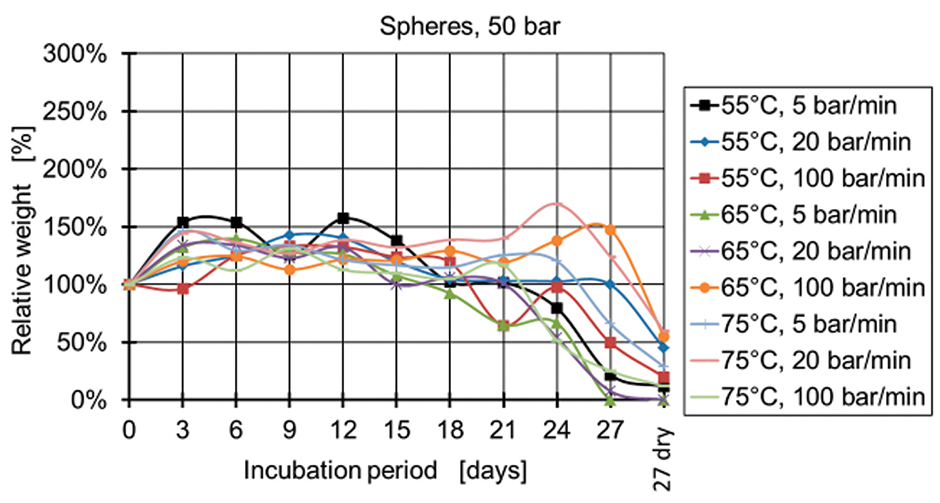
Figure 4: Relative weight of spheres manufactured at 50 bar.
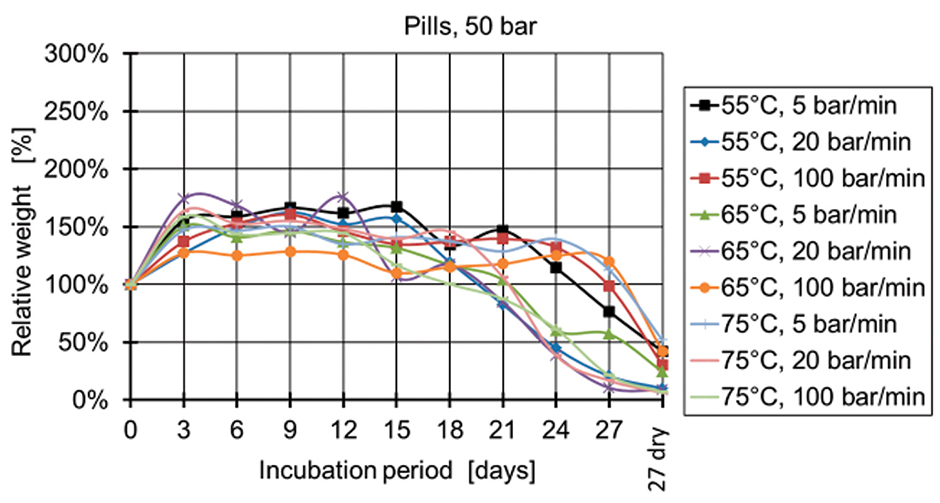
Figure 5: Relative weight of pills manufactured at 50 bar.
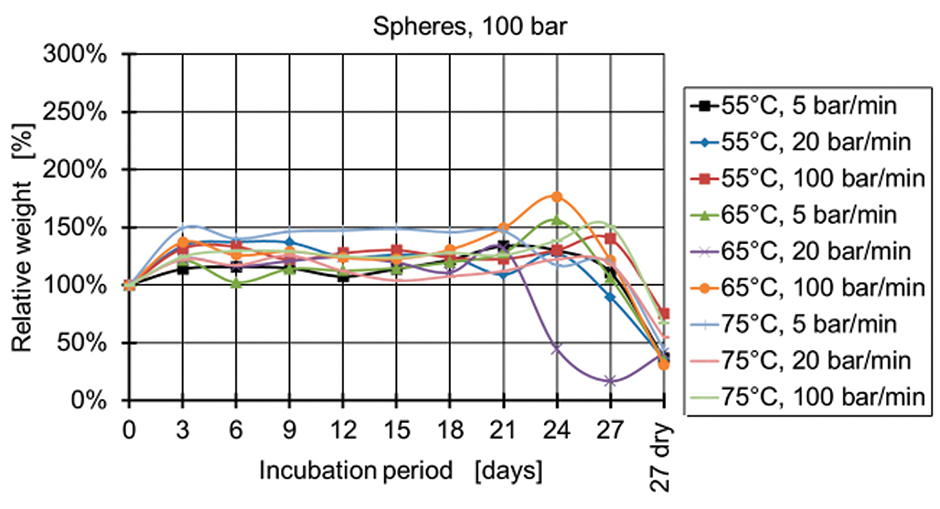
Figure 6: Relative weight of spheres manufactured at 100 bar.
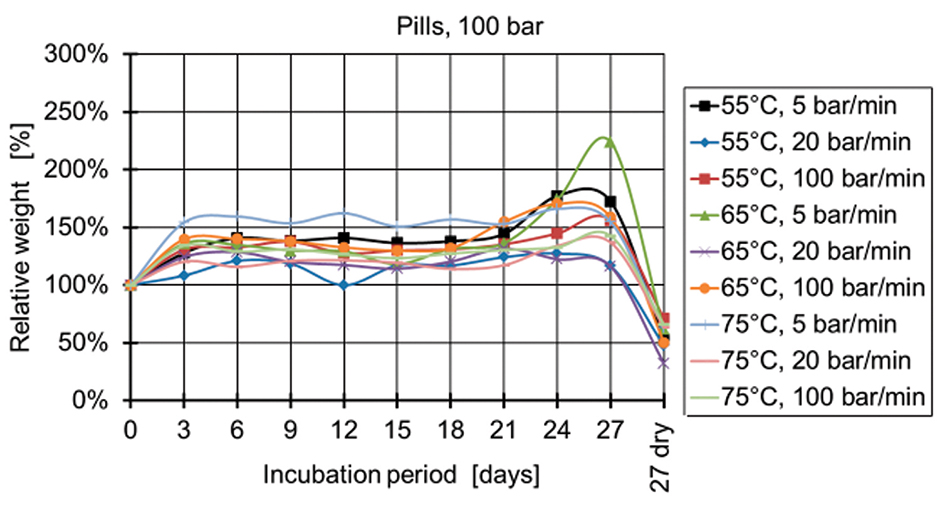
Figure 7: Relative weight of pills manufactured at 100 bar.
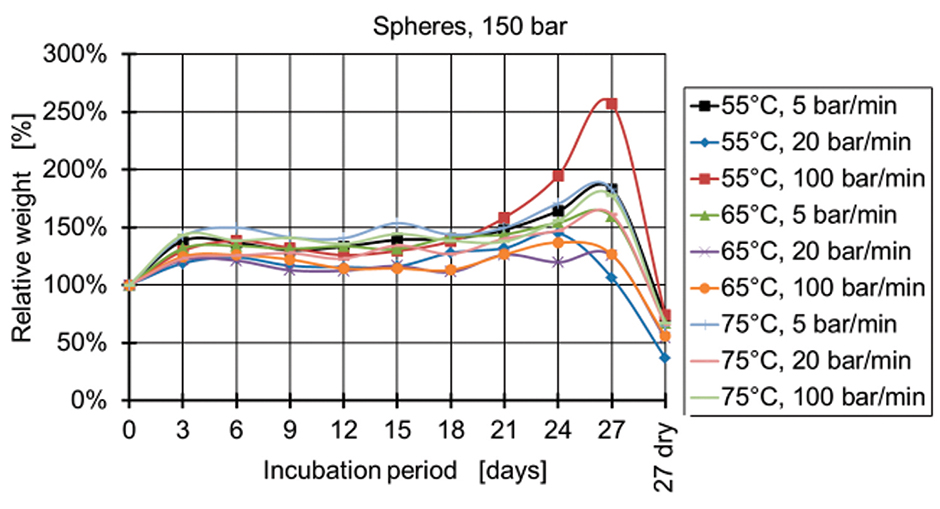
Figure 8: Relative weight of spheres manufactured at 150 bar.
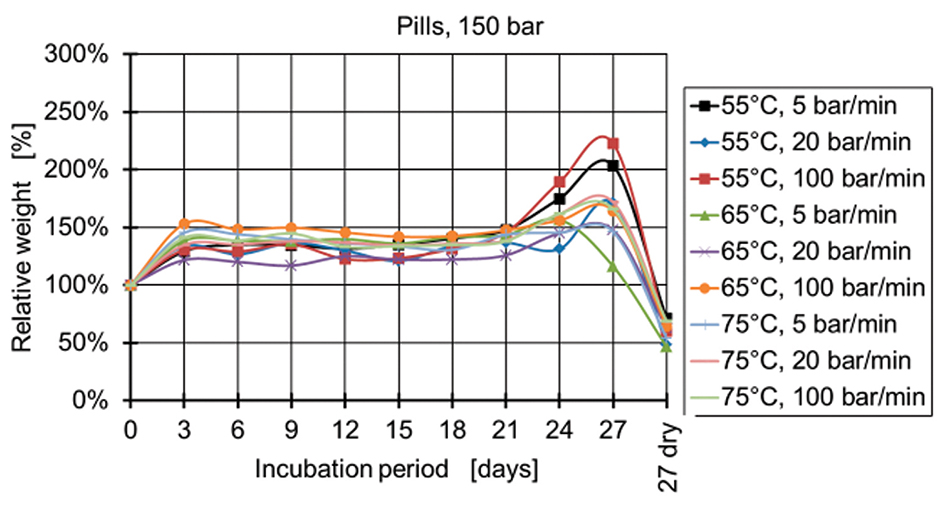
Figure 9: Relative weight of pills manufactured at 150 bar.
Note: This paper was singled out as “Best Paper” by the awards committee of the SPE Medical Plastics Division earlier this year at ANTEC® Orlando. To access other ANTEC papers, contact SPE customer service at U.S. 203-775-0471.
For the treatment of diseases of the bladder, a drug delivery system (DDS) has been developed which can be applied intravesically. The DDS is composed of multiple carriers that consist of non-absorbable, drug-carrying microspheres which are embedded in a foamed absorbable matrix. After degradation of the absorbable matrix, the non-absorbable microspheres are eliminated through the urethra.
The foamed absorbable matrix is fabricated out of a poly(D,L-lactide-co-glycolide)-co-polyethylene glycol di-block copolymer, due to its short degradation time. These polymers are temperature-sensitive and therefore manufactured by the controlled expansion of saturated polymers (CESP) process. The foam structure, which influences the degradation, is controlled by the process parameters.
Within this paper the influence of the process parameters on the degradation of the implants is investigated.
Introduction
Overactive bladder syndrome (OAB) is a symptom complex which is defined by the International Continence Society as urinary urgency, even when the bladder is not completely filled (51% of the cases), combined with or without involuntary urinary loss and frequent urination of more than eight times during daytime and two times at night (86% of the cases).1,2 In population-based studies, OAB prevalence rates range from 7 to 27% in men, and 9 to 43% in women.3
OAB is treated with anticholinergic agents.4 Anticholinergic agents affect the human body by inhibiting specific receptors, which is supposed to suppress the permanent or sudden urge to urinate. Usually the agents for the treatment of OAB are given orally, but the large number of side effects of the anticholinergic agents is a severe problem. By taking the anticholinergic agents orally, the agents are distributed over the digestive tract and the bloodstream to the whole organism. Therefore, the agents do not only inhibit the receptors of the bladder but also affect other receptors of the organism. This causes many side effects like mouth dryness, digestive disorder, impaired vision, depression, or dizziness.5,6 For that reason 70% of the patients abandon the therapy and accept the unpleasant afflictions of the OAB instead, since the side effects overburden the patient.7
By applying the agent intravesically, the side effects can be minimized significantly.4 During intravesical medication, the agent is applied to the bladder via a catheter through the urethra. Therefore, it is necessary to insert the catheter daily (possibly even several times a day) to administer the agent, which is dissolved in sodium chloride solution. Because of the catheterization, most of the patients do not choose this type of medication, though the side effects are minor.
Due to the unsatisfactory therapy options, a DDS is being developed by a consortium of different companies and research facilities (Dr. R. Pfleger GmbH, DWI at RWTH Aachen, Institute of Plastics Processing (IKV) at RWTH Aachen, Department of Urology at University Hospital Aachen, and Hemoteq AG). The DDS, which releases the anticholinergic agent trospium chloride, can be applied into the bladder through the urethra. It is composed of multiple carriers that consist of non-absorbable, drug-carrying microspheres which are embedded in an absorbable matrix. After degradation of the absorbable matrix, the non-absorbable microspheres are eliminated through the urethra. The approach focuses on a continuous drug release out of the microspheres and an elimination of the DDS after less than four weeks, to avoid side effects like incrustation due to salt deposition.
The design of the implant and its application is shown in Figure 1.
The DDS consists of multiple carriers (spheres or pills) which are connected flexibly with each other through an absorbable suture. The implants are arranged in a way that they can be expanded in the bladder after insertion through the urethra via a catheter. Due to the change in shape it stays safely in the bladder.8
Materials
The absorbable matrix is fabricated out of Resomer RGP d 5055 (Evonik Industries AG, Essen, Germany), which is a poly-D,L-lactide-co-glycolide-co-polyethylene glycol (PLGA-PEG). The material was chosen due to its short degradation time (3-5 weeks). The material has a PEG ratio of 3-7%.9 The inherent viscosity (at 25°C, 0.1% in CHCl3) is 0.93 dL/g and was specified by the manufacturer. The glass transition temperature of the polymer is 38.7°C.10
Instead of trospium chloride, the microspheres are loaded with barium sulfate, which enables the analysis of the microstructure with a micro-computer tomography scanner (μCT). Polydimethylsiloxane is used for encapsulation of the agent. The size of the microspheres is up to 30 μm.
Methods
Barium sulfate is embedded in the microspheres using a modified solvent evaporation process at DWI at RWTH Aachen.11
Due to the temperature sensitivity of the polymer and the agent, the materials are processed in the controlled expansion of saturated polymers process.12 With this CESP process, polymers can be processed into a foamed part at low temperatures. Therefore, temperature-sensitive materials like absorbable polymers or active agents can be processed without loss of their properties (Figure 2).
Within the process the powdery polymer is first mixed with microspheres with a weight ratio of 2:1. The mixture is then filled into a mold by a gravimetric dosing unit. Two different cavities are used, a spherical cavity with a diameter of 2.4 mm and a pill-shaped cavity with the same diameter and a length of 4 mm. For the spheres, 3 mg, and for the pills, 5 mg of the mixture is filled into the cavities.
The filled mold is then exposed to a carbon dioxide atmosphere under high pressure in an autoclave. Through the absorption of the carbon dioxide into the polymer, the cohesive forces between the molecules are reduced so that the polymer is in a foamable state at low temperatures. As soon as the saturation of the polymer with carbon dioxide occurs, the pressure in the autoclave is released. Hence the occurring oversaturation of the polymer with carbon dioxide leads to a foaming of the polymer and enables the shaping under reproducible conditions.
The processing parameters of pressure, temperature, pressure release gradient, and saturation time influence the structure of the resulting foam.13 The processing parameters are varied according to Table 1. Saturation time is 30 minutes for all specimens. For each parameter set, three specimens were investigated.
Figure 3 shows an example of the DDS and a μCT-scan of one implant.
The specimens are incubated individually in 5 mL of artificial urine for up to 27 days. The wet specimens are re-weighed and the fluid is exchanged every three days. Furthermore, the pH value is measured. In case of breakage of the specimens, the experiment is continued with the bigger fragment. Total degradation of the specimen is noted with a weight of 0 mg. The specimens are weighed dry before incubation, and after incubation and desiccation with silica gel and vacuum. For comparison of the specimens, the relative weight shift compared to the initial weight is used.
For statistical analysis of the influence of the process parameters on the degradation, the software Statistica (StatSoft GmbH, Hamburg, Germany) is used.
An optical microscope is used for characterization of the foam structure. The specimens are embedded in epoxide resin and cut into slices with a thickness of 50 μm by means of a microtome. For analysis the colored images are converted into binary images.
Results
The initial weight of the spheres was between 1.97 and 3.81 mg, and of the pills was in the range of 3.94 to 5.37 mg. The specimens first float in the artificial urine and sink to the bottom of the vessel between the first and the second week of incubation. Breakage of several specimens is observed between 14 and 27 days. Table 2 shows the parameter sets of specimens that totally dissolved within 27 days.
The saturation pressure is at the lowest value investigated, whereas the temperature and the release gradient of the pills demonstrate the initial point of the research plan. The release gradient of the spheres is the slowest pressure release investigated.
Figure 4 and Figure 5 show the relative weight of the specimens manufactured at 50 bar. During the first days, the weight increases and then starts decreasing after two weeks for the specimens produced at 50 bar and after three weeks for the specimens produced at 100 bar and 150 bar (Figures 6-9). The fastest degradation is observed with spheres manufactured at 65°C, 50 bar, and 5 bar/min. (ΔmMax = -100%) and pills manufactured at 65°C, 50 bar, and 20 bar/min. (ΔmMax = -100%). The least weight loss is observed with spheres manufactured at 55°C, 100 bar, and 100 bar/min. (ΔmMin = +22.22%) and pills manufactured at 55°C, 100 bar, and 100 bar/min. (ΔmMin = -19.84%).
Microscopic analysis shows that the average pore area was 1985.5 μm2 for the faster-degrading spheres (T = 65°C, p = 50 bar, Δp = 5 bar/min.) and 3812.7 μm2 for the faster-degrading pills (T = 65°C, p = 50 bar, Δp = 20 bar/min.). The average pore size of the slower-degrading spheres (T = 55°C, p = 100 bar, Δp = 100 bar/min.) was 1873.9 μm2 and of the slower degrading pills (T = 55°C, p = 100 bar, Δp = 100 bar/min.), 2386.3 μm2.
The porosity is the ratio between the pore area and the sample area. The faster-degrading specimens have a higher porosity than the slower-degrading specimens.
The pressure has the main influence on the degradation of the specimens, when statistical analysis is conducted. A lower processing pressure leads to a lower final weight after 27 days of incubation. Regarding the processing temperatures, a temperature of 65°C results for both types of specimens (spheres and pills) in the lowest weights, whereas 55°C and 75°C lead to higher final weights.
The influence of the pressure release, however, is different for the spheres and pills. The lowest weights of spheres are observed with a pressure release of 5 bar/min. closely followed by the 20 bar/min. The lowest weights of pills are observed at a pressure release of 20 bar/min. A clear reciprocal effect is observed with the pressure release and the temperature for the spheres. The manufacturing of spheres at 75°C and 20 bar/min. results in a high final weight of the specimens (less degradation).
Discussion
For the development of a DDS for the urinary bladder, the degradation and excretion of the implant within four weeks is crucial. Otherwise, incrustations due to salt depositions might lead to complications.
The degradation of the examined material, PLGA-PEG, is dominated by hydrolysis. The urine is first absorbed by the polymer and then hydrolysis starts.13 Accumulating decomposition products have a catalytic effect on the hydrolysis. During this process, on the one hand, water molecules are absorbed by the implant and water diffuses into the implant, and, on the other hand, degradation products diffuse out of the implant following the concentration gradient.
With compact materials, this leads to an accelerated degradation in the center of the implant, which finally leads to a sudden breakage of the implant with a sudden release of the acid degradation products.13 Considering a microcellular material, the diffusion path of the decomposition products is shorter, and the degradation products can accumulate in the pores. Therefore, degradation is slower, and a continuous degradation starting from the surface is promoted.13
The influence of the CESP process parameters on the morphology of the resulting foam of PDLLA was already described by Pfannschmidt.13 He found that the pressure has the main influence on the pore size. At higher pressures (130 bar) smaller pores are generated (average pore diameter of 40 μm at T= 41°C), and at lower pressures (60 bar) bigger pores arise (average pore diameter 230 μm at T = 41°C). Furthermore, at higher temperatures (T = 60°C) bigger pores are generated (average pore diameter of 75 μm at p = 130 bar), but the influence of the temperature is minor.
Pfannschmidt also investigated the influence of the foam structure on the degradation of the material. Specimens with an average pore diameter of 25 μm have a linear molecular weight reduction, whereas specimens with an average pore diameter of 75 μm first degrade slower and then faster than the specimens with smaller pores. The final molecular weights of the considered specimens are the same. The results within this paper confirm the influence of the microcellular structure of the specimens on their degradation. It was shown that a bigger pore area and a higher porosity lead to a faster degradation of the specimens. It is also confirmed that pressure has the main influence on the resulting pore structure and the degradation, respectively.
The reason for the sinking of the specimens during incubation is most likely the fluid that is soaked by the specimens. The density of the soaked specimen is higher than the density of the artificial urine. This theory also fits the observed initial increase of weight. An explanation for the constant weight after the initial increase might be a parallel degradation of the polymer during diffusion of fluid into the foam. This leads to a decrease of the polymer mass which is not measured due to the fluid infiltration. Furthermore, the weight increase observed with single specimens might be due to deposition of salt crystals from the artificial urine in the specimens and inhomogeneous infiltration of fluid.
Conclusions
Degradation of PLGA-PEG implants is influenced by their microstructure, which can be set by the process parameters pressure, temperature, and pressure release gradient of the CESP process. Spheres produced at 65°C, 50 bar, and 5 bar/min. and pills produced at 65°C, 50 bar, and 20 bar/min. degrade completely within four weeks. Due to their bigger volume, pills can be loaded with more microspheres. Therefore, pills are chosen for production of specimens for further investigations.
The development of the DDS is currently being continued by means of optimizing the drug release and the application of the DDS into the bladder.
Acknowledgement
The investigations set out in this report received financial support from the German Bundesministerium für Bildung und Forschung (Federal Ministry of Education and Research, no. 13N11306), to whom we extend our thanks.
References
- I. Milsom, P. Abrams, L. Cardozo, R.G. Roberts, J. Thüroff, A.J. Wein, “How Widespread Are the Symptoms of an Overactive Bladder and How Are They Managed? A Population-Based Prevalence Study,” BJU International 87 (2001) 9, pp. 760-766.
- P. Abrams, L. Cardozo, M. Fall, D. Griffiths, P. Rosier, U. Ulmsten, P. van Kerrebroeck, A. Victor, A. Wein, “The Standardisation of Terminology of Lower Urinary Tract Function: Report from the Standardisation Sub-Committee of the International Continence Society,” Neurourology and Urodynamics 21 (2002) 2, pp. 167-178.
- E. A. Gormley, D. J. Lightner, K. L. Burgio, T. C. Chai, J. Q. Clemens, D. J. Culkin, A. K. Das, H. E. Foster, H. M. Scarpero, C. D. Tessier, S. P. Vasavada, “Diagnosis and Treatment of Overactive Bladder (Non-Neurogenic) in Adults: AUA/SUFU Guideline,” American Urological Association (AUA) Guideline (2012).
- H. Madersbach, “Orale Anticholinergika bei überaktiver Blasé,” Der Urologe A 45 (2006) 7, pp. 830-597.
- A. Haferkamp, M. Hohenfellner, “Intravesikale Therapie des Overactive-Bladder-Syndroms,” Der Urologe A 45 (2006) 10, pp. 1283-1288.
- Optimierung der Pharmakotherapie, Eine Information nach § 73 Abs. 8 SGB V, Kassenärztliche Vereinigung Westfalen-Lippe (KVWL), December 2006.
- C. Hampel, R. Gillitzer, S. Pahernik, S.W. Melchior, J.W. Thüroff, “Drug Therapy of Female Urinary Incontinence,” Der Urologe A 44 (2005) 3, pp. 244-255.
- W. Michaeli, I. Michaelis, J. Grosse, M. von Walter, E. Wintermantel, N. Laar, “Development of an Active Agent Carrying, Biodegradable Implant for the Intravesical Therapy of the Overactive Bladder Syndrome,” SPE ANTEC Technical Papers, 55, 235 (2009).
- Resomer® RGP d 5055 technical information, Evonik Industries AG, Essen.
- H. Liedtke, Evonik Industries AG, Essen, personal communication.
- B. Dittrich, D. Klee, H. Höcker, “Hocheffiziente Verkapselung eines wasserlöslichen Wirkstoffs in Siliconmikrosphären im Solvent-Evaporationsprozess,” Aachen-Dresden International Textile Conference, Aachen, 2007.
- W. Michaeli, L.-O. Pfannschmidt, “Microporous, Resorbable Implants Produced by the CESP Process,” Advanced Engineering Materials 1 (1999) 3-4.
- L.-O. Pfannschmidt, Herstellung resorbierbarer Implantate mit mikrozellulärer Schaumstruktur, RWTH Aachen, dissertation, ISBN 3-89653-996-5, 2002.