Original Article
Significant Publications for Pharmacy Nutrition
Support Practice in 2013
Roland N. Dickerson, PharmD, BCNSP*; Vanessa J. Kumpf, PharmD, BCNSP†; Carol J. Rollins, MS, PharmD, BCNSP‡; Eric H. Frankel, MSE, PharmD, BCNSP§; Michael D. Kraft, PharmD, BCNSP§; Todd W. Canada, PharmD, BCNSP**; and Catherine M. Crill, PharmD, BCNSP††
Original Article
Significant Publications for Pharmacy Nutrition Support
Practice in 2013
Roland N. Dickerson, PharmD, BCNSP*; Vanessa J. Kumpf, PharmD, BCNSP†; Carol J. Rollins, MS, PharmD, BCNSP‡; Eric H. Frankel, MSE, PharmD, BCNSP§; Michael D. Kraft, PharmD, BCNSP§; Todd W. Canada, PharmD, BCNSP**; and Catherine M. Crill, PharmD, BCNSP††
Original Article
Significant Publications for Pharmacy Nutrition
Support Practice in 2013
Roland N. Dickerson, PharmD, BCNSP*; Vanessa J. Kumpf, PharmD, BCNSP†; Carol J. Rollins, MS, PharmD, BCNSP‡; Eric H. Frankel, MSE, PharmD, BCNSP§; Michael D. Kraft, PharmD, BCNSP§; Todd W. Canada, PharmD, BCNSP**; and Catherine M. Crill, PharmD, BCNSP††
Abstract
Purpose: To assist the pharmacy clinician engaged in nutrition support in staying current with the most pertinent literature.
Methods: Several experienced board-certified clinical pharmacists in nutrition support compiled a list of publications published in 2013 that they considered to be important to their practice. The citation list was compiled into a Web-based survey whereby pharmacist members of the American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.), GI-Liver-Nutrition Practice Research Network of the American College of Clinical Pharmacy, and the Pharmacy and Pharmacology Section of the Society of Critical Care Medicine were asked to rank each article according to level of importance in their practice.
Results: A total of 30 articles were identified by the author group. Thirty-six participants responded to the survey. The top-ranked papers by participants from the Web-based survey were reviewed by the authors. Due to its high level of importance, the parenteral nutrition safety consensus recommendations article, to be published in 2014 by A.S.P.E.N., was also reviewed. Conclusion: It is recommended that the informed pharmacist, who is engaged in nutrition support therapy, be familiar with the majority of these publications.
Key Words—consensus, enteral nutrition, guidelines, nutrition support, outcomes, parenteral nutrition
Hosp Pharm—2014; 49:717–730
Abstract
Purpose: To assist the pharmacy clinician engaged in nutrition support in staying current with the most pertinent literature.
Methods: Several experienced board-certified clinical pharmacists in nutrition support compiled a list of publications published in 2013 that they considered to be important to their practice. The citation list was compiled into a Web-based survey whereby pharmacist members of the American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.), GI-Liver-Nutrition Practice Research Network of the American College of Clinical Pharmacy, and the Pharmacy and Pharmacology Section of the Society of Critical Care Medicine were asked to rank each article according to level of importance in their practice.
Results: A total of 30 articles were identified by the author group. Thirty-six participants responded to the survey. The top-ranked papers by participants from the Web-based survey were reviewed by the authors. Due to its high level of importance, the parenteral nutrition safety consensus recommendations article, to be published in 2014 by A.S.P.E.N., was also reviewed. Conclusion: It is recommended that the informed pharmacist, who is engaged in nutrition support therapy, be familiar with the majority of these publications.
Key Words—consensus, enteral nutrition, guidelines, nutrition support, outcomes, parenteral nutrition
Hosp Pharm—2014; 49:717–730
Abstract
Purpose: To assist the pharmacy clinician engaged in nutrition support in staying current with the most pertinent literature.
Methods: Several experienced board-certified clinical pharmacists in nutrition support compiled a list of publications published in 2013 that they considered to be important to their practice. The citation list was compiled into a Web-based survey whereby pharmacist members of the American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.), GI-Liver-Nutrition Practice Research Network of the American College of Clinical Pharmacy, and the Pharmacy and Pharmacology Section of the Society of Critical Care Medicine were asked to rank each article according to level of importance in their practice.
Results: A total of 30 articles were identified by the author group. Thirty-six participants responded to the survey. The top-ranked papers by participants from the Web-based survey were reviewed by the authors. Due to its high level of importance, the parenteral nutrition safety consensus recommendations article, to be published in 2014 by A.S.P.E.N., was also reviewed. Conclusion: It is recommended that the informed pharmacist, who is engaged in nutrition support therapy, be familiar with the majority of these publications.
Key Words—consensus, enteral nutrition, guidelines, nutrition support, outcomes, parenteral nutrition
Hosp Pharm—2014; 49:717–730
Hosp Pharm 2014;49(8):717–730
2014 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj4908-717


Staying current with the literature is a requirement for the informed pharmacist who maintains an evidence-based clinical practice. This requirement has become increasingly more challenging to fulfill as a paradigm shift has changed the practice culture of a full-time pharmacy nutrition support specialist to a more integrated model whereby the clinical pharmacist provides pharmacotherapy services along with nutrition support responsibilities. This paradigm shift has arguably been partially attributed to the financially motivated decline in the provision of interdisciplinary nutrition support teams by US hospitals over the past couple of decades.1
As a result of this change, informed clinicians are responsible for staying abreast of numerous therapeutic areas that interface with their clinical practice in addition to nutrition support therapy. Keeping current with the nutrition support literature, even for the pharmacy nutrition support specialist, is a daunting if not overwhelming task. Because nutrition support therapy is integrated with many divergent specialized fields (eg, medicine, surgery, pediatrics, gastroenterology, oncology, nephrology, infectious disease, endocrinology, hepatology, transplantation, trauma, burns, home infusion, critical care, dietetics, nursing, and pharmacy), it is nearly impossible for one individual to screen the plethora of journals each month to seek out those clinical studies, position papers, or clinical guidelines that may enhance or change their clinical practice.
METHODS
To assist pharmacy clinicians engaged in nutrition support in staying current with the most pertinent literature, the primary author of this paper (R.N.D.) invited several clinical pharmacists with a long history of active involvement in pharmacy nutrition support practice and the American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) to provide citations of articles published from January 2013 to December 2013 that resulted in a change or affirmation of their current clinical practice. All invited authors are board-certified nutrition support pharmacists. The duration of individual practice experience of the group members ranges from 13 years to over 30 years. Members of this authorship group have advanced practice roles with direct patient care responsibilities for prescribing enteral and/or parenteral nutrition, laboratory analysis, and pharmacotherapy integrated with nutrition therapy (eg, fluid and electrolytes, prokinetic drugs, insulin, anti-diarrheal and laxative therapy), and some have administrative or supervisory roles with respect to nutrition support therapy. This group has a broad range of practice experiences. Most are acute care-based, but some have long-term care (home parenteral and enteral nutrition) responsibilities. Current practices of the group span the age range from pediatrics to geriatrics. Some members have a diverse patient population, whereas others have a focused patient population (eg, pediatrics, oncology, trauma/thermal injury).
The primary author asked each member of the author group to provide at least 3 citations published from January 2013 to December 2013 that changed or affirmed their current clinical practice. The cited articles had to be either a primary research article from a peer-reviewed journal or a clinical guidelines article from a national or international organization. Only those journal publications available in print were eligible for inclusion. A total of 30 significant publications for pharmacy nutrition support practice were collectively identified.2-31
The citations were compiled into a Web-based survey (www.surveymonkey.com), whereby one of the following rating scales could be selected for each article: definitely will not change/support my practice; will not likely change/support my practice; will likely change/support my practice; definitely changed/supported my practice; or not applicable to my practice. The Web link to this survey was distributed to members of the American College of Clinical Pharmacy (ACCP) GI/Liver/Nutrition and Critical Care Practice Research Networks and posted to the message boards of the Pharmacy section of A.S.P.E.N. and Clinical Pharmacy and Pharmacology section of the Society of Critical Care Medicine. Individual e-mails with a link to the survey were also sent to ACCP GI/Liver/Nutrition PRN members who listed “nutrition” as one of their primary interests in their membership profile. The first posting of the survey was on December 21, 2013, and the second call for participation was on December 30, 2013. The survey was closed on January 6, 2014.
Publications were ranked according to the compiled results of the survey and were prioritized in descending order by the greatest number of responses as “definitely changed/supported my practice” and secondarily by “will likely change/support my practice.” Nomination by survey participants of a publication that was not in the author group’s original compilation was allowed pending evaluation of the article by members of the author group to ensure its quality and practicality.
Guidelines articles utilized the GRADE system for the grading of recommendations, assessment, development, and evaluations.32,33 GRADE methodology incorporates a thorough search of available published literature to answer identified questions and uses a standardized approach to evaluate the strength of evidence. To maintain transparency, most of the guidelines articles provided a table that summarized the body of evidence presented, with recommendations for clinical practice graded based on group consensus. The GRADE system ranks evidence as strong (1) or weak (2) and grades quality from high (A) to very low (D), with some recommendations noted as ungraded (UG). For the A.S.P.E.N. guidelines, no recommendations were usually made when a clinical question had limited available evidence.
RESULTS
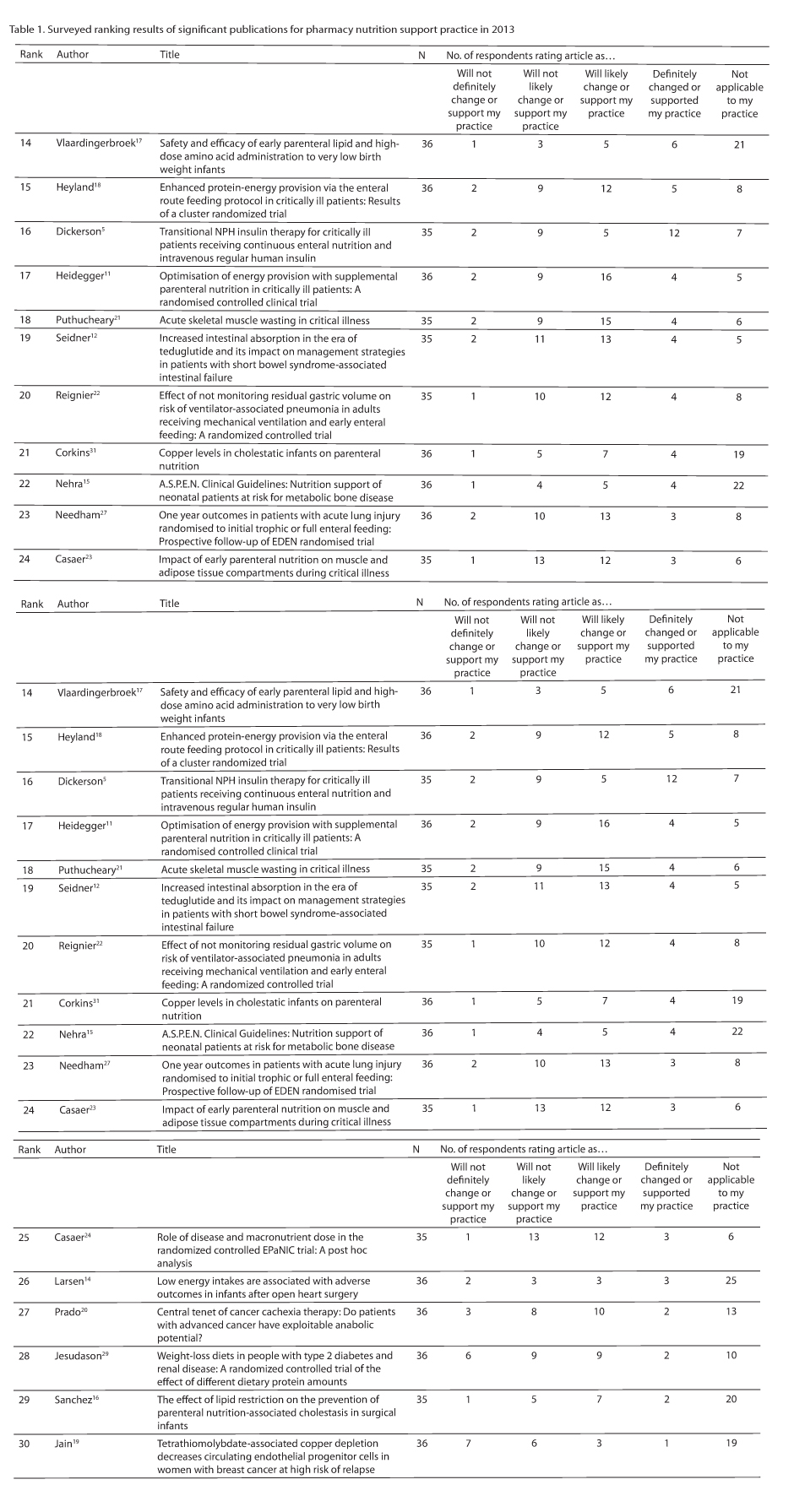
Of the 30 publications collectively selected by the author group, 11 were published in the Journal of Parenteral and Enteral Nutrition (JPEN), 3 were from Critical Care Medicine, another 3 were from the Journal of the American Medical Association, and the remaining 13 publications were from 12 different medical, pediatric, nutrition, and pharmacy journals. Thirty-six participants responded to the survey, of whom 74% claimed to be board-certified nutrition support pharmacists. Seven articles were scored by 35 participants, whereas the remaining 23 articles were ranked by all participants. Forty-three percent of respondents have practiced in pharmacy nutrition support for over 20 years, 20% for 11 to 20 years, 26% for 6 to 10 years, and 11% for less than or equal to 5 years.
Individual rankings, based on relevancy to pharmacy nutrition support clinical practice according to the respondents, are given in Table 1. The highest ranked articles are summarized in the discussion along with a narrative regarding their implications for pharmacy nutrition support practice. Due to its substantial level of relevancy and importance to our practice, the A.S.P.E.N. Parenteral Nutrition Safety Consensus Recommendations article34 was included in the discussion despite not being available in print in 2013 nor available for ranking.
DISCUSSION
1. Choban et al. A.S.P.E.N. clinical guidelines: Nutrition support of hospitalized adult patients with obesity.2
Over one-third of the adult population in the United States is classified as obese and there is a growing obesity problem throughout the world, so the nutrition support clinician must be prepared to manage obese patients. Clinical guidelines to address pertinent clinical questions regarding nutrition support of the hospitalized adult patient with obesity receiving parenteral nutrition (PN) or enteral nutrition (EN) were developed and published by A.S.P.E.N. in 2013.2
The obesity guidelines asked 4 specific questions: (1) Do clinical outcomes vary across levels of obesity in critically ill or hospitalized non–intensive care unit (ICU) patients? (2) How should energy requirements be determined in obese critically ill or hospitalized non-ICU patients? (3) Are clinical outcomes improved with hypocaloric, high-protein diets in hospital patients with obesity? (4) In obese patients who have had a malabsorptive or restrictive surgical procedure, what micronutrients should be evaluated?
Evidence was low for all recommendations except question 2, where evidence was moderate to strong. For question 1 related to clinical outcome, the recommendation for nutrition assessment within 48 hours of admission to the hospital or ICU was considered strong despite a low level of evidence. None of the studies included for evaluation assessed the impact of early nutrition assessment or intervention on outcome; they assessed the effect of obesity on morbidity and mortality. Most studies were retrospective and several had fewer than 100 patients, although a few large studies (>2,000 patients) were also included. Question 2 had the strongest data supporting the recommendations, with various comparisons between predictive equations and measured energy expenditure. As a whole, studies included only a small number of obese subjects. Equipment and procedures used to measure energy expenditure varied between studies, as did the predictive equations that were evaluated. Hypocaloric, high-protein diets were evaluated in question 3. Most studies were small, retrospective, and used an observational study design, although a couple of small randomized controlled trials (RCTs) were also included. Recommendations included adjusting the initial protein provision based on nitrogen balance, which essentially precludes patients with excessive and unmeasurable losses such as severe diarrhea or high output enterocutaneous fistulae. Evidence was low and the recommendations were classified as weak. The same is true for question 4 related to micronutrient evaluation following malabsorptive or restrictive surgical procedures for obesity. Risk of micronutrient deficiency by type of restrictive or malabsorptive procedure is not clearly differentiated in most studies; therefore, it is difficult to determine risk for a specific procedure.
Despite their limitations, the clinical guidelines for hospitalized adult patients with obesity are useful in practice. In particular, the guidelines allow the practitioner to use the best available evidence when determining nutrient goals while also noting that even the best predictive equations35 have only about 70% accuracy. The guidelines consolidate available information related to the 4 specific questions evaluated and allow the practitioner to view the many studies assessed for the guidelines in a summary format.
2. Mancl et al. Tolerability and safety of enteral nutrition in critically ill patients receiving intravenous vasopressor therapy.8
The intent of this study was to retrospectively evaluate the tolerability and safety of EN in critically ill patients receiving intravenous (IV) vasopressor therapy. Adult ICU patients who received concurrent EN and IV vasopressor therapy for 1 hour or more were evaluated. EN tolerance was defined as an absence of gastric residuals greater than or equal to 300 mL, emesis, positive finding on abdominal imaging, or evidence of bowel ischemia/perforation. Two hundred fifty-nine patients received 346 episodes of concomitant EN and IV vasopressor therapy. Overall EN tolerability was 74.9%. Adverse events included rising serum lactate (30.6%), elevated gastric residuals (14.5%), emesis (9.0%), positive finding on kidney/ureter/bladder radiograph (4.3%), and bowel ischemia/perforation (0.9%). An inverse relationship was found between maximum norepinephrine equivalent dose and EN tolerability (12.5 mcg/min for patients who tolerated EN vs 19.4 mcg/min; P = .0009). Patients who tolerated EN were less likely to have received dopamine (63.8% vs 77.6%; P = .018) or vasopressin (58.9% vs 77.9%; P = .0027). The authors concluded that patients receiving IV vasopressor therapy tolerate EN. Tolerability was related to the maximum cumulative vasopressor dose and may be related to the specific vasopressor administered.
The safety and efficacy of provision of EN during vasopressor therapy remains controversial. The reader is referred to an excellent recent review on this topic.36 Although an improvement in cardiac index with pharmacotherapy generally leads to improved splanchnic blood perfusion during shock, the pharmacologic actions of the drug as well as dosage must be considered as these can also result in -perturbations in splanchnic blood flow. The use of an inotrope such as dobutamine has improved splanchnic blood perfusion; however, dopamine (which predominately has inotropic effects at low doses) has had an inconsistent dose-dependent effect on gastric blood flow and may have a detrimental effect on antral contractions.36,37 Most studies suggest, albeit with conflicting data, that epinephrine, norepinephrine, and vasopressin tend to decrease splanchnic blood flow in a dose-dependent manner.
The authors acknowledge that their findings should be interpreted with caution. Although only 3 patients developed bowel ischemia and/or intestinal perforation, this complication is potentially life-threatening and demands a high level of attention from the clinician. Most of these intestinal ischemia and bowel perforation case reports stem from surgical or trauma patients where intra-abdominal surgery was indicated; only 6% of Mancl et al’s patient population were surgical/trauma patients. Despite these caveats, this study suggests that for the majority of ICU patients, administration of intragastric EN during low, stable doses of vasopressors may be possible with minimal risk for bowel necrosis. The risk of EN intolerance may be increased by use of dopamine or vasopressin as compared to other vasopressors. However, vigilant close monitoring for signs and symptoms of gastric feeding intolerance is mandatory regardless of the specific vasopressor administered.
3. Dickerson et al. Hypocaloric, high-protein -nutrition therapy in older vs younger critically ill patients with obesity.4
Data from unstressed, healthy older subjects indicate that aging is associated with an increased requirement for protein compared to that required in younger subjects to achieve nitrogen equilibrium.38 Provision of a hypocaloric nutrition regimen to obese patients necessitates a high-protein intake to achieve nitrogen equilibrium. One small study in surgical patients with obesity suggested that older patients had reduced nutritional efficacy from hypocaloric, high-protein PN as exhibited by a lower nitrogen balance.39 Additionally, age-associated renal dysfunction may limit the amount of protein that can be safely provided. Thus, it is not clear whether sufficient protein can be safely given to older, critically ill patients with obesity who are prescribed a hypocaloric regimen.
This study examined whether older, critically ill trauma patients with obesity can achieve nitrogen equilibrium and have positive clinical outcomes similar to younger obese patients during hypocaloric, high-protein nutrition therapy. Adult patients with traumatic injury and obesity (body mass index [BMI] ≥30 kg/m2) admitted to the ICU were retrospectively evaluated. Patients received hypocaloric, high-protein nutrition therapy (≤25 kcal/kg ideal body weight [IBW]/d and ≥2 g/kg IBW/d of protein) for at least 10 days. Patients were stratified as older (≥60 years) or younger (18-59 years). Seventy-four patients (33 older, 41 younger) were studied. Older and younger patients were similar in BMI (35 ± 6 vs 35 ± 5 kg/m2; P = .876) and injury severity (injury severity scores of 27 ± 10 vs 29 ± 13; P = .461). When given isonitrogenous regimens (2.3 ± 0.2 g/kg IBW/d), nitrogen balance was similar between older and younger patients (-3.2 ± 5.7 g/d vs -4.9 ± 9.0 g/d; P = .363). Older patients experienced a greater mean serum urea nitrogen concentration than younger patients (30 ± 14 mg/dL vs 20 ± 9 mg/dL; P = .001) during nutrition therapy. Clinical and nutritional outcomes were not different between groups.
The investigators indicated that older critically ill trauma patients exhibited an equivalent net protein response compared to younger patients during hypocaloric, high-protein nutrition therapy when adequate protein intake was given. These data confirmed that a hypocaloric, high-protein nutrition regimen is safe and efficacious when used for older critically ill patients with obesity. However, it was noted that older patients are at greater risk for developing an increase in serum urea nitrogen concentration and close monitoring is warranted.
4. McMahon et al. A.S.P.E.N. clinical guidelines: Nutrition support of adult patients with hyperglycemia.10
Clinical guidelines to address pertinent clinical questions regarding glucose management in adult hospitalized patients receiving PN or EN have been developed by A.S.P.E.N.10 The first question asks, “What is the desired blood glucose range in adult hospitalized patients receiving nutrition support?” This is a relevant clinical question because treatment to minimize the adverse outcomes associated with hyperglycemia must be balanced by risk of hypoglycemia that can result from intensive insulin therapy for glucose control. The recommendation for a target blood glucose goal range of 140 to 180 mg/dL is graded as strong. The body of evidence to support this recommendation is based on 7 RCTs, 2 historical control trials, and 5 retrospective reviews that compared tight to standard glycemic control. The next question asks, “How is hypoglycemia defined in adult hospitalized patients receiving nutrition support?” Concern for identifying an accepted definition is raised because certain studies done in critically ill patients define hypoglycemia as less than 40 mg/dL and this low threshold may compromise patient safety. The guidelines recommend that hypoglycemia be defined as a blood glucose concentration less than 70 mg/dL, and this is consistent with other available practice guidelines. The body of evidence to support this recommendation is graded as low quality based on its retrospective design, but the overall recommendation is graded as strong due to the significant patient risk associated with hypoglycemia in the setting of intensive glycemic control. The final question asked in this guideline is, “Should diabetes-specific enteral formulas be used for hospitalized patients with hyperglycemia?” Although widely used in this patient population, the impact of enteral formulas with lower carbohydrate and higher monounsaturated fat content (with or without added fiber) on glycemic control in hospitalized patients is questioned. Most available studies are conducted in the outpatient or long-term care setting, and the 2 RCTs in hospitalized patients that are included for evaluation demonstrate conflicting results. Unfortunately, the working group was unable to provide practice recommendations due to the low quality of available evidence.
5. Tenner et al. American College of Gastroenterology guideline: Management of acute pancreatitis.9
The definition and classification of acute pancreatitis has changed significantly since previously published guidelines from 1992. This article discusses the diagnosis, etiology, and severity of acute pancreatitis and then discusses management of early and complicated acute pancreatitis. Recommendations were made in regard to diagnosis, etiology, initial assessment and risk stratification, initial management, the use of endoscopic retrograde cholangiopancreatography, antibiotics, nutrition, and surgery. Of particular interest to pharmacists working in nutrition support is the recommendation for checking the serum triglyceride concentration and the need for early aggressive hydration for the first 12 to 24 hours.
Recommendations are made for immediate oral feeding with a low-fat solid diet for mild acute pancreatitis once the abdominal pain has resolved and in the absence of nausea and vomiting. A low-fat solid diet appears to be as safe as a clear liquid diet. In all cases of acute pancreatitis, a recommendation is made to avoid PN due to infections and other complications unless the enteral route is not available, not tolerated, or not able to meet caloric requirements. The final recommendation declaring comparable efficacy and safety for nasogastric and nasojejunal feeding may be controversial. It has been preferred to administer jejunal feedings beyond an anatomical loci (ligament of Trietz) where proximal feedings are known to stimulate exocrine pancreatic response. This is consistent with the traditional approach to provide pancreatic “rest” to diminish further injury and allow recovery. This traditional approach requires evidential support and is being challenged.40 A separate 2013 meta-analysis pooled 3 RCTs that supported the safety of early jejunal feeding and also suggests the need for additional study.41 The authors of these guidelines acknowledge that additional evidence is currently being accumulated in a large multicenter trial sponsored by the National Institutes of Health. Currently, results of this trial are not available and it is hoped that this large trial will provide clarity regarding gastric versus jejunal feeding for these patients.
6. Dickerson et al. Safety and efficacy of intravenous hypotonic 0.225% sodium chloride infusion for the treatment of hypernatremia in critically ill patients.3
Significant hypernatremia, acquired in the ICU, has been associated with increased mortality. It has been common in clinical practice at some institutions to intravenously administer hypotonic 0.225% sodium chloride (one-quarter normal saline [¼ NS]) when the critically ill patient is unresponsive to or unable to receive intragastric water administration or dextrose-containing, low sodium IV fluids. Hypotonic ¼ NS has to be compounded by the pharmacy service and is not commercially available in the United States presumably due to its low tonicity and the theoretical risk for causing intravascular hemolysis. Despite its common use in practice and recommendation for its use in the literature, data to support the safety and efficacy for its use in clinical practice are lacking.
The purpose of this study was to evaluate the safety and efficacy of central venous administration of ¼ NS infusion for ICU patients with hypernatremia. Critically ill, adult patients with traumatic injuries and hypernatremia (serum sodium concentration >150 mEq/L) who received ¼ NS were retrospectively studied. The ¼ NS infusion was given at 1.5 ± 1.0 L/d for 4.6 ± 1.6 days. Serum sodium concentration decreased from 156 ± 4 to 143 ± 6 mEq/L (P < 0.001) over 3 to 7 days. Plasma free hemoglobin concentration, a marker of intravascular hemolysis, increased from 4.9 ± 5.4 mg/dL pre infusion to 8.9 ± 7.4 mg/dL after 2.6 ± 1.3 days of continuous IV infusion of ¼ NS in 10 patients (P = .055). An additional 10 patients, without a baseline plasma free hemoglobin concentration determination, had a plasma free hemoglobin concentration of 10.2 ± 9.0 mg/dL during the infusion. Hematocrit and hemoglobin concentrations decreased (26% ± 3% to 24% ± 2%, P < .001, and 9.1 ± 1.1 to 8.2 ± 0.8 g/dL, P < .001, respectively). The authors concluded that IV ¼ NS was effective for decreasing serum sodium concentration, and evidence for minor hemolysis warrants further research to establish its safety before its routine use can be recommended.
This study is limited by the small number of patients and lack of pre-infusion plasma free hemoglobin concentration determinations for half of the patients. Additionally, it is unclear whether the observed minor hemolysis was clinically relevant. Because of these minor safety concerns, Dickerson et al3 have abandoned the use of IV ¼ NS in lieu of 5% dextrose in water (D5W) or D5 ¼ NS with additional insulin therapy if necessary.
7. Heyland et al. A randomized trial of glutamine and antioxidants in critically ill patients.7
Critically ill patients demonstrate substantial oxidative stress. In a multicenter, blinded factorial design, 1,223 patients were randomized to receive placebo, glutamine (0.35 g/kg ideal body weight/d intravenously and 30 g/d enterally), an antioxidant cocktail (500 mcg/d of IV selenium plus enteral administration of 300 mcg/d of selenium, 20 mg of elemental zinc, 10 mg of beta-carotene, 500 mg of vitamin E, and 1500 mg of vitamin C), or both glutamine and antioxidants within 24 hours after admission to the ICU. Patients were included if they had 2 or more organ failures related to their acute illness as defined by PaO2/FiO2 less than or equal to 300, vasopressor therapy, acute kidney injury, or thrombocytopenia (platelets <50,000 cells/mm3). Those with liver disease, traumatic brain injury, thermal injury of greater than 30% total body surface area, cardiothoracic surgery, seizures, or a body weight less than 50 kg or greater than 200 kg were excluded. The supplements were provided continuously and administered for a maximum of 28 days or until discharge from the ICU. There was a trend toward increased mortality at 28 days among patients who received glutamine as compared with those who did not receive glutamine (32.4% vs 27.2%; adjusted odds ratio [aOR], 1.28; 95% confidence interval [CI], 1.00 to 1.64; P = .05). In-hospital mortality and mortality at 6 months were significantly higher among those who received glutamine than those who did not. Glutamine had no effect on rates of organ failure or infectious complications. Antioxidants had no effect on 28-day mortality (30.8% vs 28.8% with no antioxidants; aOR, 1.09; 95% CI, 0.86 to 1.40; P = .48) or any other secondary endpoint. The authors concluded that early provision of glutamine or antioxidants did not improve clinical outcomes and glutamine administration was associated with an increase in mortality among critically ill patients with multi-organ failure.
This article illustrates that aggressive glutamine dosing may be harmful when used in the critically ill patient with multiple organ dysfunction syndrome. Forty percent of patients had renal dysfunction. The dose of glutamine was about 65 g daily or approximately 0.7 to 0.8 g/kg/d for many patients in this study. This glutamine dose significantly exceeded the 0.3 to 0.5 g/kg/d doses that were previously shown to be of benefit.42,43 The investigators also assumed that critically ill patients would be glutamine depleted; however, in a subset of 66 patients, only 31% of patients exhibited a low baseline (pretreatment) plasma glutamine concentration. Their data may have also been skewed in that the number of patients with more than 2 failing organs at baseline was higher in the groups receiving glutamine versus not receiving glutamine (187 vs 148), which likely contributed to a higher mortality. Finally, 79% of the patient population was medical ICU patients. The current body of literature, performed in smaller studies, indicates that glutamine supplementation appears to be beneficial for thermally injured and trauma patients.42-45 However, only 3% of the study population were trauma patients, and those with significant thermal injury were excluded. Due to these unfavorable results, the principal investigator has recommended, in a separate publication, that glutamine supplementation be reserved for thermally injured or trauma patients at a dosage of 0.35 to 0.5 g/kg/d.46 We would also suggest that glutamine supplementation not be given to patients with significant renal or hepatic disease.
8. Dellinger et al. Surviving sepsis campaign: I-nternational guidelines for management of severe sepsis and septic shock: 2012.25
This publication is an update to the Surviving Sepsis Campaign Guidelines for Management of Severe Sepsis and Septic Shock47 from 2008 including sections focusing on selenium, glycemic control, and nutrition support in adults and pediatrics. The recommendations related to nutrition support were categorized as those targeting the general care of the critically ill and a priority in severe sepsis.
Intravenous selenium is not recommended in the guidelines to treat severe sepsis (grade 2C), despite an observed reduction in plasma selenium concentrations in septic patients. This was based on its lack of effect in reducing ICU or hospital mortality, shock reversal, days on mechanical ventilation, renal replacement therapy, days of antibiotic use, or length of hospitalization. Additionally, the optimal dose of selenium and dosage route in severe sepsis remains to be determined; however, the guidelines do recommend continuing low-dose selenium as a component of standard nutrition support in these patients.
A protocol approach to blood glucose (BG) management in patients with severe sepsis using IV insulin is recommended when 2 consecutive blood glucose levels are greater than 180 mg/dL with an upper target blood glucose level less than or equal to 180 mg/dL (grade 1A). This recommendation is based upon the lack of reduction in mortality and potential for increased mortality among heterogeneous ICU populations with intensive insulin therapy targeting blood glucose levels less than or equal to 110 mg/dL. Monitoring is recommended every 1 to 2 hours until blood glucose levels and insulin infusion rates are stable, then every 4 hours is considered reasonable (grade 1C). It should be noted that the continuation of insulin infusions in the critically ill, especially with cessation of nutrition, is a risk factor for hypoglycemia.48 The authors note that blood glucose levels obtained with point-of-care testing of capillary blood may not accurately estimate arterial or plasma glucose values (ungraded) in this patient population.
Oral or enteral (if necessary) nutrition is preferred within the first 48 hours after the diagnosis of severe sepsis or septic shock as opposed to either complete starvation or provision of IV dextrose (grade 2C). This recommendation is based on the lack of clinical trials of early feeding in septic patients. Low-dose feeding (approximately 500 kcal/day) is suggested in the first week after severe sepsis or septic shock (grade 2B). The observation of no harm, but reduced incidence of infectious complications, mechanical ventilator days, and ICU and hospital days, was the basis for this degree of underfeeding. The use of IV dextrose and EN is recommended over PN alone or in combination with EN within the first week (grade 2B). There does not appear to be any direct evidence that PN is beneficial or harmful within the first 48 hours of sepsis. Of note, a recent trial from Switzerland of supplemental PN started on ICU day 4 with mixed medical and surgical ICU patients (approximately 20% with shock) has shown an 11% reduction in nosocomial infections between hospital days 9 and 28 for patients unable to reach more than 60% of their EN goal by ICU day 3.11 This is one of the first trials to demonstrate supplemental PN with partial EN to deliver a mean of 28 kcal/kg/day with 1.2 g protein/kg/day compared with 20 kcal/kg/day with 0.8 g protein/kg/day via EN alone on ICU days 4 through 8 may be beneficial.11 The last recommendation regarding adult nutrition support suggested avoiding specific immunomodulating (eg, arginine, glutamine, eicosapentaenoic acid, gamma-linolenic acid) products in severe sepsis (grade 2C). Regarding nutrition support in pediatric patients with severe sepsis or septic shock, EN should be utilized if tolerated, otherwise PN should be provided for those patients unable to tolerate enteral feeding (grade 2C). This is primarily based upon the glucose needs of children with sepsis.
These guidelines, as related to nutrition support, show weak strength of the existing published ICU studies, noting that most had inconsistent results and problems with subgroup analyses, as well as heterogeneity. The management of glycemic control in the ICU patient with severe sepsis or septic shock may have a greater impact than the nutrition provided based upon the published literature.
9. Klang et al. Osmolality, pH, and compatibility of selected oral liquid medications with an enteral nutrition product.28
In an effort to prevent the clogging of the feeding tube from incomplete crushing of a solid medication dosage form, liquid or suspended medication products are preferred. However, liquid medications can present issues of intolerance and incompatibility when administered via a feeding tube mainly due to osmolality, sorbitol content, or pH value. This study examined 62 liquid formulations for their osmolality, pH, and physical compatibility with EN formulas. Although the osmolality of common liquid medications were evaluated over 20 years ago,49 this article provides an important update for practitioners; those past findings are potentially outdated as newer medications are available and excipient ingredient concentrations may have changed. Seventeen of the 62 tested products had an osmolality greater than 5,000 mOsm/kg. Depending on the dose, the osmotic load of a liquid medication may cause cramping and diarrhea, particularly when administered directly into the small bowel. Common liquid medications with a high osmotic load (which considers osmolality and dosage volume) included acetaminophen, potassium chloride, and metoclopramide. The pH value may be predictive of potential interactions with the EN formula that can result in tube clogging, as acidic liquids are especially reactive with enteral formulas. Common liquid medications that caused enteral formula clumping in vitro include acetaminophen, ferrous sulfate, levofloxacin, and metoclopramide.
10. Ayers et al. Parenteral nutrition safety consensus recommendations.34
PN is a life-saving therapy for patients who cannot maintain adequate nutritional intake via oral or EN. However, PN is a complex and high-risk therapy with many significant safety concerns and potential complications. In addition, PN is used across the spectrum of patient care from critical care/intensive care to the home setting, further adding to the complexity of the PN-use process. The A.S.P.E.N. Safe Practices for Parenteral Nutrition is an essential document for any health care professional involved in the care of patients receiving PN.50 However, revisions were needed given that this document was last revised and published in 2004. In 2011, A.S.P.E.N. convened a group of experts and leaders in nutrition support along with representatives from other organizations at a PN safety summit to address safety issues and identify processes to improve the safety of PN. Findings from this summit and questions regarding PN safety were divided into 2 groups of questions: those that could be answered and supported by evidence using the GRADE process, and those for which evidence did not support GRADE-level recommendations. The PN Safety Task Force was charged with addressing those questions where evidence did not support GRADE-level recommendations and with developing consensus recommendations regarding PN safety and opportunities for future research based on expert opinion. The PN Safety Task Force was comprised of experts and leaders in nutrition support and within A.S.P.E.N. representing all of the major disciplines and practice areas, and all members are certified in nutrition support by one of the major certification organizations (eg, the Board of Pharmacy Specialties [BPS], the National Board of Nutrition Support Certification [NBNSC], or the National Board of Physician Nutrition Specialties [NBPNS]).
The PN safety consensus recommendations document is separated into 4 sections: Prescribing and Communicating the PN Order, PN Order Review and Verification, Compounding, and PN Administration. Each section is formatted with questions developed from the PN Safety Summit, the recommendations to address those questions and rationale supporting the recommendations, and a list of topics for future research. A summary of key recommendations includes:
- Using standardized processes and developing policies, procedures, and protocols for all aspects of the PN-use process
- Providing standardized education and competency assessments (at least annually) for all health care professionals involved in the PN-use process
- Using integrated electronic systems for PN order entry, order verification, and automated compounding devices (ACDs)
- Avoiding handwritten, verbal, and telephone orders; unapproved abbreviations; and manual transcription of PN orders
- Ordering and listing PN ingredients in amounts per day (or amounts per kg per day); ordering electrolytes as the complete salt rather than individual ions
- Including required information on PN order templates and PN labels
- Using standardized PN order templates, and matching the sequence of ingredients in order templates, ACDs, and PN labels
- Using clinical decision support and warnings within electronic systems
- Developing processes to manage and communicate information about PN-related medication shortages
- Adopting and complying with standards within United States Pharmacopeia (USP) Chapter
- Documenting appropriate information at all steps in the PN-use process
- Including clinicians with expertise in nutrition support in all aspects of the PN-use process.
These consensus recommendations are important for all pharmacists and health care professionals to improve the safety of PN therapy. However, the recommendations are limited because they are largely based on expert opinion and extrapolated from other literature. This leaves significant opportunities to generate further data to either support or refute these recommendations.
CONCLUSION
With the volume of publications and the appearance of these publications in differing journals, it is extremely difficult for the pharmacist to stay current with studies applicable to pharmacy nutrition support practice. Although only the “top” rated papers were discussed, the other identified articles may be important to an individual’s clinical practice depending on patient population and the role of the pharmacist at a specific institution. Thus, it is recommended that the informed pharmacist, engaged in nutrition support therapy, be familiar with the majority of these publications as applicable to their clinical practice.
REFERENCES
- Schneider PJ. Nutrition support teams: An evidence-based practice. Nutr Clin Pract. 2006;21:62-67.
- Choban P, Dickerson R, Malone A, et al. A.S.P.E.N. clinical guidelines: Nutrition support of hospitalized adult patients with obesity. J Parenter Enteral Nutr. 2013;37:714-744.
- Dickerson RN, Maish GO 3rd, Weinberg JA, Croce MA, Minard G, Brown RO. Safety and efficacy of intravenous hypotonic 0.225% sodium chloride infusion for the treatment of hypernatremia in critically ill patients. Nutr Clin Pract. 2013;28:400-408.
- Dickerson RN, Medling TL, Smith AC, et al. Hypocaloric, high-protein nutrition therapy in older vs younger critically ill patients with obesity. J Parenter Enteral Nutr. 2013;37:
342-351. - Dickerson RN, Wilson VC, Maish GO 3rd, Croce MA, Minard G, Brown RO. Transitional NPH insulin therapy for critically ill patients receiving continuous enteral nutrition and intravenous regular human insulin. J Parenter Enteral Nutr. 2013;37:506-516.
- Frankenfield DC, Ashcraft CM, Galvan DA. Prediction of resting metabolic rate in critically ill patients at the extremes of body mass index. J Parenter Enteral Nutr. 2013;37:361-367.
- Heyland D, Muscedere J, Wischmeyer PE, et al. A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med. 2013;368:1489-1497.
- Mancl EE, Muzevich KM. Tolerability and safety of enteral nutrition in critically ill patients receiving intravenous vasopressor therapy. J Parenter Enteral Nutr. 2013;37:641-651.
- Tenner S, Baillie J, DeWitt J, Vege SS, American College of Gastroenterology. American College of Gastroenterology guideline: Management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400-1416.
- McMahon MM, Nystrom E, Braunschweig C, et al. A.S.P.E.N. clinical guidelines: Nutrition support of adult patients with hyperglycemia. J Parenter Enteral Nutr. 2013;37:23-36.
- Heidegger CP, Berger MM, Graf S, et al. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: A randomised controlled clinical trial. Lancet. 2013;381:385-393.
- Seidner DL, Schwartz LK, Winkler MF, Jeejeebhoy K, Boullata JI, Tappenden KA. Increased intestinal absorption in the era of teduglutide and its impact on management strategies in patients with short bowel syndrome-associated intestinal failure. J Parenter Enteral Nutr. 2013;37:201-211.
- Doig GS, Simpson F, Sweetman EA, et al. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: A randomized controlled trial. JAMA. 2013;309:2130-2138.
- Larsen BM, Goonewardene LA, Field CJ, et al. Low energy intakes are associated with adverse outcomes in infants after open heart surgery. J Parenter Enteral Nutr. 2013;37:254-260.
- Nehra D, Carlson SJ, Fallon EM, et al. A.S.P.E.N. clinical guidelines: Nutrition support of neonatal patients at risk for metabolic bone disease. J Parenter Enteral Nutr. 2013;37:
570-598. - Sanchez SE, Braun LP, Mercer LD, Sherrill M, Stevens J, Javid PJ. The effect of lipid restriction on the prevention of parenteral nutrition-associated cholestasis in surgical infants. J Pediatr Surg. 2013;48:573-578.
- Vlaardingerbroek H, Vermeulen MJ, Rook D, et al. Safety and efficacy of early parenteral lipid and high-dose amino acid administration to very low birth weight infants. J Pediatr. 2013;163:638-644.
- Heyland DK, Murch L, Cahill N, et al. Enhanced protein-energy provision via the enteral route feeding protocol in critically ill patients: Results of a cluster randomized trial. Crit Care Med. 2013;41:2743-2753.
- Jain S, Cohen J, Ward MM, et al. Tetrathiomolybdate-associated copper depletion decreases circulating endothelial progenitor cells in women with breast cancer at high risk of relapse. Ann Oncol. 2013;24:1491-1498.
- Prado CM, Sawyer MB, Ghosh S, et al. Central tenet of cancer cachexia therapy: Do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr. 2013;98:1012-1019.
- Puthucheary ZA, Rawal J, McPhail M, et al. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591-1600.
- Reignier J, Mercier E, Le Gouge A, et al. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: A randomized controlled trial. JAMA. 2013;309:249-256.
- Casaer MP, Langouche L, Coudyzer W, et al. Impact of early parenteral nutrition on muscle and adipose tissue compartments during critical illness. Crit Care Med. 2013;41:2298-2309.
- Casaer MP, Wilmer A, Hermans G, Wouters PJ, Mesotten D, Van den Berghe G. Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: A post hoc analysis. Am J Respir Crit Care Med. 2013;187:247-255.
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580-637.
- Fox LM, Wilder AG, Foushee JA. Physical compatibility of various drugs with neonatal total parenteral nutrient solution during simulated Y-site administration. Am J Health Syst Pharm. 2013;70:520-524.
- Needham DM, Dinglas VD, Bienvenu OJ, et al. One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: Prospective follow-up of EDEN randomised trial. BMJ. 2013;346:f1532.
- Klang M, McLymont V, Ng N. Osmolality, pH, and compatibility of selected oral liquid medications with an enteral nutrition product. J Parenter Enteral Nutr. 2013;37:689-694.
- Jesudason DR, Pedersen E, Clifton PM. Weight-loss diets in people with type 2 diabetes and renal disease: A randomized controlled trial of the effect of different dietary protein amounts. Am J Clin Nutr. 2013;98:494-501.
- Olveira G, Tapia MJ, Ocon J, et al. Parenteral nutrition-associated hyperglycemia in non-critically ill inpatients increases the risk of in-hospital mortality (multicenter study). Diabetes Care. 2013;36:1061-1066.
- Corkins MR, Martin VA, Szeszycki EE. Copper levels in cholestatic infants on parenteral nutrition. J Parenter Enteral Nutr. 2013;37:92-96.
- Druyan ME, Compher C, Boullata JI, et al. Clinical guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients: Applying the GRADE system to development of A.S.P.E.N. clinical guidelines. J Parenter Enteral Nutr. 2012;36:77-80.
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924-926.
- Ayers P, Adams S, Boullata J, et al. A.S.P.E.N. parenteral nutrition safety consensus recommendations. J Parenter Enteral Nutr. 2014;38(3):296-333.
- Frankenfield D. Validation of an equation for resting metabolic rate in older obese, critically ill patients. J Parenter Enteral Nutr. 2011;35:264-269.
- Wells DL. Provision of enteral nutrition during vasopressor therapy for hemodynamic instability: An evidence-based review. Nutr Clin Pract. 2012;27:521-526.
- Dive A, Foret F, Jamart J, Bulpa P, Installe E. Effect of dopamine on gastrointestinal motility during critical illness. Intensive Care Med. 2000;26:901-907.
- Campbell WW, Johnson CA, McCabe GP, Carnell NS. Dietary protein requirements of younger and older adults. Am J Clin Nutr. 2008;88:1322-1329.
- Liu KJ, Cho MJ, Atten MJ, et al. Hypocaloric parenteral nutrition support in elderly obese patients. Am Surg. 2000;66:394-400.
- Spanier BW, Bruno MJ, Mathus-Vliegen EM. Enteral nutrition and acute pancreatitis: A review. Gastroenterol Res Pract. 2011;2011.
- Chang YS, Fu HQ, Xiao YM, Liu JC. Nasogastric or nasojejunal feeding in predicted severe acute pancreatitis: A meta-analysis. Crit Care. 2013;17:R118.
- Garrel D, Patenaude J, Nedelec B, et al. Decreased mortality and infectious morbidity in adult burn patients given enteral glutamine supplements: A prospective, controlled, randomized clinical trial. Crit Care Med. 2003;31:2444-2449.
- Wischmeyer PE, Lynch J, Liedel J, et al. Glutamine administration reduces Gram-negative bacteremia in severely burned patients: A prospective, randomized, double-blind trial versus isonitrogenous control. Crit Care Med. 2001;29:2075-2080.
- Kudsk KA, Minard G, Croce MA, et al. A randomized trial of isonitrogenous enteral diets after severe trauma. An immune-enhancing diet reduces septic complications. Ann Surg. 1996;224:531-543.
- Vanek VW, Matarese LE, Robinson M, et al. A.S.P.E.N. position paper: Parenteral nutrition glutamine supplementation. Nutr Clin Pract. 2011;26:479-494.
- Heyland DK, Dhaliwal R. Role of glutamine supplementation in critical illness given the results of the REDOXS study. J Parenter Enteral Nutr. 2013;37:442-443.
- Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:
296-327. - Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: The Glucontrol study. Intensive Care Med. 2009;35:
1738-1748. - Dickerson RN, Melnik G. Osmolality of oral drug solutions and suspensions. Am J Hosp Pharm. 1988;45:832-834.
- Mirtallo J, Canada T, Johnson D, et al. Safe practices for parenteral nutrition. J Parenter Enteral Nutr. 2004;28:S39-70.

*Professor of Clinical Pharmacy, University of Tennessee College of Pharmacy, Memphis, Tennessee; †Clinical Specialist, Nutrition Support, Center for Human Nutrition, Vanderbilt University Medical Center, Nashville, Tennessee; ‡Clinical Coordinator, Nutrition Support Team, The University of Arizona Medical Center, Tucson, Arizona; §Neonatal Clinical Lead & Metabolic Support Service Clinical Pharmacist, Truman Medical Center Hospital Hill, Kansas City, Missouri; ¶Assistant Director, Education and Research, Department of Pharmacy Services, University of Michigan Hospitals and Health Centers, Ann Arbor, Michigan; **Clinical Pharmacy Services Manager, University of Texas, MD Anderson Cancer Center, Houston, Texas; ††Associate Professor of Clinical Pharmacy and Pediatrics, University of Tennessee College of Pharmacy and Medicine, Memphis, Tennessee. Corresponding author: Roland N. Dickerson, PharmD, BCNSP, FCCP, FASHP, FCCM, Department of Clinical Pharmacy, University of Tennessee College of Pharmacy, 881 Madison Avenue, Suite 345, Memphis, TN 38163; e-mail: rdickerson@uthsc.edu