Current FDA-Related Drug Information
Approvals, Submission, and Important Labeling Changes for US Marketed Pharmaceuticals
Danial E. Baker, PharmD, FASHP, FASCP*
Current FDA-Related Drug Information
Approvals, Submission, and Important Labeling Changes for US Marketed Pharmaceuticals
Danial E. Baker, PharmD, FASHP, FASCP*
Current FDA-Related Drug Information
Approvals, Submission, and Important Labeling Changes for US Marketed Pharmaceuticals
Danial E. Baker, PharmD, FASHP, FASCP*
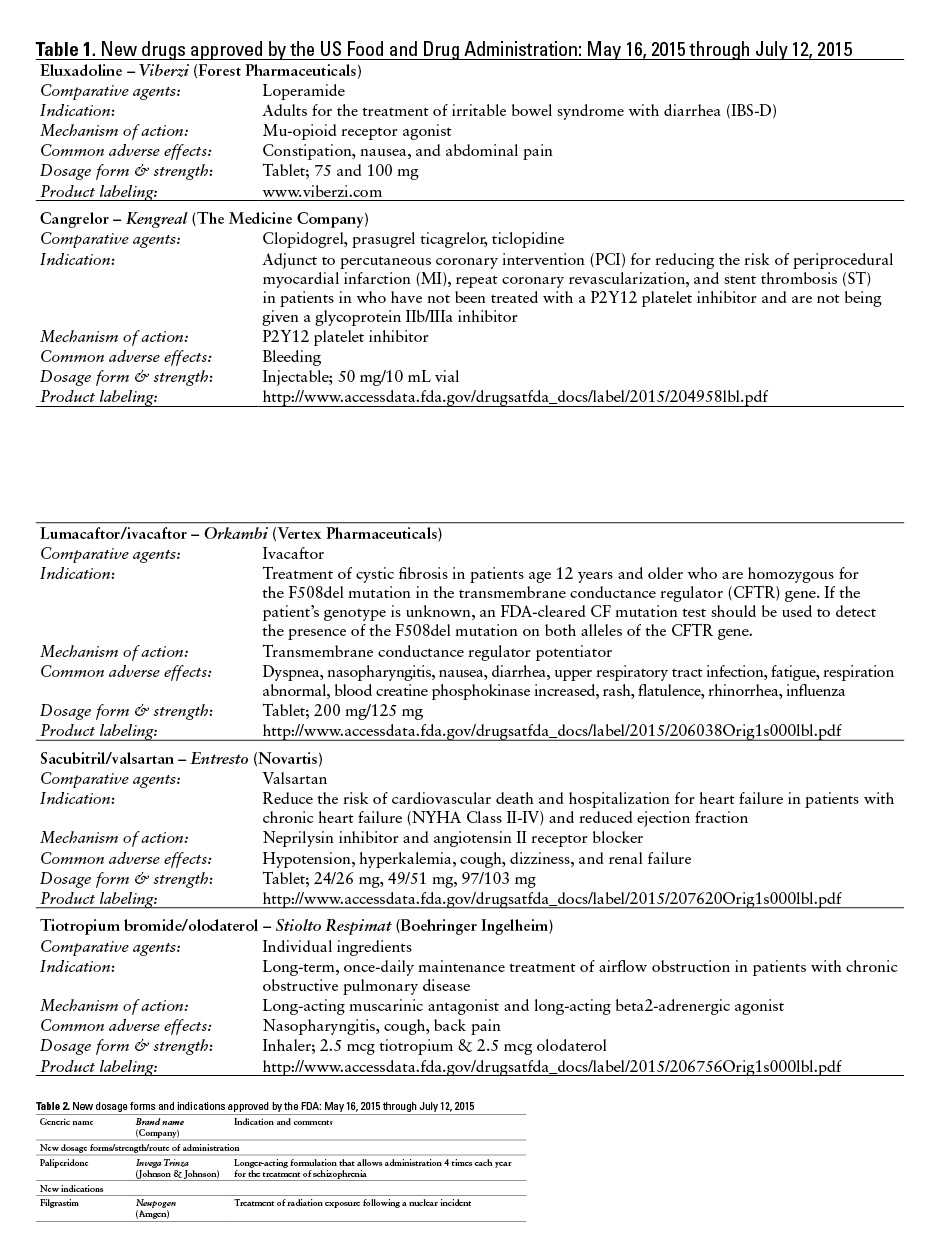
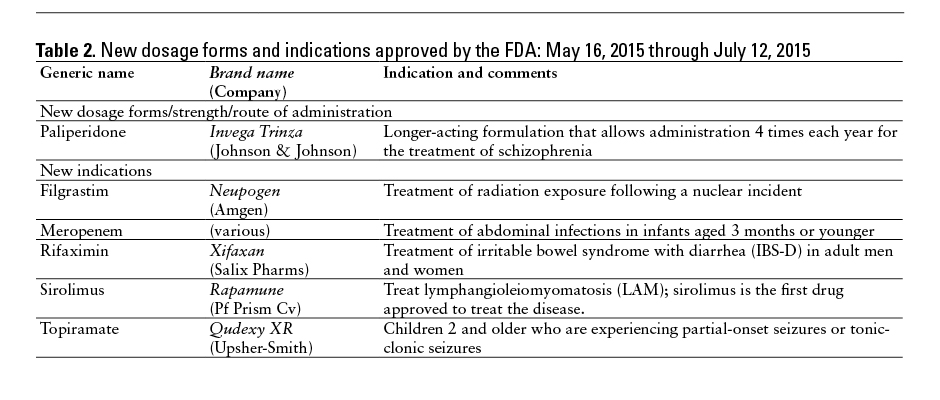
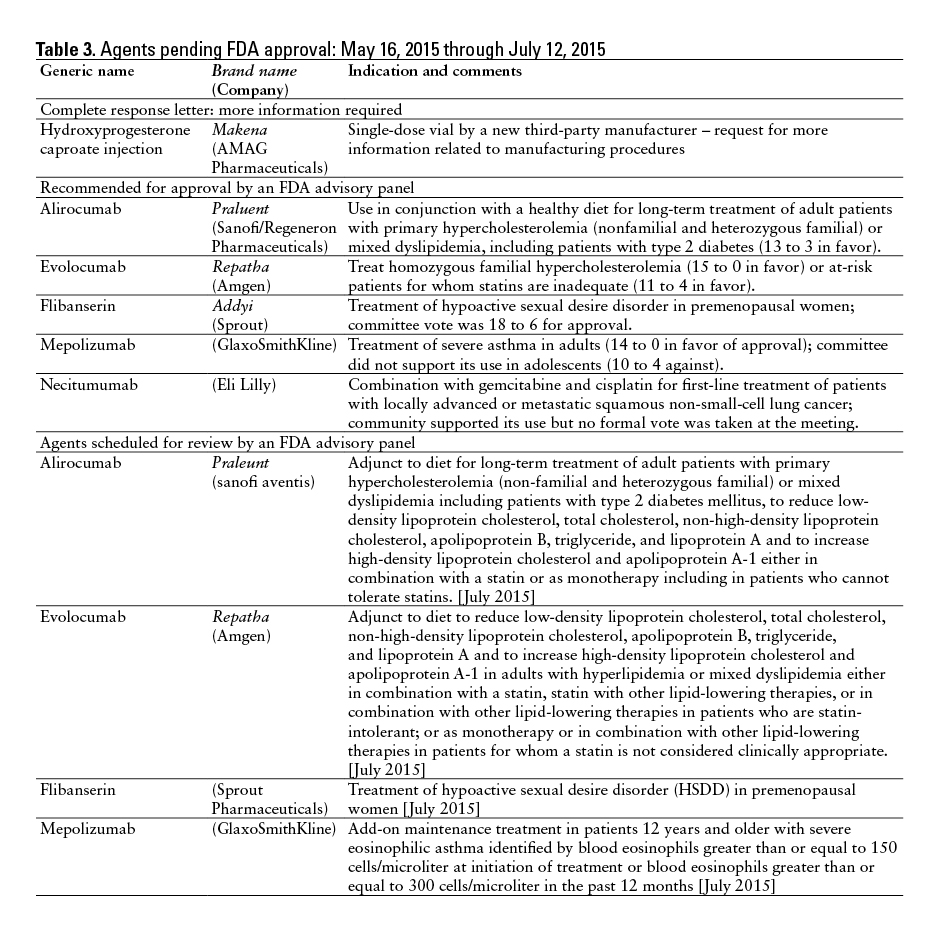
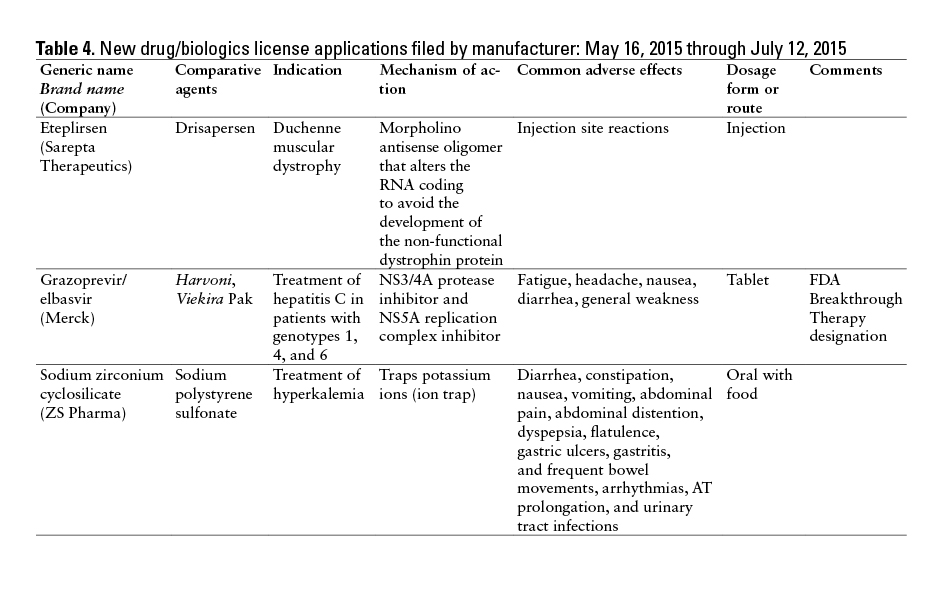
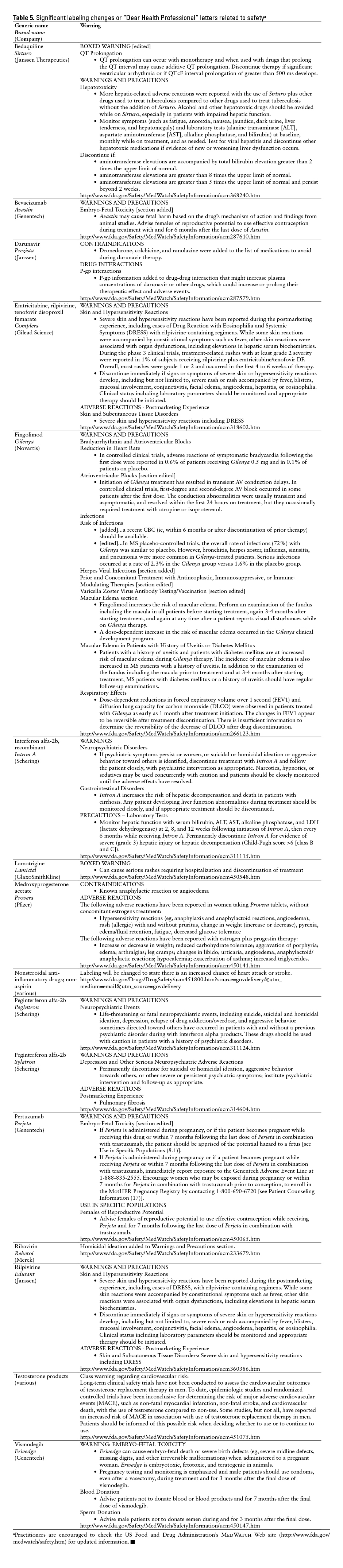
This monthly feature will help readers keep current on new drugs, new indications, dosage forms, and safety-related changes in labeling or use. Efforts have been made to ensure the accuracy of this information; however, if there are any questions, please let me know at danial.baker@wsu.edu.
This monthly feature will help readers keep current on new drugs, new indications, dosage forms, and safety-related changes in labeling or use. Efforts have been made to ensure the accuracy of this information; however, if there are any questions, please let me know at danial.baker@wsu.edu.
This monthly feature will help readers keep current on new drugs, new indications, dosage forms, and safety-related changes in labeling or use. Efforts have been made to ensure the accuracy of this information; however, if there are any questions, please let me know at danial.baker@wsu.edu.
Hosp Pharm 2015;50(8):731–738
2015 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj5008-731










*Director, Drug Information Center, College of Pharmacy, Washington State University Spokane, PO Box 1495, Spokane, WA 99210-1495. ![]()