Medical Plastics: Well and Good
Previous Article Next Article
By Pat Toensmeier

BASF has commercialized both flexible and rigid medical grades recently (photo courtesy of BASF).
Plastics have been doing well, and doing good, in medical and healthcare markets for years. Major advances in many medical procedures often go hand-in-hand with developments in resins and compounds, thereby enabling the design, engineering, and cost-efficient production of instruments, devices, and related components that facilitate short- and long-term treatments.
Demographically, the benefits that developments in plastics provide in many areas are especially apparent where aging populations ratchet up demand for innovative treatment systems—and for devices that allow the elderly to meet their medical needs without undue and costly reliance on doctors and hospitals.
Ongoing advances in plastics address another demographic: developing countries where access to hospitals and medical services is limited—or unavailable—for many people, especially children and infants. Devices for testing, vaccination, accurate dispensing of antibiotics and other medicines, and self-medication bring vital treatments to people who live in areas where there is little or no access to healthcare.
The growing range of medical resins and compounds also addresses enhancements to conventional device requirements such as clarity, chemical resistance, sterilization, and of course compliance to mandates from the U.S. FDA, U.S. Pharmacopeia (USP), the European Union’s REACH protocols, ISO standards, adherence to Good Manufacturing Practices (GMP), and other regulations.
These concerns influence the relentless intra-material competition that drives plastics use in medical devices. The market has never stood still in this area, of course, and has always demonstrated that there is no status quo when it comes to material selection. Formulation advancements improve processability, upgrade device performance, and allow part consolidation, multi-material molding, and structural enhancements like thinwalling—often at lower cost than competing materials.
Below are representative examples of suppliers whose new and recent developments in materials affect the design and performance of medical and healthcare devices. The capabilities that these resins and compounds bring to this large, diverse and challenging market showcase the ability of plastics to enhance applications and make possible the devices and procedures that improve individual treatments, and ultimately, the quality of life for people around the world.
High Flow, with Design Flexibility
One area where compounds excel in properties and performance is devices and components that require metal-like stiffness along with high flow that facilitates molding. SABIC Innovative Plastics recently unveiled two compounds that combine high-modulus carbon fiber technology with high-performance resins, for use in disposable or reusable (and sterilizable) parts.
The grades, LNP Lubricomp DCI06APW and LNP Thermocomp EC006AQW are alternatives to metal and fiber-reinforced polymers, says Cathie Hess, director of healthcare marketing. Lubricomp is a 30% fiber-reinforced grade of polycarbonate (PC), and Thermocomp is a 30% reinforced grade of polyetherimide.

LNP Lubricomp is a 30% carbon fiberreinforced PC resin from SABIC for replacing metal in scalpels and other components (photo courtesy of SABIC).
Hess describes Lubricomp as a “high-flow material for high-strength components where the use of metals or other fiber-filled thermoplastics with poor flow can create design and manufacturing challenges.” The compound achieves a balance between design flexibility and ease of manufacturing that enables complex part design and part consolidation, while allowing molders “to reduce waste [and] manufacturing and maintenance costs, and improve cycle times,” she notes.
Target applications for Lubricomp include disposable surgical instruments, medical device housings, and drug-delivery components.
Thermocomp, also touted as an alternative to metal, provides high strength and stiffness, chemical compatibility, and mechanical stability in parts, Hess says. Applications include disposable or reusable surgical instruments, fixation devices like those used to secure broken bones, housings, and patient transport devices.
Importantly, the two compounds allow designers, OEMs, and molders to reduce development and manufacturing costs. “Cost effectiveness continues to be a major driver of design decisions in the healthcare industry,” Hess affirms. “These reinforced materials can help optimize medical device system costs as well as improve processability.”
Tiny, Precision Parts for Drug Delivery
Drug-delivery devices are a major part of the healthcare market. These products allow users to accurately and safely self-medicate without the need for repeat visits to doctors or hospitals, and with little or no disruption to their daily routines. As the design of these devices trends toward ever-more compact, lightweight, and even stylish assemblies, resin suppliers are targeting the market with enhanced materials.
One resin producer that specializes in the drug-delivery device market is DuPont Performance Polymers. William Hassink, global healthcare segment leader, says the global device segment of the market alone (excluding the value of delivery systems with medicine) is huge, accounting for an estimated $60 to $80 billion annually, and growing. When medicine is factored in, the value of these devices could be as much as $400 billion.
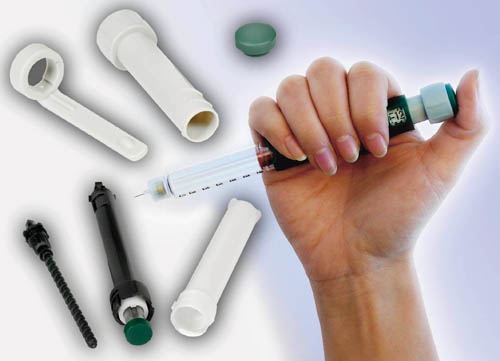
Portable drug-delivery systems make increasing use of internal parts molded from resins such as Delrin acetals from DuPont (photo courtesy of DuPont).
DuPont supplies semi-crystalline engineering thermoplastics for these devices, among them grades of polybutylene terephthalate, acetal, and polyamide. A major challenge to the design and engineering of these systems is the ability to accurately mold tiny precision parts that comprise the inner workings of the devices—gears, plungers, and similar components.
“If you open a dry-powder inhaler, you see a complex device for managing one month of doses,” Hassink says. Since the portability of these devices means they will get no bigger than they are now (typically pocket size), and likely will become more compact and lightweight, the precise and reliable operation of interior components, as well as their ability to assume multifunctional roles, are crucial to use and safety.
But that’s not all. Hassink notes that the ability to differentiate devices is often limited by patent protection. In other words, there’s only so much a designer can do with the look and basic operation of a drug-delivery device to set it apart from competitors or improve its manufacture.
One option could be different ways of constructing internal features using multifunctionality and other techniques to set the operation of a device apart from competitive products. Examples Hassink cites include:
- achieving a well-defined “click” from a dose indicator so users know their administration of medicine is accurate;
- internal energy storage for mechanical movements from the addition of a spring or other part; and
- the use of snap fits to improve both the positioning of internal components like pumps and the assembly of devices.
One resin DuPont recently added to address these needs is Delrin SC699, an acetal homopolymer with inherent silicone lubrication. The lubrication ensures that internal parts molded with the grade generate uniform force in the hands of different users, thereby ensuring consistent dosages. The grade also eliminates the need for molders to undertake costly secondary operations to lubricate parts.
The lubricity of SC699 and a companion Delrin grade, PC699, suit them for applications where surfaces slide, roll, or rub against each other. Hassink says the grades additionally have high flow, multi-cavity molding properties, creep resistance, and printability, among other properties. “They work, and they are cost effective.”

Makroblend M525 PC/polyester from Bayer MaterialScience allows designers to develop compact, lightweight housings for wearable medicine pumps (photo courtesy of Bayer).
One OEM that specified SC699 is Ypsomed AG of Switzerland, which uses it for a dose dial sleeve in its UnoPen variable-dose injector pen for insulin and other medicines. The sleeve, positioned between the housing and piston rod, is used by patients to set required doses for injection. It interacts mechanically with the piston rod, which dispenses the medicine. The lubricity of the grade ensures consistent dispensing of accurate doses.
Enhancing Wearable Pumps
Closely related to portable, self-administered drug-delivery systems are wearable pumps that automatically inject insulin, painkillers, and other vital medicines to patients with chronic conditions. These devices, which are typically worn on a belt or under a shirt or blouse, use subcutaneous injection sites to administer medicine, and have battery-powered pumps, timers, alarms, and display windows in compact, lightweight housings.
The resin requirements for these units are different than for self-administered delivery systems. For one thing, says Bruce Fine, North American medical segment market leader at Bayer MaterialScience, the housings need chemical resistance, especially to body oils, lotions, and creams, since they contact a wearer’s skin, and to hospital disinfectants.
They must also be biocompatible. Relevant tests here include ISO-10993-5, for cytotoxicity, and ISO 10993-10, for skin irritation and sensitivity.
Resins must accommodate electrical components and power sources, which usually mean batteries. They don’t need a UL-VO rating but should meet UL 94 horizontal burn requirements.
High-flow properties and thinwalling are important, as is the ability to overmold features like display windows and tubing connections. Color options also figure in resin specification since OEMs want wearable pumps to be stylish and aesthetically pleasing to users.
Bayer officially launched a new grade in February for such applications. Makroblend M525 is a PC/polyester blend that meets all these requirements, and additionally provides toughness, moldability, and dimensional stability. Fine declined to reveal details about the blend beyond saying that the polyester component provides good chemical resistance. PC, of course, is tough, heat resistant, and dimensionally stable.
As with self-administered drug-delivery systems, Fine notes a trend in wearable pumps toward lighter weights, enhanced portability, and simplicity of operation—for “getting as much healthcare as possible delivered outside healthcare settings.”
High-Performance “Cyclic” Elastomers
Clarity and molded-in detail are essential properties in a number of applications, notably flexible membranes, tubing, and other parts that are used in flow-control systems and microfluidic devices. One company with a high-performance elastomer for these products is TOPAS Advanced Polymers Inc., the U.S. business unit of parent TOPAS Advanced Polymers GmbH in Germany.
TOPAS supplies cyclic olefin copolymers, or COCs. These are transparent, amorphous resins copolymerized from norbornene and ethylene with a metallocene catalyst. (TOPAS stands for “thermoplastic olefin polymer of amorphous structure.”) The result is a material with inherent properties such as glass-like optics, heat resistance, and high dimensional stability.
Timothy Kneale, president of TOPAS, points to grade E-140, a COC elastomer that provides a “performance advantage” to applications requiring clarity and molded-in or extruded detail, along with high levels of barrier, purity (low leachables and extractables), and chemical resistance.
“This is not a commodity item,” he notes. “It is specified when ordinary materials will not do the job.”
Though Kneale declines to provide application details, citing non-disclosure agreements with end-users, he explains that the E-140 grade can be used in drug storage or delivery devices and as a flexible membrane in demanding diagnostic and flow-control applications. It can also replace
specialty silicones such as polydimethylsiloxane, which is inert, non-toxic, optically clear, and has good rheological properties. He adds that the E-140 elastomer also lends itself to faster production scale-up than most silicones.
Importantly, the elastomer is compatible in assemblies with a rigid COC, like grade 5013L-10. Among other advantages, this property facilitates multi-material molding and eliminates concerns about cracking and other structural deficiencies that may develop from incompatible materials. The resin, which is also touted for microfluidic applications, reportedly has exceptional clarity, high flow without loss of material strength or optical properties, and heat resistance to 127°C.
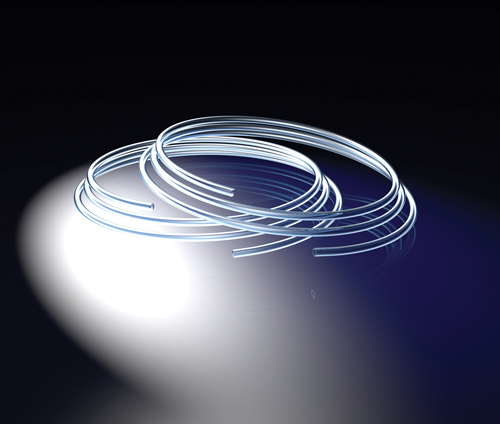
TOPAS Advanced Polymers produces a cyclic olefin copolymer elastomer for flexible applications that require high barrier properties, purity, and chemical resistance (photo courtesy of TOPAS).
Kneale says the rigid grade can be molded with “sub-micron details,” which is a plus for small parts. Customers, moreover, report achieving novel molding capabilities like zero draft angles on parts. Grade 5013L-10 also reportedly maintains dimensional tolerances of 0.0004 inch (0.01 mm) in molded parts.
TPEs for Diverse Needs
Elastomeric grades are essential for many applications, among them closures, gaskets, tubing, valves, infusion stoppers, and some forms of packaging. One supplier of medical elastomers, Kraiburg TPE Corp., specializes in styrenic block copolymers, which it markets as Thermolast M. These provide important benefits such as translucence and transparency, low compression set, good adhesion to polypropylene and polyethylene, and phthalate- and latex-free formulations, which avoid end-use concerns.
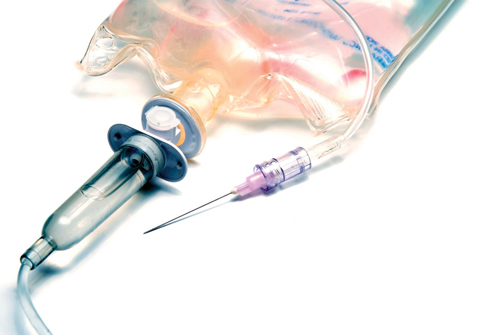
Kraiburg TPE’s Thermolast M styrene block copolymer elastomers are used in diverse products like tubing, drip chambers, luer locks, and gaskets (photo courtesy of Kraiburg TPE).
While Thermolast M grades have been commercial for some time, a recent formulation development allows for direct body and blood contact on a limited basis, generally 1 to 30 days, says Katherine Olano, distribution and marketing specialist. The exact time depends on the application.
Kraiburg supplies four medical-grade TPEs. They include a translucent general-purpose elastomer for stand-alone parts that is not designed for adhesion to other materials. Another grade designed for adhesion bonds to PC, ABS, PC/ABS, and PETG (polyethylene terephthalate-glycol) resins, and reportedly has no issues with stress cracking at the materials interface. A high-elasticity version has a low coefficient of friction for mechanical components and sealants. The fourth grade reportedly can replace costlier silicone as a bottle seal. Here the material can be punctured with a needle to draw out fluid, after which it reseals itself when the needle is withdrawn.

The PEEK-Optima Ultra-Reinforced distal femoral plate, fabricated of carbon fiber-reinforced PEEK and developed by Victrex Invibio, is used to heal bone trauma (photo courtesy of Invibio).
Typical applications for Kraiburg TPEs include nebulizer masks (whose soft-touch properties improve comfort), syringe stoppers, bottle caps, tubing, and bags. Olano says the company is developing formulations that chemically bond to different resins, a property that will facilitate multi-material molding and applications that specify the elastomers as substrates.
Reinforced Implants
Implants for fracture fixation are a growing and dynamic application for high-performance resins and compounds, which enable devices with higher fatigue life, greater interoperative visibility, and flexible use. One company active in this sector is Victrex Plc, which supplies ketone-based materials through its medical-focused Invibio division for orthopedic trauma devices.
One material from Invibio is PEEK-Optima Ultra-Reinforced, which is used in plates fabricated from PEEK (polyetheretherketone) resin reinforced with continuous PAN-based (polyacrylonitrile) carbon fiber. When used to treat a fractured bone, the plate provides advantages over conventional metal versions (titanium and stainless steel) and has the potential to accelerate healing due to its decreased bending stiffness.
A PEEK-Optima plate reportedly has at least 50 times greater fatigue resistance than metal, can be fabricated with tailored levels of stiffness to meet individual needs, has a modulus of elasticity closer to that of cortical bone than metal, and weighs less than metal versions.
The formulation and fabrication technology that Invibio provides can also help OEMs bring PEEK-Optima Ultra-Reinforced plates to market faster than metal versions and thereby help them reduce costly R&D programs.
Looking Ahead
Resins and compounds will remain vital enabling materials for a growing range of medical and healthcare devices. Research underway, however, suggests that the properties of plastics will soon be applied to the use of microscopic materials such as bacteria and nanoparticles used to treat cell disorders and infirmities at the molecular level within the body. Coated tubing and other implantable devices could be the mechanism by which these treatments are delivered and controlled.
For now, though, product designers, OEMs, and processors can expect to see growing demand for plastics in a multitude of institutional and personal treatment devices, as healthcare procedures become increasingly tailored for lifestyle needs.
Show & Well
Medical device trade fairs are becoming common places where resin suppliers can show off their new grades and applications. For instance, BASF recently promoted a number of materials serving in various healthcare roles at the annual Compamed trade fair in Germany, including the following:
- Thermoplastic polyurethane (TPU) for transparent, thin-walled elements and films: The company’s Elastollan TPU is used for “flexibly foldable” tube elements, balloons, elastically deformable foam suppositories, and drainage tubes, with some parts having a thickness of a few micrometers.
- Non-phthalate plasticizers, like Hexamoll DINCH, a low-migrating alternative to traditional phthalates used in PVC medical applications. The plasticizer is said to be a fit for soft PVC feeding tubes, catheters, breathing masks, and blood bags. The PVC/plasticizer’s technical properties are maintained after sterilization, the company says.
- “Skin-stretching” polyoxymeth-ylene: BASF’s Ultraform S2320 003 PRO’s first application is reportedly in the Skin Stretcher, a device from BioWim GmbH. The device is used to close the skin when treating large wounds following operations or accidents. The resin is said to minimize the friction between parts in contact. In injection molding, the high-flowing material produces parts that are rigid and strong, supplying the resilience and spring characteristics needed by the device, says BASF.
—Ed.
Medical Plastics: Well and Good
Previous Article Next Article
By Pat Toensmeier

BASF has commercialized both flexible and rigid medical grades recently (photo courtesy of BASF).
Plastics have been doing well, and doing good, in medical and healthcare markets for years. Major advances in many medical procedures often go hand-in-hand with developments in resins and compounds, thereby enabling the design, engineering, and cost-efficient production of instruments, devices, and related components that facilitate short- and long-term treatments.
Demographically, the benefits that developments in plastics provide in many areas are especially apparent where aging populations ratchet up demand for innovative treatment systems—and for devices that allow the elderly to meet their medical needs without undue and costly reliance on doctors and hospitals.
Ongoing advances in plastics address another demographic: developing countries where access to hospitals and medical services is limited—or unavailable—for many people, especially children and infants. Devices for testing, vaccination, accurate dispensing of antibiotics and other medicines, and self-medication bring vital treatments to people who live in areas where there is little or no access to healthcare.
The growing range of medical resins and compounds also addresses enhancements to conventional device requirements such as clarity, chemical resistance, sterilization, and of course compliance to mandates from the U.S. FDA, U.S. Pharmacopeia (USP), the European Union’s REACH protocols, ISO standards, adherence to Good Manufacturing Practices (GMP), and other regulations.
These concerns influence the relentless intra-material competition that drives plastics use in medical devices. The market has never stood still in this area, of course, and has always demonstrated that there is no status quo when it comes to material selection. Formulation advancements improve processability, upgrade device performance, and allow part consolidation, multi-material molding, and structural enhancements like thinwalling—often at lower cost than competing materials.
Below are representative examples of suppliers whose new and recent developments in materials affect the design and performance of medical and healthcare devices. The capabilities that these resins and compounds bring to this large, diverse and challenging market showcase the ability of plastics to enhance applications and make possible the devices and procedures that improve individual treatments, and ultimately, the quality of life for people around the world.
High Flow, with Design Flexibility
One area where compounds excel in properties and performance is devices and components that require metal-like stiffness along with high flow that facilitates molding. SABIC Innovative Plastics recently unveiled two compounds that combine high-modulus carbon fiber technology with high-performance resins, for use in disposable or reusable (and sterilizable) parts.
The grades, LNP Lubricomp DCI06APW and LNP Thermocomp EC006AQW are alternatives to metal and fiber-reinforced polymers, says Cathie Hess, director of healthcare marketing. Lubricomp is a 30% fiber-reinforced grade of polycarbonate (PC), and Thermocomp is a 30% reinforced grade of polyetherimide.

LNP Lubricomp is a 30% carbon fiberreinforced PC resin from SABIC for replacing metal in scalpels and other components (photo courtesy of SABIC).
Hess describes Lubricomp as a “high-flow material for high-strength components where the use of metals or other fiber-filled thermoplastics with poor flow can create design and manufacturing challenges.” The compound achieves a balance between design flexibility and ease of manufacturing that enables complex part design and part consolidation, while allowing molders “to reduce waste [and] manufacturing and maintenance costs, and improve cycle times,” she notes.
Target applications for Lubricomp include disposable surgical instruments, medical device housings, and drug-delivery components.
Thermocomp, also touted as an alternative to metal, provides high strength and stiffness, chemical compatibility, and mechanical stability in parts, Hess says. Applications include disposable or reusable surgical instruments, fixation devices like those used to secure broken bones, housings, and patient transport devices.
Importantly, the two compounds allow designers, OEMs, and molders to reduce development and manufacturing costs. “Cost effectiveness continues to be a major driver of design decisions in the healthcare industry,” Hess affirms. “These reinforced materials can help optimize medical device system costs as well as improve processability.”
Tiny, Precision Parts for Drug Delivery
Drug-delivery devices are a major part of the healthcare market. These products allow users to accurately and safely self-medicate without the need for repeat visits to doctors or hospitals, and with little or no disruption to their daily routines. As the design of these devices trends toward ever-more compact, lightweight, and even stylish assemblies, resin suppliers are targeting the market with enhanced materials.
One resin producer that specializes in the drug-delivery device market is DuPont Performance Polymers. William Hassink, global healthcare segment leader, says the global device segment of the market alone (excluding the value of delivery systems with medicine) is huge, accounting for an estimated $60 to $80 billion annually, and growing. When medicine is factored in, the value of these devices could be as much as $400 billion.

Portable drug-delivery systems make increasing use of internal parts molded from resins such as Delrin acetals from DuPont (photo courtesy of DuPont).
DuPont supplies semi-crystalline engineering thermoplastics for these devices, among them grades of polybutylene terephthalate, acetal, and polyamide. A major challenge to the design and engineering of these systems is the ability to accurately mold tiny precision parts that comprise the inner workings of the devices—gears, plungers, and similar components.
“If you open a dry-powder inhaler, you see a complex device for managing one month of doses,” Hassink says. Since the portability of these devices means they will get no bigger than they are now (typically pocket size), and likely will become more compact and lightweight, the precise and reliable operation of interior components, as well as their ability to assume multifunctional roles, are crucial to use and safety.
But that’s not all. Hassink notes that the ability to differentiate devices is often limited by patent protection. In other words, there’s only so much a designer can do with the look and basic operation of a drug-delivery device to set it apart from competitors or improve its manufacture.
One option could be different ways of constructing internal features using multifunctionality and other techniques to set the operation of a device apart from competitive products. Examples Hassink cites include:
- achieving a well-defined “click” from a dose indicator so users know their administration of medicine is accurate;
- internal energy storage for mechanical movements from the addition of a spring or other part; and
- the use of snap fits to improve both the positioning of internal components like pumps and the assembly of devices.
One resin DuPont recently added to address these needs is Delrin SC699, an acetal homopolymer with inherent silicone lubrication. The lubrication ensures that internal parts molded with the grade generate uniform force in the hands of different users, thereby ensuring consistent dosages. The grade also eliminates the need for molders to undertake costly secondary operations to lubricate parts.
The lubricity of SC699 and a companion Delrin grade, PC699, suit them for applications where surfaces slide, roll, or rub against each other. Hassink says the grades additionally have high flow, multi-cavity molding properties, creep resistance, and printability, among other properties. “They work, and they are cost effective.”

Makroblend M525 PC/polyester from Bayer MaterialScience allows designers to develop compact, lightweight housings for wearable medicine pumps (photo courtesy of Bayer).
One OEM that specified SC699 is Ypsomed AG of Switzerland, which uses it for a dose dial sleeve in its UnoPen variable-dose injector pen for insulin and other medicines. The sleeve, positioned between the housing and piston rod, is used by patients to set required doses for injection. It interacts mechanically with the piston rod, which dispenses the medicine. The lubricity of the grade ensures consistent dispensing of accurate doses.
Enhancing Wearable Pumps
Closely related to portable, self-administered drug-delivery systems are wearable pumps that automatically inject insulin, painkillers, and other vital medicines to patients with chronic conditions. These devices, which are typically worn on a belt or under a shirt or blouse, use subcutaneous injection sites to administer medicine, and have battery-powered pumps, timers, alarms, and display windows in compact, lightweight housings.
The resin requirements for these units are different than for self-administered delivery systems. For one thing, says Bruce Fine, North American medical segment market leader at Bayer MaterialScience, the housings need chemical resistance, especially to body oils, lotions, and creams, since they contact a wearer’s skin, and to hospital disinfectants.
They must also be biocompatible. Relevant tests here include ISO-10993-5, for cytotoxicity, and ISO 10993-10, for skin irritation and sensitivity.
Resins must accommodate electrical components and power sources, which usually mean batteries. They don’t need a UL-VO rating but should meet UL 94 horizontal burn requirements.
High-flow properties and thinwalling are important, as is the ability to overmold features like display windows and tubing connections. Color options also figure in resin specification since OEMs want wearable pumps to be stylish and aesthetically pleasing to users.
Bayer officially launched a new grade in February for such applications. Makroblend M525 is a PC/polyester blend that meets all these requirements, and additionally provides toughness, moldability, and dimensional stability. Fine declined to reveal details about the blend beyond saying that the polyester component provides good chemical resistance. PC, of course, is tough, heat resistant, and dimensionally stable.
As with self-administered drug-delivery systems, Fine notes a trend in wearable pumps toward lighter weights, enhanced portability, and simplicity of operation—for “getting as much healthcare as possible delivered outside healthcare settings.”
High-Performance “Cyclic” Elastomers
Clarity and molded-in detail are essential properties in a number of applications, notably flexible membranes, tubing, and other parts that are used in flow-control systems and microfluidic devices. One company with a high-performance elastomer for these products is TOPAS Advanced Polymers Inc., the U.S. business unit of parent TOPAS Advanced Polymers GmbH in Germany.
TOPAS supplies cyclic olefin copolymers, or COCs. These are transparent, amorphous resins copolymerized from norbornene and ethylene with a metallocene catalyst. (TOPAS stands for “thermoplastic olefin polymer of amorphous structure.”) The result is a material with inherent properties such as glass-like optics, heat resistance, and high dimensional stability.
Timothy Kneale, president of TOPAS, points to grade E-140, a COC elastomer that provides a “performance advantage” to applications requiring clarity and molded-in or extruded detail, along with high levels of barrier, purity (low leachables and extractables), and chemical resistance.
“This is not a commodity item,” he notes. “It is specified when ordinary materials will not do the job.”
Though Kneale declines to provide application details, citing non-disclosure agreements with end-users, he explains that the E-140 grade can be used in drug storage or delivery devices and as a flexible membrane in demanding diagnostic and flow-control applications. It can also replace
specialty silicones such as polydimethylsiloxane, which is inert, non-toxic, optically clear, and has good rheological properties. He adds that the E-140 elastomer also lends itself to faster production scale-up than most silicones.
Importantly, the elastomer is compatible in assemblies with a rigid COC, like grade 5013L-10. Among other advantages, this property facilitates multi-material molding and eliminates concerns about cracking and other structural deficiencies that may develop from incompatible materials. The resin, which is also touted for microfluidic applications, reportedly has exceptional clarity, high flow without loss of material strength or optical properties, and heat resistance to 127°C.

TOPAS Advanced Polymers produces a cyclic olefin copolymer elastomer for flexible applications that require high barrier properties, purity, and chemical resistance (photo courtesy of TOPAS).
Kneale says the rigid grade can be molded with “sub-micron details,” which is a plus for small parts. Customers, moreover, report achieving novel molding capabilities like zero draft angles on parts. Grade 5013L-10 also reportedly maintains dimensional tolerances of 0.0004 inch (0.01 mm) in molded parts.
TPEs for Diverse Needs
Elastomeric grades are essential for many applications, among them closures, gaskets, tubing, valves, infusion stoppers, and some forms of packaging. One supplier of medical elastomers, Kraiburg TPE Corp., specializes in styrenic block copolymers, which it markets as Thermolast M. These provide important benefits such as translucence and transparency, low compression set, good adhesion to polypropylene and polyethylene, and phthalate- and latex-free formulations, which avoid end-use concerns.

Kraiburg TPE’s Thermolast M styrene block copolymer elastomers are used in diverse products like tubing, drip chambers, luer locks, and gaskets (photo courtesy of Kraiburg TPE).
While Thermolast M grades have been commercial for some time, a recent formulation development allows for direct body and blood contact on a limited basis, generally 1 to 30 days, says Katherine Olano, distribution and marketing specialist. The exact time depends on the application.
Kraiburg supplies four medical-grade TPEs. They include a translucent general-purpose elastomer for stand-alone parts that is not designed for adhesion to other materials. Another grade designed for adhesion bonds to PC, ABS, PC/ABS, and PETG (polyethylene terephthalate-glycol) resins, and reportedly has no issues with stress cracking at the materials interface. A high-elasticity version has a low coefficient of friction for mechanical components and sealants. The fourth grade reportedly can replace costlier silicone as a bottle seal. Here the material can be punctured with a needle to draw out fluid, after which it reseals itself when the needle is withdrawn.

The PEEK-Optima Ultra-Reinforced distal femoral plate, fabricated of carbon fiber-reinforced PEEK and developed by Victrex Invibio, is used to heal bone trauma (photo courtesy of Invibio).
Typical applications for Kraiburg TPEs include nebulizer masks (whose soft-touch properties improve comfort), syringe stoppers, bottle caps, tubing, and bags. Olano says the company is developing formulations that chemically bond to different resins, a property that will facilitate multi-material molding and applications that specify the elastomers as substrates.
Reinforced Implants
Implants for fracture fixation are a growing and dynamic application for high-performance resins and compounds, which enable devices with higher fatigue life, greater interoperative visibility, and flexible use. One company active in this sector is Victrex Plc, which supplies ketone-based materials through its medical-focused Invibio division for orthopedic trauma devices.
One material from Invibio is PEEK-Optima Ultra-Reinforced, which is used in plates fabricated from PEEK (polyetheretherketone) resin reinforced with continuous PAN-based (polyacrylonitrile) carbon fiber. When used to treat a fractured bone, the plate provides advantages over conventional metal versions (titanium and stainless steel) and has the potential to accelerate healing due to its decreased bending stiffness.
A PEEK-Optima plate reportedly has at least 50 times greater fatigue resistance than metal, can be fabricated with tailored levels of stiffness to meet individual needs, has a modulus of elasticity closer to that of cortical bone than metal, and weighs less than metal versions.
The formulation and fabrication technology that Invibio provides can also help OEMs bring PEEK-Optima Ultra-Reinforced plates to market faster than metal versions and thereby help them reduce costly R&D programs.
Looking Ahead
Resins and compounds will remain vital enabling materials for a growing range of medical and healthcare devices. Research underway, however, suggests that the properties of plastics will soon be applied to the use of microscopic materials such as bacteria and nanoparticles used to treat cell disorders and infirmities at the molecular level within the body. Coated tubing and other implantable devices could be the mechanism by which these treatments are delivered and controlled.
For now, though, product designers, OEMs, and processors can expect to see growing demand for plastics in a multitude of institutional and personal treatment devices, as healthcare procedures become increasingly tailored for lifestyle needs.
Show & Well
Medical device trade fairs are becoming common places where resin suppliers can show off their new grades and applications. For instance, BASF recently promoted a number of materials serving in various healthcare roles at the annual Compamed trade fair in Germany, including the following:
- Thermoplastic polyurethane (TPU) for transparent, thin-walled elements and films: The company’s Elastollan TPU is used for “flexibly foldable” tube elements, balloons, elastically deformable foam suppositories, and drainage tubes, with some parts having a thickness of a few micrometers.
- Non-phthalate plasticizers, like Hexamoll DINCH, a low-migrating alternative to traditional phthalates used in PVC medical applications. The plasticizer is said to be a fit for soft PVC feeding tubes, catheters, breathing masks, and blood bags. The PVC/plasticizer’s technical properties are maintained after sterilization, the company says.
- “Skin-stretching” polyoxymeth-ylene: BASF’s Ultraform S2320 003 PRO’s first application is reportedly in the Skin Stretcher, a device from BioWim GmbH. The device is used to close the skin when treating large wounds following operations or accidents. The resin is said to minimize the friction between parts in contact. In injection molding, the high-flowing material produces parts that are rigid and strong, supplying the resilience and spring characteristics needed by the device, says BASF.
—Ed.
Medical Plastics: Well and Good
Previous Article Next Article
By Pat Toensmeier

BASF has commercialized both flexible and rigid medical grades recently (photo courtesy of BASF).
Plastics have been doing well, and doing good, in medical and healthcare markets for years. Major advances in many medical procedures often go hand-in-hand with developments in resins and compounds, thereby enabling the design, engineering, and cost-efficient production of instruments, devices, and related components that facilitate short- and long-term treatments.
Demographically, the benefits that developments in plastics provide in many areas are especially apparent where aging populations ratchet up demand for innovative treatment systems—and for devices that allow the elderly to meet their medical needs without undue and costly reliance on doctors and hospitals.
Ongoing advances in plastics address another demographic: developing countries where access to hospitals and medical services is limited—or unavailable—for many people, especially children and infants. Devices for testing, vaccination, accurate dispensing of antibiotics and other medicines, and self-medication bring vital treatments to people who live in areas where there is little or no access to healthcare.
The growing range of medical resins and compounds also addresses enhancements to conventional device requirements such as clarity, chemical resistance, sterilization, and of course compliance to mandates from the U.S. FDA, U.S. Pharmacopeia (USP), the European Union’s REACH protocols, ISO standards, adherence to Good Manufacturing Practices (GMP), and other regulations.
These concerns influence the relentless intra-material competition that drives plastics use in medical devices. The market has never stood still in this area, of course, and has always demonstrated that there is no status quo when it comes to material selection. Formulation advancements improve processability, upgrade device performance, and allow part consolidation, multi-material molding, and structural enhancements like thinwalling—often at lower cost than competing materials.
Below are representative examples of suppliers whose new and recent developments in materials affect the design and performance of medical and healthcare devices. The capabilities that these resins and compounds bring to this large, diverse and challenging market showcase the ability of plastics to enhance applications and make possible the devices and procedures that improve individual treatments, and ultimately, the quality of life for people around the world.
High Flow, with Design Flexibility
One area where compounds excel in properties and performance is devices and components that require metal-like stiffness along with high flow that facilitates molding. SABIC Innovative Plastics recently unveiled two compounds that combine high-modulus carbon fiber technology with high-performance resins, for use in disposable or reusable (and sterilizable) parts.
The grades, LNP Lubricomp DCI06APW and LNP Thermocomp EC006AQW are alternatives to metal and fiber-reinforced polymers, says Cathie Hess, director of healthcare marketing. Lubricomp is a 30% fiber-reinforced grade of polycarbonate (PC), and Thermocomp is a 30% reinforced grade of polyetherimide.

LNP Lubricomp is a 30% carbon fiberreinforced PC resin from SABIC for replacing metal in scalpels and other components (photo courtesy of SABIC).
Hess describes Lubricomp as a “high-flow material for high-strength components where the use of metals or other fiber-filled thermoplastics with poor flow can create design and manufacturing challenges.” The compound achieves a balance between design flexibility and ease of manufacturing that enables complex part design and part consolidation, while allowing molders “to reduce waste [and] manufacturing and maintenance costs, and improve cycle times,” she notes.
Target applications for Lubricomp include disposable surgical instruments, medical device housings, and drug-delivery components.
Thermocomp, also touted as an alternative to metal, provides high strength and stiffness, chemical compatibility, and mechanical stability in parts, Hess says. Applications include disposable or reusable surgical instruments, fixation devices like those used to secure broken bones, housings, and patient transport devices.
Importantly, the two compounds allow designers, OEMs, and molders to reduce development and manufacturing costs. “Cost effectiveness continues to be a major driver of design decisions in the healthcare industry,” Hess affirms. “These reinforced materials can help optimize medical device system costs as well as improve processability.”
Tiny, Precision Parts for Drug Delivery
Drug-delivery devices are a major part of the healthcare market. These products allow users to accurately and safely self-medicate without the need for repeat visits to doctors or hospitals, and with little or no disruption to their daily routines. As the design of these devices trends toward ever-more compact, lightweight, and even stylish assemblies, resin suppliers are targeting the market with enhanced materials.
One resin producer that specializes in the drug-delivery device market is DuPont Performance Polymers. William Hassink, global healthcare segment leader, says the global device segment of the market alone (excluding the value of delivery systems with medicine) is huge, accounting for an estimated $60 to $80 billion annually, and growing. When medicine is factored in, the value of these devices could be as much as $400 billion.

Portable drug-delivery systems make increasing use of internal parts molded from resins such as Delrin acetals from DuPont (photo courtesy of DuPont).
DuPont supplies semi-crystalline engineering thermoplastics for these devices, among them grades of polybutylene terephthalate, acetal, and polyamide. A major challenge to the design and engineering of these systems is the ability to accurately mold tiny precision parts that comprise the inner workings of the devices—gears, plungers, and similar components.
“If you open a dry-powder inhaler, you see a complex device for managing one month of doses,” Hassink says. Since the portability of these devices means they will get no bigger than they are now (typically pocket size), and likely will become more compact and lightweight, the precise and reliable operation of interior components, as well as their ability to assume multifunctional roles, are crucial to use and safety.
But that’s not all. Hassink notes that the ability to differentiate devices is often limited by patent protection. In other words, there’s only so much a designer can do with the look and basic operation of a drug-delivery device to set it apart from competitors or improve its manufacture.
One option could be different ways of constructing internal features using multifunctionality and other techniques to set the operation of a device apart from competitive products. Examples Hassink cites include:
- achieving a well-defined “click” from a dose indicator so users know their administration of medicine is accurate;
- internal energy storage for mechanical movements from the addition of a spring or other part; and
- the use of snap fits to improve both the positioning of internal components like pumps and the assembly of devices.
One resin DuPont recently added to address these needs is Delrin SC699, an acetal homopolymer with inherent silicone lubrication. The lubrication ensures that internal parts molded with the grade generate uniform force in the hands of different users, thereby ensuring consistent dosages. The grade also eliminates the need for molders to undertake costly secondary operations to lubricate parts.
The lubricity of SC699 and a companion Delrin grade, PC699, suit them for applications where surfaces slide, roll, or rub against each other. Hassink says the grades additionally have high flow, multi-cavity molding properties, creep resistance, and printability, among other properties. “They work, and they are cost effective.”

Makroblend M525 PC/polyester from Bayer MaterialScience allows designers to develop compact, lightweight housings for wearable medicine pumps (photo courtesy of Bayer).
One OEM that specified SC699 is Ypsomed AG of Switzerland, which uses it for a dose dial sleeve in its UnoPen variable-dose injector pen for insulin and other medicines. The sleeve, positioned between the housing and piston rod, is used by patients to set required doses for injection. It interacts mechanically with the piston rod, which dispenses the medicine. The lubricity of the grade ensures consistent dispensing of accurate doses.
Enhancing Wearable Pumps
Closely related to portable, self-administered drug-delivery systems are wearable pumps that automatically inject insulin, painkillers, and other vital medicines to patients with chronic conditions. These devices, which are typically worn on a belt or under a shirt or blouse, use subcutaneous injection sites to administer medicine, and have battery-powered pumps, timers, alarms, and display windows in compact, lightweight housings.
The resin requirements for these units are different than for self-administered delivery systems. For one thing, says Bruce Fine, North American medical segment market leader at Bayer MaterialScience, the housings need chemical resistance, especially to body oils, lotions, and creams, since they contact a wearer’s skin, and to hospital disinfectants.
They must also be biocompatible. Relevant tests here include ISO-10993-5, for cytotoxicity, and ISO 10993-10, for skin irritation and sensitivity.
Resins must accommodate electrical components and power sources, which usually mean batteries. They don’t need a UL-VO rating but should meet UL 94 horizontal burn requirements.
High-flow properties and thinwalling are important, as is the ability to overmold features like display windows and tubing connections. Color options also figure in resin specification since OEMs want wearable pumps to be stylish and aesthetically pleasing to users.
Bayer officially launched a new grade in February for such applications. Makroblend M525 is a PC/polyester blend that meets all these requirements, and additionally provides toughness, moldability, and dimensional stability. Fine declined to reveal details about the blend beyond saying that the polyester component provides good chemical resistance. PC, of course, is tough, heat resistant, and dimensionally stable.
As with self-administered drug-delivery systems, Fine notes a trend in wearable pumps toward lighter weights, enhanced portability, and simplicity of operation—for “getting as much healthcare as possible delivered outside healthcare settings.”
High-Performance “Cyclic” Elastomers
Clarity and molded-in detail are essential properties in a number of applications, notably flexible membranes, tubing, and other parts that are used in flow-control systems and microfluidic devices. One company with a high-performance elastomer for these products is TOPAS Advanced Polymers Inc., the U.S. business unit of parent TOPAS Advanced Polymers GmbH in Germany.
TOPAS supplies cyclic olefin copolymers, or COCs. These are transparent, amorphous resins copolymerized from norbornene and ethylene with a metallocene catalyst. (TOPAS stands for “thermoplastic olefin polymer of amorphous structure.”) The result is a material with inherent properties such as glass-like optics, heat resistance, and high dimensional stability.
Timothy Kneale, president of TOPAS, points to grade E-140, a COC elastomer that provides a “performance advantage” to applications requiring clarity and molded-in or extruded detail, along with high levels of barrier, purity (low leachables and extractables), and chemical resistance.
“This is not a commodity item,” he notes. “It is specified when ordinary materials will not do the job.”
Though Kneale declines to provide application details, citing non-disclosure agreements with end-users, he explains that the E-140 grade can be used in drug storage or delivery devices and as a flexible membrane in demanding diagnostic and flow-control applications. It can also replace
specialty silicones such as polydimethylsiloxane, which is inert, non-toxic, optically clear, and has good rheological properties. He adds that the E-140 elastomer also lends itself to faster production scale-up than most silicones.
Importantly, the elastomer is compatible in assemblies with a rigid COC, like grade 5013L-10. Among other advantages, this property facilitates multi-material molding and eliminates concerns about cracking and other structural deficiencies that may develop from incompatible materials. The resin, which is also touted for microfluidic applications, reportedly has exceptional clarity, high flow without loss of material strength or optical properties, and heat resistance to 127°C.

TOPAS Advanced Polymers produces a cyclic olefin copolymer elastomer for flexible applications that require high barrier properties, purity, and chemical resistance (photo courtesy of TOPAS).
Kneale says the rigid grade can be molded with “sub-micron details,” which is a plus for small parts. Customers, moreover, report achieving novel molding capabilities like zero draft angles on parts. Grade 5013L-10 also reportedly maintains dimensional tolerances of 0.0004 inch (0.01 mm) in molded parts.
TPEs for Diverse Needs
Elastomeric grades are essential for many applications, among them closures, gaskets, tubing, valves, infusion stoppers, and some forms of packaging. One supplier of medical elastomers, Kraiburg TPE Corp., specializes in styrenic block copolymers, which it markets as Thermolast M. These provide important benefits such as translucence and transparency, low compression set, good adhesion to polypropylene and polyethylene, and phthalate- and latex-free formulations, which avoid end-use concerns.

Kraiburg TPE’s Thermolast M styrene block copolymer elastomers are used in diverse products like tubing, drip chambers, luer locks, and gaskets (photo courtesy of Kraiburg TPE).
While Thermolast M grades have been commercial for some time, a recent formulation development allows for direct body and blood contact on a limited basis, generally 1 to 30 days, says Katherine Olano, distribution and marketing specialist. The exact time depends on the application.
Kraiburg supplies four medical-grade TPEs. They include a translucent general-purpose elastomer for stand-alone parts that is not designed for adhesion to other materials. Another grade designed for adhesion bonds to PC, ABS, PC/ABS, and PETG (polyethylene terephthalate-glycol) resins, and reportedly has no issues with stress cracking at the materials interface. A high-elasticity version has a low coefficient of friction for mechanical components and sealants. The fourth grade reportedly can replace costlier silicone as a bottle seal. Here the material can be punctured with a needle to draw out fluid, after which it reseals itself when the needle is withdrawn.

The PEEK-Optima Ultra-Reinforced distal femoral plate, fabricated of carbon fiber-reinforced PEEK and developed by Victrex Invibio, is used to heal bone trauma (photo courtesy of Invibio).
Typical applications for Kraiburg TPEs include nebulizer masks (whose soft-touch properties improve comfort), syringe stoppers, bottle caps, tubing, and bags. Olano says the company is developing formulations that chemically bond to different resins, a property that will facilitate multi-material molding and applications that specify the elastomers as substrates.
Reinforced Implants
Implants for fracture fixation are a growing and dynamic application for high-performance resins and compounds, which enable devices with higher fatigue life, greater interoperative visibility, and flexible use. One company active in this sector is Victrex Plc, which supplies ketone-based materials through its medical-focused Invibio division for orthopedic trauma devices.
One material from Invibio is PEEK-Optima Ultra-Reinforced, which is used in plates fabricated from PEEK (polyetheretherketone) resin reinforced with continuous PAN-based (polyacrylonitrile) carbon fiber. When used to treat a fractured bone, the plate provides advantages over conventional metal versions (titanium and stainless steel) and has the potential to accelerate healing due to its decreased bending stiffness.
A PEEK-Optima plate reportedly has at least 50 times greater fatigue resistance than metal, can be fabricated with tailored levels of stiffness to meet individual needs, has a modulus of elasticity closer to that of cortical bone than metal, and weighs less than metal versions.
The formulation and fabrication technology that Invibio provides can also help OEMs bring PEEK-Optima Ultra-Reinforced plates to market faster than metal versions and thereby help them reduce costly R&D programs.
Looking Ahead
Resins and compounds will remain vital enabling materials for a growing range of medical and healthcare devices. Research underway, however, suggests that the properties of plastics will soon be applied to the use of microscopic materials such as bacteria and nanoparticles used to treat cell disorders and infirmities at the molecular level within the body. Coated tubing and other implantable devices could be the mechanism by which these treatments are delivered and controlled.
For now, though, product designers, OEMs, and processors can expect to see growing demand for plastics in a multitude of institutional and personal treatment devices, as healthcare procedures become increasingly tailored for lifestyle needs.
Show & Well
Medical device trade fairs are becoming common places where resin suppliers can show off their new grades and applications. For instance, BASF recently promoted a number of materials serving in various healthcare roles at the annual Compamed trade fair in Germany, including the following:
- Thermoplastic polyurethane (TPU) for transparent, thin-walled elements and films: The company’s Elastollan TPU is used for “flexibly foldable” tube elements, balloons, elastically deformable foam suppositories, and drainage tubes, with some parts having a thickness of a few micrometers.
- Non-phthalate plasticizers, like Hexamoll DINCH, a low-migrating alternative to traditional phthalates used in PVC medical applications. The plasticizer is said to be a fit for soft PVC feeding tubes, catheters, breathing masks, and blood bags. The PVC/plasticizer’s technical properties are maintained after sterilization, the company says.
- “Skin-stretching” polyoxymeth-ylene: BASF’s Ultraform S2320 003 PRO’s first application is reportedly in the Skin Stretcher, a device from BioWim GmbH. The device is used to close the skin when treating large wounds following operations or accidents. The resin is said to minimize the friction between parts in contact. In injection molding, the high-flowing material produces parts that are rigid and strong, supplying the resilience and spring characteristics needed by the device, says BASF.
—Ed.