Original Article
Compatibility of Cloxacillin Sodium with Selected Intravenous Drugs During Simulated Y-Site Administration
Thomas Sullivan, PharmD*; Jean-Marc Forest, MSc†; and Grégoire Leclair, PhD‡
Original Article
Compatibility of Cloxacillin Sodium with Selected Intravenous Drugs During Simulated Y-Site Administration
Thomas Sullivan, PharmD*; Jean-Marc Forest, MSc†; and Grégoire Leclair, PhD‡
Original Article
Compatibility of Cloxacillin Sodium with Selected Intravenous Drugs During Simulated Y-Site Administration
Thomas Sullivan, PharmD*; Jean-Marc Forest, MSc†; and Grégoire Leclair, PhD‡
Abstract
Background: Data regarding Y-site compatibility of intravenous (IV) cloxacillin sodium with other drugs are scarce and incomplete.
Objective: To establish the compatibility of IV cloxacillin with 89 injectable drugs during simulated Y-site administration.
Methods: Cloxacillin sodium (10 mL, 100 mg/mL) was combined with 89 undiluted IV drugs (10 mL, each). Tests were duplicated and performed at room temperature. Visual evaluation and a light obscuration particle count test were performed on 1 of the 2 solutions immediately after mixing. The second mixture underwent visual evaluation after 15 minutes, 1 hour, and 4 hours, followed by a particle count test at 4 hours. Drugs were considered incompatible if the mixture precipitated or became turbid within the 4-hour period or exceeded the particle count limit allowed by Test 1.B of USP initially or at 4 hours.
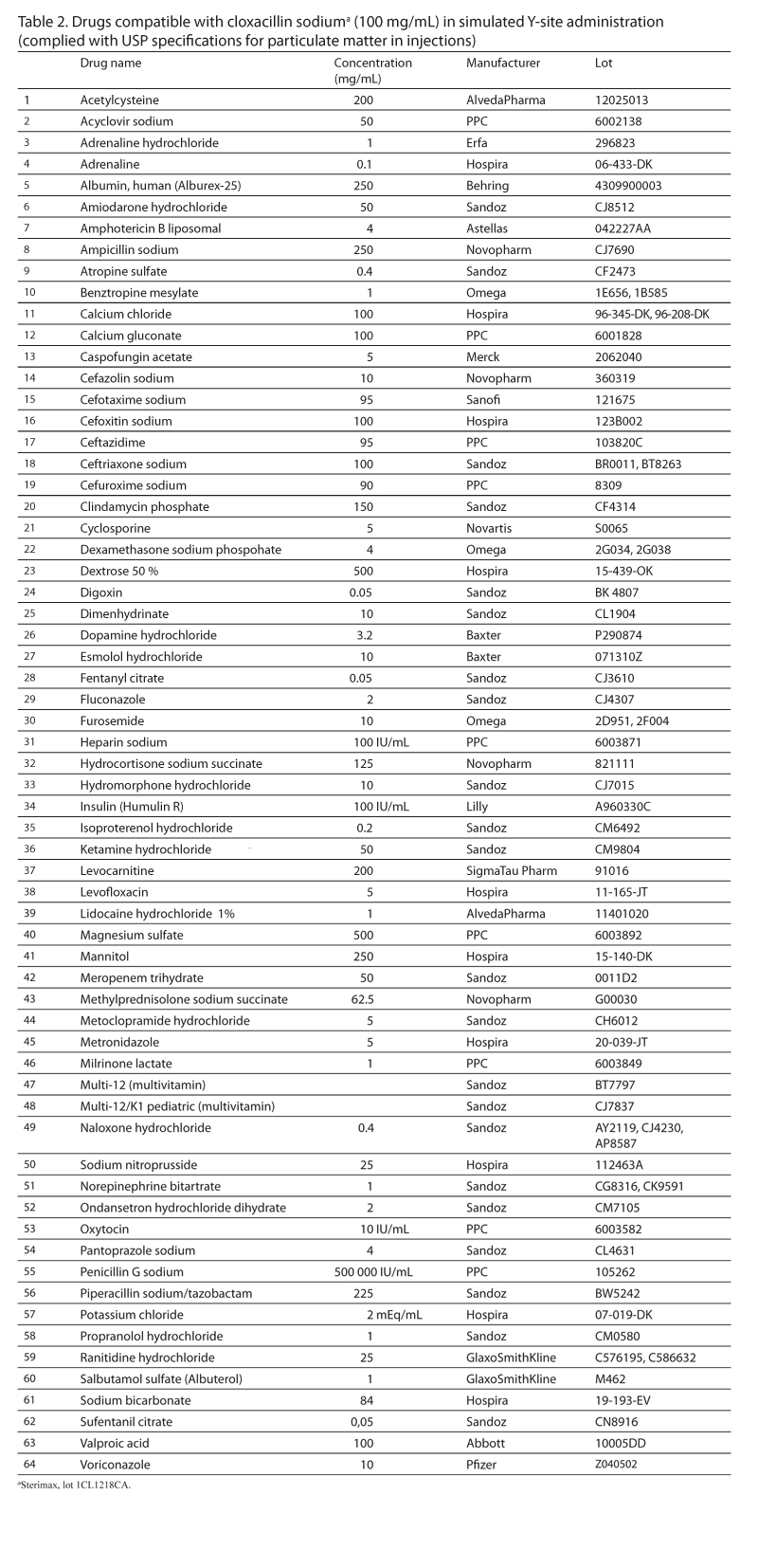
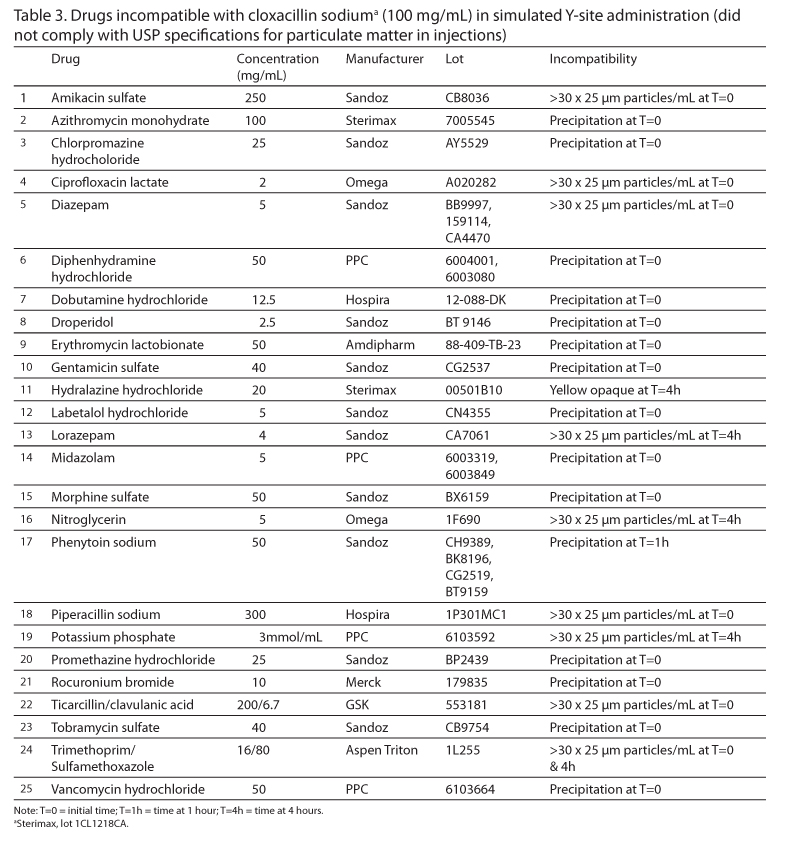
Results: Of the 89 tested drugs, 64 were compatible for up to 4 hours. The remaining 25 drugs were incompatible. Of these incompatible drugs, 16 were identified visually, and 9 were identified by the light obscuration particle count test.
Conclusions: Sixty-four IV drugs were found to be compatible with cloxacillin via simulated Y-site, whereas 25 drugs were found to be incompatible with the antibiotic. The light obscuration particle count test should be used to complement visual evaluation when samples do not precipitate immediately.
Key Words—cloxacillin, compatibility, Y-site
Hosp Pharm—2015;2015;50:214–220
Abstract
Background: Data regarding Y-site compatibility of intravenous (IV) cloxacillin sodium with other drugs are scarce and incomplete.
Objective: To establish the compatibility of IV cloxacillin with 89 injectable drugs during simulated Y-site administration.
Methods: Cloxacillin sodium (10 mL, 100 mg/mL) was combined with 89 undiluted IV drugs (10 mL, each). Tests were duplicated and performed at room temperature. Visual evaluation and a light obscuration particle count test were performed on 1 of the 2 solutions immediately after mixing. The second mixture underwent visual evaluation after 15 minutes, 1 hour, and 4 hours, followed by a particle count test at 4 hours. Drugs were considered incompatible if the mixture precipitated or became turbid within the 4-hour period or exceeded the particle count limit allowed by Test 1.B of USP initially or at 4 hours.
Results: Of the 89 tested drugs, 64 were compatible for up to 4 hours. The remaining 25 drugs were incompatible. Of these incompatible drugs, 16 were identified visually, and 9 were identified by the light obscuration particle count test.
Conclusions: Sixty-four IV drugs were found to be compatible with cloxacillin via simulated Y-site, whereas 25 drugs were found to be incompatible with the antibiotic. The light obscuration particle count test should be used to complement visual evaluation when samples do not precipitate immediately.
Key Words—cloxacillin, compatibility, Y-site
Hosp Pharm—2015;2015;50:214–220
Abstract
Background: Data regarding Y-site compatibility of intravenous (IV) cloxacillin sodium with other drugs are scarce and incomplete.
Objective: To establish the compatibility of IV cloxacillin with 89 injectable drugs during simulated Y-site administration.
Methods: Cloxacillin sodium (10 mL, 100 mg/mL) was combined with 89 undiluted IV drugs (10 mL, each). Tests were duplicated and performed at room temperature. Visual evaluation and a light obscuration particle count test were performed on 1 of the 2 solutions immediately after mixing. The second mixture underwent visual evaluation after 15 minutes, 1 hour, and 4 hours, followed by a particle count test at 4 hours. Drugs were considered incompatible if the mixture precipitated or became turbid within the 4-hour period or exceeded the particle count limit allowed by Test 1.B of USP initially or at 4 hours.
Results: Of the 89 tested drugs, 64 were compatible for up to 4 hours. The remaining 25 drugs were incompatible. Of these incompatible drugs, 16 were identified visually, and 9 were identified by the light obscuration particle count test.
Conclusions: Sixty-four IV drugs were found to be compatible with cloxacillin via simulated Y-site, whereas 25 drugs were found to be incompatible with the antibiotic. The light obscuration particle count test should be used to complement visual evaluation when samples do not precipitate immediately.
Key Words—cloxacillin, compatibility, Y-site
Hosp Pharm—2015;2015;50:214–220
Hosp Pharm 2015;50(3):214–220
2015 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj5003-214




Cloxacillin sodium is a penicillin derivative antibiotic indicated for staphylococcus and streptococcus infections. This antibiotic can be administered orally and intravenously.1 In hospital settings, cloxacillin can be administered via Y-site injection with other intravenous (IV) drugs. Data regarding the Y-site compatibility of cloxacillin with other drugs are scarce and incomplete. Oxacillin sodium is the most chemically similar drug available in the United States, differing from cloxacillin only in the absence of a chlorine atom on the benzene ring.2,3 Despite the structural similarities, Y-site compatibility data for oxacillin cannot be extrapolated to cloxacilli-n. There is therefore a need to evaluate the Y-site compatibility of cloxacillin with other IV drugs.
Chapter 788 of the United States Pharmacopeia (USP) recommends that injectable solutions be analyzed using a light obscuration particle count test.4 The USP criteria establish limits on the number of particles greater than 10 and 25 µm that are allowed in an injectable solution. USP has 2 sets of limits, depending on the volume of the solution to be administered; Test 1.A applies to volumes greater than 100 mL, and Test 1.B applies to volumes less than 100 mL. The USP particle limits are described in Table 1. For this study, the combined volumes of the solutions did not exceed 100 mL, therefore the Test 1.B limits were utilized.
The primary objective of this study was to determine the Y-site compatibility of cloxacillin with 89 IV drugs using visual evaluation and light obscuration particle counting.
METHODS
Test Materials
Cloxacillin sodium (Sterimax, lot 1CL1218CA) was supplied as a 2 g powder per vial and reconstituted with 18.8 mL of sterile water for injection, USP (Baxter, lot C880864) to yield a nominal concentration of 100 mg/mL. This reconstitution represents the practice at CHU Ste-Justine hospital center and does not match the recommendations of the manufacturer. The manufacturer suggests diluting with 6.8 mL to yield a nominal concentration of 250 mg/mL.5 CHU Ste-Justine hospital center has preferred to reconstitute to a 100 mg/mL concentration in order to simplify all dosing calculations.
A total of 89 secondary drugs, listed in Table 2 and Table 3, were tested with cloxacillin. The secondary drugs requiring reconstitution were prepared using sterile water for injection unless otherwise indicated in the product’s monograph. All liquid secondary drugs, with the exception of cyclosporine, were used without further dilution. Cyclosporine was diluted to a concentration of 5 mg/mL using sterile water for injection because the 50 mg/mL solution was too viscous for analysis. Drugs stored in breakable glass ampules were filtered using a 0.45 µm filter.
Test Solutions
All experiments were conducted in a laminar flow hood at room temperature under normal fluorescent light. Solutions were mixed in sterile 50 mL centrifuge tubes (Sarstedt, no. 62.547.004) until homogeneous. Upon mixing, each sample was immediately inspected visually, against both black and white backgrounds, with the unaided eye under normal fluorescent light for color changes, gas bubbles, haze, or precipitation.6 In the absence of precipitatio-n, the sample was immediately analyzed using the liquid particle counter and a second sample was similarly prepared. These second samples underwent further visual evaluation at 15 minutes, 1 hour, and 4 hours, followed by a final particle count test at 4 hours. Drugs that precipitated immediately were discarded without further evaluation.
Control Solutions
On each test day, 2 control solutions containing 10 mL of cloxacillin sodium and 10 mL of sterile water for injection, USP were prepared to ensure the quality of the cloxacillin solution. The first solution was immediately analyzed using the particle counter and the second solution was similarly analyzed after 4 hours.
Particle Count Test
Particle counts were determined using the LS-20 Liquid Particle Counter (Lighthouse Worldwide Solutions, Medford, OR). Chapter 788 of the USP recommen-ds using a minimum volume of 20 mL and analyzing four 5 mL aliquots, discarding the results of the first aliquot.4 In this study, the first 4 mL were discarded and 3 analyses were performed using 5 mL; the last 1 mL was discarded. This minor modification was required to limit the aspiration of air bubbles at the end of each run, which are mistaken for particles by the apparatus.
The accuracy of the particle counter was verified once during the experiment using a control solution with a specific concentration of 15 µm particles (Pharm-Trol Particle Count Control solution, Thermo Scientific, lot 38713). The number of particles measured in the control solution was within the acceptable range, as described in the USP Reference Standard (USP Reference Standard: Particle Count - Suspension 25 mL, cat. no. 1500502. lot K0H089), therefore the particle counter was deemed accurate for the duration of the experiment.
Definition of Compatibility
Visual compatibility was defined as the absence of any particulate matter visible to the naked eye, substantial increase in turbidity or haze, or production of gas bubbles.7 Particulate matter compatibilit-y was defined as an average number of particles no more than 300 per milliliter equal to or greater than 10 µm and no more than 30 per milliliter equal to or greater than 25 µm. These criteria are based on USP Test 1.B specifications using a container volume of 20 mL: “The preparation complies with the test if the average number of particles present in the units tested does not exceed 6000 per container equal to or greater than 10 µm and does not exceed 600 per container equal to or greater than 25 µm.”4(p399)
RESULTS
Control solutions made up of 10 mL of cloxacillin sodium 100 mg/mL and 10 mL of sterile water for injection, USP passed both the visual evaluation and the particle count test initially and after 4 hours during each day of testing. The average particle count of these control solutions immediately after mixing on all days was no more than 30 particles per milliliter equal to or greater than 10 µm and no more than 0.7 particles per milliliter equal to or greater than 25 µm. After 4 hours, the average particle count of control solutions on all days was no more than 15 particles per milliliter equal to or greater than 10 µm and no more than 1.1 particles per milliliter equal to or greater than 25 µm.
Of the 89 tested drugs, 64 were compatible (Table 2). These drugs were visibly compatible throughout the 4-hour period and complied with the particle count criteria immediately after mixing and also after 4 hours. The number of particles per milliliter equal to or greater than 10 µm of these 64 compatible drugs ranged from 0.9 to 72.1 at the time of mixing and from 0.9 to 191.7 after 4 hours. The number of particles per milliliter equal to or greater than 25 µm of these same drugs ranged from 0.0 to 28.5 at the time of mixing and from 0.0 to 21.4 after 4 hours.
A total of 25 drugs were incompatible (Table 3). Visual incompatibility was observed for 16 of these drugs (14 precipitated immediately, phenytoin sodium precipitated after 1 hour, and hydralazine hydrochloride became turbid after 4 hours). A total of 6 drugs passed the visual evaluation throughout the 4-hour period, but failed the particle count test either initially or at 4 hours. These drugs included amikacin sulfate, diazepam, lorazepam, nitroglycerin, piperacillin sodium, and potassium phosphate. The 3 remaining incompatible drugs (ciprofloxacin lactate, ticarcillin/clavulanic acid, and trimethoprim/sulfamethoxazole) were suspected incompatible when examined visually and were confirmed to be incompatible using the particle count test. Of the incompatible drugs that underwent the particle count test, the number of particles per milliliter equal to or greater than 10 µm ranged from 31.3 to 247.5 at the time of mixing and from 55.7 to 20756 after 4 hours. For these same solutions, the number of particles per milliliter equal to or greater than 25 µm ranged from 11.1 to 243.1 at the time of mixing and from 25.9 to 7003 after 4 hours.
DISCUSSION
The simulated Y-injection site model used in this study is based on those used in previous compatibility studies.6,7 The models from these previous studies stem from the findings of Allen et al. They suggested that when 2 solutions are simultaneously administered via Y-injection, those solutions will mix in a 1:1 ratio.8 The solutions were tested at high concentrations following the principle that, in general, 2 drugs that do not precipitate at high concentration are unlikely to precipitate at lower concentrations. It is understood that this methodology may not accurately reflect the conditions in Y-site administration and may be interpreted by some to reflect the conditions of 2 drugs mixed in a syringe.9 Samples were analyzed for up to 4 hours as is typical in Y-site compatibility experiments.6,7,10
The results from this study concerning amikacin sulfate and pantoprazole sodium are not consistent with published data on these 2 molecules.11,12 Amikacin was previously listed as compatible with cloxacillin,11 but it failed the particle count test in this study. Pantoprazole has been previously listed as incompatible, but it passed both the visual examination and the particle count test in this study.12 Consultation with one of the authors (J.M. Forest, oral communication, August 2013) responsible for the previous pantoprazole data confirms that a different formulation of pantoprazole was used in their study. These findings reinforce the disparity in compatibility of different formulations of the same drug and the importance of retesting new formulations for compatibility.
The results of this study demonstrate the value of the light obscuration particle test in evaluating the compatibility of 2 drugs. Nine of the 89 drug mixtures tested would have been considered compatible if evaluated by visual observation alone. The light obscuration particle test should be considered for those drugs that do not precipitate immediately upon mixing.
CONCLUSION
Sixty-four IV drugs were found to be compatible with cloxacillin via simulated Y-site, whereas 25 drugs were found to be incompatible with the antibiotic. The light obscuration particle count test should be used to complement visual evaluation when samples do not precipitate immediately.
ACKNOWLEDGMENTS
This study was jointly funded by CHU Ste-Justine and Université de Montréal.
The authors declare no conflicts of interest.
REFERENCES
- Katzung BG, Masters SB, Trevor AJ. Basic and Clinical Pharmacology. 11th ed. New York: McGraw-Hill; 2010:779.
- National Center for Biotechnology Information (US). PubChem Compound Database; CID=6098 (cloxacillin). Bethesda, MD: National Center for Biotechnology Information (US). 2005. http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6098. Accessed February 22, 2013.
- National Center for Biotechnology Information (US). PubChem Compound Database; CID=6196 (oxacillin). Bethesda, MD: National Center for Biotechnology Information (US). 2005. http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6196. Accessed February 22, 2013.
- Particulate matter in injections. In: United States Pharmacopeia. USP 36. Rockville, MD: United States Pharmacopeia; 2012:350-353.
- SteriMax Inc. Cloxacillin for injection [prescribing information]. Mississauga, ON: Author; 2012.
- Kumar A, Mann HJ. Visual compatibility of oritavancin diphosphate with selected coadministered drugs during simulated Y-site administration. Am J Health Syst Pharm. 2010;67:1640-1644.
- Bramm MK, Chan P, Heatherly K, et al. Compatibility of doripenem with other drugs during simulated Y-site administration. Am J Health Syst Pharm. 2008;65:1261-1265.
- Allen LV, Levinson RS, Phisutsinthrop D. Compatibility of various admixtures with secondary additives at Y-injection sites of intravenous administration sets. Am J Hosp Pharm. 1977;34:939-943.
- Truven Health Analytics: Trissel’s 2 clinical pharmaceutic-s database (parenteral compatibility): Cloxacillin - pantoprazole compatibility detail. www.micromedexsolutions.com/micromedex2. Accessed February 24, 2013.
- Trissel LA, Leissing NC. Trissel’s Tables of Physical Compatibility. Lake Forest, IL: MultiMatrix, Inc.; 1996.
- Nunning BC, Granatek AP, Ricci RA. Physical compatibility and chemical stability of amikacin sulfate in combination with antibiotics in large-volume parenteral solutions − part III. Curr Ther Res Clin Exp. 1976;20:369-416.
- Péré H, Chassé V, Forest JM, Hildgen P. Compatibilité du pantoprazole injectable lors d’administration en Y.
Pharmactuel. 2004;37:193-196.
*Pharmacist, Jewish General Hospital, Montreal, Quebec, Canada; †Pharmacist, CHU Ste-Justine, Montreal, Quebec, Canada; ‡Assistant Professor, Université de Montréal, Montreal, Quebec, Canada. Corresponding author: Grégoire Leclair, PhD, Université de Montréal, C.P. 6128, succursale Centre-ville, Montréal, Quebec, H3C 3J7 Canada; phone: 514-343-6111; e-mail: gregoire.leclair@umontreal.ca