Original Article
Pharmacy Management of Postoperative Blood Glucose in Open Heart Surgery Patients: Evaluation of an Intravenous to Subcutaneous Insulin Protocol
Amanda Stahnke, PharmD, BCACP*; Kelly Struemph, PharmD†; Erin Behnen, PharmD, BCPS‡; and Julia Schimmelpfennig, PharmD, MS, BCPS, CDE§
Abstract
Purpose: To develop and implement a protocol to improve blood glucose (BG) control during transition from intravenous (IV) to subcutaneous (SC) insulin, increase compliance with Surgical Care Improvement Project (SCIP) measures, and decrease sternal wound infections post open heart surgery (OHS).
Methods:An IV to SC protocol was developed and implemented. A retrospective chart review of patients who underwent OHS was conducted from January 2, 2009 to September 30, 2010 (pre protocol) and from October 1, 2010 to December 31, 2011 (post protocol). Data collected included age, sex, history of diabetes mellitus (DM), BG values, hypoglycemia incidence, length of stay, and incidence of sternal wound infections.
Results: A total of 243 patients were included in the study. Compliance with SCIP postoperative day 1 and 2 BG goals was similar pre and post protocol (P = .24 and .248). One sternal wound infection occurred after protocol implementation, whereas 6 occurred pre protocol (P = .046). Change in BG when transitioning from IV to SC insulin was similar between the groups, however there were significantly fewer hypoglycemia episodes post protocol (P < .001).
Conclusion: Though differences were not found in compliance with SCIP postoperative day 1 and 2 measures, fewer sternal wound infections and hypoglycemic episodes were reported, indicating that the pharmacy protocol may have a positive impact on patient outcomes.
Key Words—hyperglycemia, hypoglycemia, postoperative complications, surgical wound infection
Hosp Pharm—2014;49(2):164–169
Hosp Pharm 2014;49(2):164–169
2014 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj4902-164

Insulin resistance and elevated blood glucose (BG) levels are commonly seen in hospitalized patients irrespective of a history of diabetes mellitus (DM).1 These issues arise in almost all critically ill patients, especially those who have undergone open heart surgery (OHS), coronary artery bypass graft (CABG), and/or valve replacement (VR). Hyperglycemia and insulin resistance increase morbidity, specifically the incidence of sternal wound infections, and mortality.1-3 Hyperglycemia and increased insulin resistance may be due to many factors, but they are highly associated with the release of inflammatory substances, such as cytokines, and stress hormones, such as cortisol.4 To help reduce morbidity and mortality, guidelines and recommendations regarding BG goals have been put forth by the Society of Thoracic Surgeons (STS) and the Surgical Care Improvement Project (SCIP). Though these guidelines and recommendations have similar objectives, their BG goals differ slightly. The STS recommends maintaining BG levels 1,5
At St. Elizabeth’s Hospital (SEH), a 500-bed facility located in Belleville, Illinois, an increase in sternal wound infections was seen within the OHS patient population during the spring months of 2010 compared to previous months. After an analysis of other factors affecting infection rates such as preoperative antibiotic administration, surgeon, surgical technicians, and operating rooms showed no association with the increase in sternal wound infections, pharmacy was consulted to evaluate postoperative BG control. An initial pilot study of 7 patients was conducted in June 2010 to evaluate current BG control throughout the identified patients’ entire stay. The data collected showed that patients post OHS were meeting the POD 1 goal of
Objective
The primary outcome of this study was to safely and effectively improve glucose control post OHS. Compliance with SCIP measures, the mean change in BG during transition from IV to SC insulin, incidence of hypoglycemia (BG ≤ 70mg/dL), and incidence of sternal wound infections before and after protocol implementation were assessed.
Protocol Development
Other institutions have increased compliance with SCIP measures and decreased sternal wound infections by adjusting their current protocols to include continuing IV insulin through POD 2.6 However, at our institution, transfer typically occurs on POD 1 and issues related to the level of care that is required to manage an insulin infusion result in conversion from IV to SC insulin prior to POD 2. This is consistent with the STS guidelines statement that the insulin infusion should be maintained for a minimum of 24 hours postoperatively.1
Based on these factors and information revealed during the pilot study, a new protocol was developed and education regarding insulin therapy and cardiac surgery complications was provided to pharmacy, nursing, and physician staff. Despite the plethora of evidence regarding complications of hyperglycemia, there are few sources that provide recommendations regarding the transition from IV to SC insulin, specifically in post-OHS patients. Three such references were identified: the Cleveland Clinic Cardiovascular Intensive Care Unit Insulin Conversion Protocol (Cleveland Clinic) and 2 consensus statements set forth by the American College of Endocrinology (ACE) and jointly by the American Association of Clinical Endocrinologists (AACE) and the American Diabetes Association (ADA).7-9 These sources were taken into consideration during development of the new IV to SC protocol at SEH. The protocol put forth by Hemerson and colleagues regarding conversion from IV to SC insulin was not available at the time of our protocol development.10 The hospital’s protocol is provided in Appendix 1.
Methods
A retrospective chart review of 243 patients who had undergone OHS at SEH was conducted from January 2,2009 to September 30, 2010 (pre protocol) and from October 1, 2010 to December 31, 2011 (post protocol). To meet inclusion criteria, patients had to be at least 18 years of age and had undergone CABG and/or VR at SEH within the time frame of the study. Institutional review board approval was granted, and informed consent was waived due to the nature of the study. Data collected included age, sex, history of DM, IV insulin drip rates, SC insulin doses, BG values, incidence of hypoglycemia while on SC insulin, length of stay, and incidence of sternal wound infections within the first 30 days following surgery. Patient charts were reviewed 30 days post surgery to determine whether readmissions had occurred and to ensure that, if a diagnosis of sternal wound infection was made, it was documented. A Student t test was utilized to evaluate most baseline characteristics, whereas chi-square test was used to evaluate sex, history of DM, compliance with POD 2 SCIP measure, occurrence of hypoglycemia, and incidence of sternal wound infections.
Results
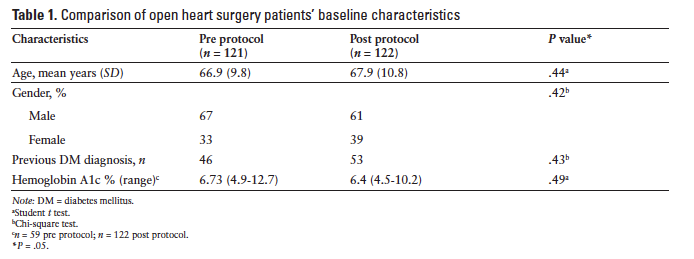
Baseline characteristics were not significantly different between the pre- and postprotocol groups (Table 1). Hemoglobin A1c (A1c) was only available in 49 of the 121 preprotocol patients, whereas all 122 postprotocol patients had recorded preoperative A1c (Table 1). Based on the available data, average A1c was similar between both groups at baseline (P = .49).
Analysis of compliance with SCIP measures on POD 1 and 2 indicated that POD 1 BG goal of P = .248). Comparison of average fasting blood glucose (FBG) on POD 1 and 2 was not significantly different between the groups, but FBG levels were lower on both days in the postprotocol group. Additionally, there was only 1 sternal wound infection post protocol compared to 6 pre protocol (P = .046). Specific findings are reported in Table 2.
Although a statistically significant difference was not seen when transitioning from IV to SC insulin using the new protocol, there was a smaller increase in BG during this transition (61.6 mg/dL vs 58 mg/dL). Hypoglycemia (BG P < .001) (Table 2).
Conclusion
Although a significant reduction in hypoglycemia was seen after protocol implementation, there is no indication from the current study that the protocol resulted in better BG control POD 1 or POD 2 or that it improved the transition from IV to SC insulin. Fewer sternal wound infections occurred, but this cannot be attributed to the protocol alone due to multiple other factors that may potentially affect this outcome (eg, antibiotic administration timing). Despite the lack of statistical significance, the greater number of patients meeting the POD 2 6:00 a.m. BG
There are several limitations to this study. First, the sample size was small. SEH performs a small number of OHS, which results in limited data availability and external validity. Additionally, other factors that may influence BG control and infection rates, such as use of catecholamines, steroids, and antibiotics, were not evaluated during this study. The use of sliding scale insulin is also a potential limitation. Scheduled meal-time and correction-dose insulin is preferred, and this is currently being addressed through the development of a new bolus insulin protocol at SEH.9 Patients could have sought postsurgery complication care at an alternative facility, which would limit our ability to determine true incidence of sternal wound infections within 30 days post OHS.
Despite the fact that hyperglycemia and insulin resistance are well documented in the literature, few studies evaluate transitioning patients from IV to SC insulin. The protocol discussed throughout this article did not statistically improve BG control, but improvements were seen (eg, decreased incidence of hypoglycemia) and facility standards in need of updating were identified (eg, acquiring A1c in patients with risk factors for diabetes previously undiagnosed).11 Additionally, the data collected will help in modification of the protocol to better suit our patient needs and will be a useful tool for other facilities looking to implement similar practices.
Acknowledgments
The authors have no conflicts of interest.
References
- Lazar HL, McDonnell M, Chipkin SR, et al. The Society of Thoracic Surgeons practice guideline series: Blood glucose management during adult cardiac surgery. Ann Thorac Surg. 2009;87:663-669.
- Breithaupt T. Postoperative glycemic control in cardiac surgery patients. Proc (Bayl Univ Med Cent). 2010;23(1):
79-82. - Zerr KJ, Furnary AP, Grunkemeier GL, Bookin S, Kanhere V, Starr A. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg. 1997;63:356-361.
- Kremen J, Dolinkova M, Krajickova J, et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: Possible role in postoperative insulin resistance. J Clin Endocrinol Metab. 2006;91(11):4620-4627.
- Centers for Medicare & Medicaid Services and The Joint Commission. Cardiac surgery patients with controlled 6 a.m. postoperative blood glucose. In: Specifications Manual for National Hospital Inpatient Quality Measures (SCIP-Inf-4). Version 3.2. Washington, DC: Author; 2011.
- Warrington L, Ayers P, Baldwin AM, et al. Implementation of a pharmacist-led multidisciplinary diabetes management team. Am J Health Syst Pharm. 2012;69(14):1240-1245.
- Olansky L, Sam S, Lober C, Yared J, Hoogwerf B. Cleveland Clinic cardiovascular intensive care unit insulin conversion protocol. J Diabetes Sci Technol. 2009;3(3):478-486.
- Bode BW, Braithwaite SS, Steed RD, Davidson PC. Intravenous insulin infusion therapy indications, methods, and transition to subcutaneous insulin therapy. Endocr Pract. 2004;10(suppl 2):71-80.
- Moghissi ES, Korytkowski MT, DiNardo M, et al.
American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):1-17. - Hemerson P, Banarova A, Izakovic M, Clancy GM, Richenbacher WE, Beireis L. Transitioning postoperative
cardiovascular surgery patients from intravenous to subcutaneous insulin: An improvement project. J Clin Outcomes Manage. 2011;18(12):563-567. - American Diabetes Association. Standards of medical care in diabetes–2013. Diabetes Care. 2013;36(suppl 1):S11-66.
Appendix-1
IV to SC Insulin
Nursing
To calculate the patient’s subcutaneous insulin requirements, base the initial dose on final insulin drip rate:
If final insulin drip rate < 1 unit/h:
1) Start Sliding Scale Insulin Protocol at low-dose insulin regimen for rapid-acting insulin only
2) Do NOT initiate glargine (Lantus;basal insulin) orders
If final insulin drip rate > 1 unit/h:
1) Insulin dose determination:
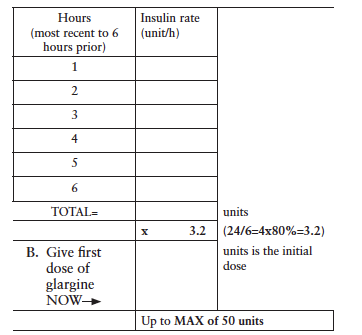
A. Add the hourly insulin drip rate for the last 6 hours to calculate initial glargine dose:
2) Stop IV insulin drip 2 hours after the glargine dose is given.
Administer daily glargine (basal) insulin dose at:
a. 0900, if initial basal dose is given prior to 1300 on 1st day OR
b. 2100, if initial basal dose is given at 1300 or after on 1st day
3) Use Sliding Scale Insulin Protocol only for dosing of the rapid-acting insulin based on low-dose regimen. Do not use the glargine dosing of the Sliding Scale Insulin Protocol.
4) Blood glucose measurement will be performed as follows*:
a. Q6hours if on tube feeds
b. Q6hours if NPO
c. AC and HS if taking oral diet
5) Pharmacy will adjust insulin orders for subsequent glargine (basal) insulin based on the following scale*:
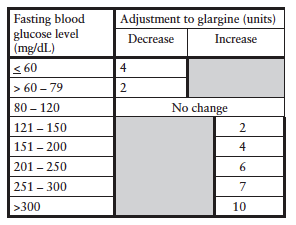
6) Pharmacy will write insulin orders for adjustment of aspart (rapid-acting) insulin to the low-, mid-, or high-dose regimens based on previous day’s blood glucose measurements and insulin requirements.
*Modifications to above protocol will be based on unique patient characteristics for which the best clinical judgment will be used.
Consults
Dietitian consult: Carbohydrate
Pharmacy consult: Insulin adjustments based on blood glucose levels
Note: AC = before meals; HS = bedtime; IV = intravenous; Q6hours = every 6 hours; SC = subcutaneous.
*Clinical Assistant Professor, University of Missouri-Kansas City School of Pharmacy, Kansas City, Missouri; †Clinical Pharmacy Lead, Menorah Medical Center, Overland Park, Kansas; ‡Associate Professor, Southern Illinois University Edwardsville School of Pharmacy, Edwardsville, Illinois; §Pharmacy Manager - Clinical Services, PGY-1 Residency Program Director, St. Elizabeth’s Hospital, Belleville, Illinois. Corresponding author: Amanda Stahnke, PharmD, University of Missouri-Kansas City School of Pharmacy, Health Sciences Building, Room 3248, 2464 Charlotte Street, Kansas City, MO 64108-2718; phone: 314-800-4128; e-mail: stahnkea@umkc.edu