Plugging Into the Power of Polymers
Lithium-ion and other battery technologies will increasingly be getting more solid support and energy density from polymers
Previous Article Next Article
By Jon Evans
Plugging Into the Power of Polymers
Lithium-ion and other battery technologies will increasingly be getting more solid support and energy density from polymers
Previous Article Next Article
By Jon Evans
Plugging Into the Power of Polymers
Lithium-ion and other battery technologies will increasingly be getting more solid support and energy density from polymers
Previous Article Next Article
By Jon Evans

Electric vehicles plugged into one of the 36 charging stations in the U.S. National Renewable Energy Laboratory’s parking garage. (Photo by Dennis Schroeder courtesy of the NREL.)
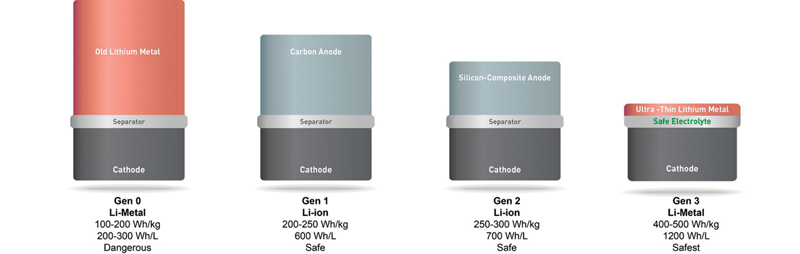
SolidEnergy says its advanced battery has around double the energy density of a conventional Li-ion battery (graphic courtesy of the company).

SolidEnergy says its advanced battery has around double the energy density of a conventional Li-ion battery (graphic courtesy of the company).

A plug-in electric vehicle in its charging station at the NREL’s Vehicle Testing and Integration Facility. (Photo by Dennis Schroeder courtesy of NREL.)
University of Jena researchers (left to right) Ulrich Schubert, Tobias Janoschka, and Martin Hager holding their redoxflow battery (photo courtesy of Anne Guenther/FSU).

University of Jena researchers (left to right) Ulrich Schubert, Tobias Janoschka, and Martin Hager holding their redoxflow battery (photo courtesy of Anne Guenther/FSU).
Our modern, always-on, 24/7 lifestyle is powered by lithium-ion (Li-ion) batteries, without which we wouldn’t be able to use all our portable electronic devices, from laptops to tablets to smartphones. Nevertheless, anyone who uses these portable electronic devices knows that the current generation of Li-ion batteries are not without their problems.
For a start, they still need to be recharged fairly regularly, especially with our electronic devices becoming ever more powerful and therefore power-hungry, and this usually takes a few hours. Furthermore, their energy capacity steadily degrades over time, requiring them to be recharged more and more regularly until eventually they need to be replaced.
Solving these problems would not just prove beneficial for users of electronic devices, they could also help usher in the widespread adoption of electric cars and renewable energy. Ideally, electric cars need batteries that are powerful enough to take them the same kind of distances between charges as a full tank of gasoline. And renewable energy technologies such as solar and wind require batteries that can efficiently store excess energy produced when it’s sunny or windy, and then quickly release that energy when it’s not.
Researchers in both academia and industry are hard at work exploring various approaches for solving these problems and improving Li-ion batteries, as well as developing alternative battery technologies. And polymers are at the heart of many of these efforts.
The Guts of Li-Ion Batteries
As with any battery, Li-ion batteries consist of a negative electrode (anode) and a positive electrode (cathode) separated by a liquid electrolyte. When releasing power, lithium ions travel from the anode through the electrolyte to the cathode, driving an associated flow of electrons through an external circuit. This process goes into reverse when the battery is charged by an external power source, with the opposite flow of electrons driving lithium ions from the cathode to the anode.
The amount of charge that a Li-ion battery can hold depends on how many lithium ions can be stored in each of the electrodes. The power the battery can generate when discharging and the speed at which it can be recharged depends on how fast the lithium ions travel through the electrolyte. In the current generation of Li-ion batteries, the anode is graphite, while the cathode is a metal oxide such as lithium cobalt oxide or lithium manganese oxide. The liquid electrolyte comprises a lithium salt such as lithium hexafluorophosphate dissolved in an organic solvent like ethylene carbonate or propylene carbonate.
Researchers know of several substances that would make better electrode materials for Li-ion batteries, but they each come with problems. A pure lithium metal anode would be better than a graphite anode, and a sulfur-based cathode would be better than a metal oxide cathode, as both could theoretically store many more lithium ions.
The problem with lithium metal anodes is that the lithium ions tend to arrive faster than they can be incorporated within the anodes and thus build up as deposits on the surface. Given enough time, these deposits, known as dendrites, can extend right across the electrolyte to the cathode, causing the battery to short out. These dendrites form on graphite anodes as well, but they’re more of an issue with lithium metal anodes. The problem with sulfur-based cathodes is that the sulfur and lithium react to produce various compounds that dissolve in the electrolyte, contaminating it and causing the sulfur-based electrode to degrade over time.
Liquid Electrolytes Become Solid Polymers
Handily, though, both of these problems could be resolved by replacing the liquid electrolyte with a solid polymer one, which would physically stop the spread of dendrites and the dissolving of compounds produced by the reaction between sulfur and lithium. Solid polymer electrolytes would also have several other advantages.
For a start, they would be safer, because the liquid electrolyte used in current Li-ion batteries is flammable and has been known to burst into flames if the battery gets too hot. They could also allow the creation of flexible batteries with a range of different shapes, as the liquid electrolyte would not need to encased in robust packaging. (As this packaging is usually made from a polymer, current Li-ion batteries are sometimes rather confusingly known as polymer Li-ion batteries, even though they contain liquid electrolytes.)
Conductive polymers such as polyethylene oxide (PEO) doped with lithium ions have shown promise as solid polymer electrolytes, but scientists have struggled to develop PEO materials that transport lithium ions anywhere near as well as liquid electrolytes. Nevertheless, several companies, including the U.S. companies Seeo and SolidEnergy, are actively developing advanced versions of Li-ion batteries that incorporate solid polymer electrolytes.
Seeo’s polymer electrolyte technology is called DryLyte and was originally developed by researchers at Lawrence Berkeley National Laboratory. Seeo is combining DryLyte with a lithium metal anode to produce batteries for electric vehicles and claims that these batteries have an energy density of 350 watt-hours per kilogram. This is more than double the energy density of a conventional Li-ion battery and would be sufficient to take an electric car as far as a full tank of gasoline on a single charge. (The German technology company Bosch is obviously convinced, because it acquired Seeo in September 2015.)
SolidEnergy is a spin-off from the Massachusetts Institute of Technology. It’s developing an advanced Li-ion battery that incorporates an ultra-thin metal anode, made of thin lithium on copper, and a solid polymer electrolyte. The company claims that its advanced battery also has around double the energy density of a conventional Li-ion battery.
PEO Progress
Academic researchers, meanwhile, are busy finding ever more innovative ways to formulate PEO as a solid electrolyte. For example, chemical engineers at Cornell University have tethered short-chain PEO oligomers to silica particles and linked these particles together with polypropylene oxide. This produces a crosslinked nanoparticle-polymer composite that is then soaked in a conventional liquid electrolyte comprising a lithium salt dissolved in propylene carbonate.1
The great advantage of this crosslink structure is that it creates a system of porous conductive pathways which lithium ions can pass through almost as easily as through a conventional liquid electrolyte. Combining this composite with a lithium metal anode and a metal oxide cathode produces a battery that maintains a high current density and discharge capacity for over 150 charge/discharge cycles.
PEO could also help in the development of perhaps the most promising class of lithium-based battery. This is the lithium-air battery, which uses gaseous oxygen as the cathode. In addition to being much lighter than conventional Li-ion batteries (as it no longer has the weight of a solid cathode), a lithium-air battery can theoretically store ten times as much energy. This is because rather than lithium ions being incorporated in a solid material, they are stored via a reversible reaction with oxygen that creates lithium peroxide.
Unfortunately, this process can also generate oxygen radicals that gradually oxidize liquid electrolytes over time, meaning that lithium-air batteries tend to stop working after only a few charge/discharge cycles. Recently, though, two chemists from Sapienza University in Italy showed that a plasticized PEO-based material doped with lithium ions made a very effective solid electrolyte for lithium-air batteries.2
Not only is this plasticized PEO-based material chemically stable, preventing it from being oxidized by the oxygen radicals, but it’s also more conductive than most other solid electrolytes developed from PEO. When used with a lithium metal anode, this plasticized PEO produced a lithium-air battery with a likely energy density of more than 300 watt-hours per kilogram.
Redox-Flow Batteries
Lithium-based batteries are not the only option for polymers in batteries, for there’s an alternative battery technology that not only does away with lithium but also does away with solid electrodes. Known as a redox-flow battery, it works by flowing two liquids, each of which contains ions that can exist in one of two oxidation states, on either side of a proton-exchange membrane. As the ions in each liquid shift between oxidation states, they drive a flow of protons through the membrane and an associated flow of electrons through an external circuit. Charging the battery from an external power source reverses the flow of electrons and protons, causing the ions to revert to their original oxidation states.
The liquids are held in external tanks and then pumped past the membrane. This means that the energy capacity of a redox-flow battery can be increased simply by increasing the size of the tanks, which makes them an excellent battery technology for storing excess energy generated by wind and solar power.
Most of the redox-flow battery systems that have so far been installed for this purpose use ions of vanadium, a heavy metal, and the well-known perfluorinated polymer Nafion as the membrane. Because vanadium ions can exist in one of four oxidation states, they can be used in both liquids, with the ions in each liquid alternating between a different pair of oxidation states.
Unfortunately, vanadium is expensive, as is the Nafion membrane, and this cost is hampering the widespread adoption of redox-flow batteries. In addition, the vanadium ion liquids are produced by dissolving vanadium salts in sulfuric acid, which is highly corrosive and limits the lifetime of the battery. The use of sulfuric acid also explains why a robust, expensive membrane such as Nafion is required.
Recently, however, a team of chemists from the University of Jena and a spin-off company from the university called JenaBatteries have discovered two redox-active polymers that work just as well.3 In addition to being much cheaper than vanadium, these polymers can also be dissolved in salt water rather than sulfuric acid, allowing the Nafion membrane to be replaced by a much cheaper cellulose membrane.
Their initial resulting redox-flow battery only had an energy density of 10 watt-hours per liter, but it could withstand up to 10,000 charge/discharge cycles without losing any of this capacity. The team is already working on larger, more efficient systems—all of which should help ensure our electronic devices stay charged in the future.
References
1. Nature Communications, 2015, 6, 10101 (DOI: 10.1038/ ncomms10101).
2. Scientific Reports, 2015, 5, 12307 (DOI: 10.1038/srep12307).
3. Nature, 2015, 527, 78–81 (DOI: 10.1038/nature15746).



