Formulary Drug Reviews
Ceftolozane/Tazobactam
Dennis J. Cada, PharmD, FASHP, FASCP (Editor)*; Jesse Wageman, PharmD†; and Danial E. Baker, PharmD, FASHP, FASCP‡; and Terri L. Levien, PharmD§
Formulary Drug Reviews
Ceftolozane/Tazobactam
Dennis J. Cada, PharmD, FASHP, FASCP (Editor)*; Jesse Wageman, PharmD†; and Danial E. Baker, PharmD, FASHP, FASCP‡; and Terri L. Levien, PharmD§
Formulary Drug Reviews
Ceftolozane/Tazobactam
Dennis J. Cada, PharmD, FASHP, FASCP (Editor)*; Jesse Wageman, PharmD†; and Danial E. Baker, PharmD, FASHP, FASCP‡; and Terri L. Levien, PharmD§
Each month, subscribers to The Formulary Monograph Service receive 5 to 6 well-documented monographs on drugs that are newly released or are in late phase 3 trials. The monographs are targeted to Pharmacy & Therapeutics Committees. Subscribers also receive monthly 1-page summary monographs on agents that are useful for agendas and pharmacy/nursing in-services. A comprehensive target drug utilization evaluation/medication use evaluation (DUE/MUE) is also provided each month. With a subscription, the monographs are sent in print and are also available on-line. Monographs can be customized to meet the needs of a facility. A drug class review is now published monthly with The Formulary Monograph Service. Through the cooperation of The Formulary, Hospital Pharmacy publishes selected reviews in this column. For more information about The Formulary Monograph Service, call The Formulary at 800-322-4349. The June 2015 monograph topics are ceftazidime-avibactam, isavuconazonium sulfate, panobinostat, levodopa-carbidopa intestinal gel, and LCZ696. The Safety MUE is on ceftazidime-avibactam.
Generic Name: Ceftolozane/Tazobactam
Proprietary Name: Zerbaxa (Cubist Pharmaceuticals)
Approval Rating: 4P
Therapeutic Class: Anti-Infective Agents; Cephalosporins
Similar Drugs: Ceftazidime, Piperacillin/Tazobactam (Zosyn)
Sound- or Look-Alike Names: Ceftazidime
Each month, subscribers to The Formulary Monograph Service receive 5 to 6 well-documented monographs on drugs that are newly released or are in late phase 3 trials. The monographs are targeted to Pharmacy & Therapeutics Committees. Subscribers also receive monthly 1-page summary monographs on agents that are useful for agendas and pharmacy/nursing in-services. A comprehensive target drug utilization evaluation/medication use evaluation (DUE/MUE) is also provided each month. With a subscription, the monographs are sent in print and are also available on-line. Monographs can be customized to meet the needs of a facility. A drug class review is now published monthly with The Formulary Monograph Service. Through the cooperation of The Formulary, Hospital Pharmacy publishes selected reviews in this column. For more information about The Formulary Monograph Service, call The Formulary at 800-322-4349. The June 2015 monograph topics are ceftazidime-avibactam, isavuconazonium sulfate, panobinostat, levodopa-carbidopa intestinal gel, and LCZ696. The Safety MUE is on ceftazidime-avibactam.
Generic Name: Ceftolozane/Tazobactam
Proprietary Name: Zerbaxa (Cubist Pharmaceuticals)
Approval Rating: 4P
Therapeutic Class: Anti-Infective Agents; Cephalosporins
Similar Drugs: Ceftazidime, Piperacillin/Tazobactam (Zosyn)
Sound- or Look-Alike Names: Ceftazidime
Each month, subscribers to The Formulary Monograph Service receive 5 to 6 well-documented monographs on drugs that are newly released or are in late phase 3 trials. The monographs are targeted to Pharmacy & Therapeutics Committees. Subscribers also receive monthly 1-page summary monographs on agents that are useful for agendas and pharmacy/nursing in-services. A comprehensive target drug utilization evaluation/medication use evaluation (DUE/MUE) is also provided each month. With a subscription, the monographs are sent in print and are also available on-line. Monographs can be customized to meet the needs of a facility. A drug class review is now published monthly with The Formulary Monograph Service. Through the cooperation of The Formulary, Hospital Pharmacy publishes selected reviews in this column. For more information about The Formulary Monograph Service, call The Formulary at 800-322-4349. The June 2015 monograph topics are ceftazidime-avibactam, isavuconazonium sulfate, panobinostat, levodopa-carbidopa intestinal gel, and LCZ696. The Safety MUE is on ceftazidime-avibactam.
Generic Name: Ceftolozane/Tazobactam
Proprietary Name: Zerbaxa (Cubist Pharmaceuticals)
Approval Rating: 4P
Therapeutic Class: Anti-Infective Agents; Cephalosporins
Similar Drugs: Ceftazidime, Piperacillin/Tazobactam (Zosyn)
Sound- or Look-Alike Names: Ceftazidime
Hosp Pharm 2015;50(6):526–533
2015 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj5006-526




INDICATIONS
Ceftolozane/tazobactam is indicated for the treatment of adults (18 years and older) with complicated urinary tract infections (UTIs), including pyelonephritis, and complicated intra-abdominal infections (used in combination with metronidazole).1 Efficacy has been documented in patients with complicated UTIs, including pyelonephritis, caused by Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Pseudomonas aeruginosa. Efficacy has been documented in patients with complicated intra-abdominal infections caused by Enterobacter cloacae, E. coli, Klebsiella oxytoca, K. pneumoniae, P. mirabilis, P. aeruginosa, Bacteroides fragilis, Streptococcus anginosus, Streptococcus constellatus, and Streptococcus salivarius.1 Treatment in both medical conditions should be adjusted based on culture and susceptibility information.
CLINICAL PHARMACOLOGY
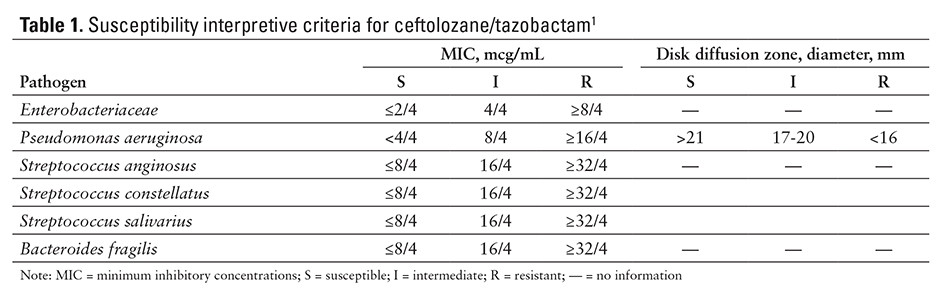
Ceftolozane/tazobactam is a combination of a cephalosporin and a beta-lactamase inhibitor.1 Ceftolozane works by binding to penicillin-binding proteins, resulting in an inhibition of bacterial cell wall biosynthesis. Tazobactam has little antimicrobial activity; instead, it irreversibly inhibits some beta-lactamases (eg, penicillinases, cephalosporinases) and binds some chromosomal and plasmid-mediated bacterial beta-lactamases.1 This combination (ceftolozane plus tazobactam) has shown activity against P. aeruginosa and other common gram-negative pathogens, including extended-spectrum, beta-lactamase–producing Enterobacteriaceae.1,2 The in vitro minimum inhibitory concentration (MIC) varies by organisms and method (see Table 1).1
Ceftolozane/tazobactam has activity against gram-negative bacilli, including beta-lactamase–producing bacteria P. aeruginosa; various drug-resistant phenotypes such as carbapenem, piperacillin/tazobactam, and ceftazidime–resistant isolates; strains that are multidrug-resistant; most extended-spectrum beta-lactamase producers; and some anaerobic species.1
No antagonism has been observed when ceftolozane/tazobactam was used in vitro with other antibacterial drugs (eg, meropenem, amikacin, aztreonam, levofloxacin, tigecycline, rifampin, linezolid, daptomycin, vancomycin, metronidazole).1
PHARMACOKINETICS
Peak plasma concentrations (Cmax) of ceftolozane and tazobactam occur within minutes of completion of the 1-hour intravenous (IV) infusion.1,3 The average area under the curve (AUC) for ceftolozane 1 g/tazobactam 0.5 g on days 1 and 10 were similar: 172 mcg•h/mL on day 1 and 182 mcg•h/mL on day 10 for ceftolozane, 24.4 mcg•h/mL on day 1 and 25 mcg•h/mL on day 10 for tazobactam. The average half-life was 2.77 hours on day 1 and 3.12 hours on day 10 for ceftolozane, and 0.91 hours on day 1 and 1.03 hours on day 10 for tazobactam.1 Overall, the terminal half-life is approximately 2.5 hours for ceftolozane and 1 hour for tazobactam.2-4 The Cmax and AUC increased in proportion to dose, while the elimination half-life was independent of dose.1,3 In patients with normal renal function, the pharmacokinetics of ceftolozane/tazobactam are linear across a large range of doses.2-4 Steady-state volume of distribution in healthy adults was 13.5 L for ceftolozane and 18.2 L
for tazobactam.1
Moderate renal function impairment led to higher concentrations of ceftolozane and tazobactam and increased AUC by 2.5-fold for ceftolozane and 1.6-fold tazobactam. For patients with severe renal impairment, the average AUC increased for ceftolozane and tazobactam by 4.5- and 3.8-fold, respectively.4 The drug plasma concentration is lower after a 3- to 4-hour hemodialysis session, with almost 90% of the initial drug concentration removed from the plasma.4
Protein binding is low for both compounds (approximately 20% for ceftolozane and 30% for tazobactam) and both are mostly excreted in the urine. Ceftolozane is excreted as unchanged drug, which suggests minimal metabolism; 80% of tazobactam is excreted unchanged and the other 20% is hydrolyzed to form an inactive tazobactam metabolite M1.1,2,3,5
COMPARATIVE EFFICACY
Indication: Complicated Urinary Tract Infections
Guidelines
Guideline: Guidelines on urological infections, European Association of Urology
Reference: Grabe M, et al, 20146
Comments: Treatment of complicated UTI should be with empiric or directed antibiotic therapy for
7 to 14 days. Drug regimens might consist of a combination of 2 antibiotics. Patients with complicated UTIs with circulatory and organ failure should be treated for 10 to 14 days with a combination of
2 antibiotics. Empiric treatment should be based on knowledge of the spectrum of possible pathogens and local antibiotic resistance patterns, combined with assessment of the severity of the infection and renal function. Generally, empiric treatment consists of a fluoroquinolone, an aminopenicillin plus a beta-lactamase inhibitor, a cephalosporin, an aminoglycoside, or a carbapenem.
Sulfamethoxazole/trimethoprim should be avoided as first-line treatment because of the frequency of resistance. When possible, the empiric therapy is only used until the results of urine cultures are available. Therapy should then be adjusted based on the culture results and antibiotic sensitivities.
Studies
Drug: Ceftolozane/Tazobactam vs Levofloxacin
Reference: Wagenlehner F, et al, 20147
Study Design: Two phase 3, global, multicenter, double-blind, randomized, noninferiority studies
Study Funding: Cubist Pharmaceuticals
Patients: Two identical phase 3 studies were combined (NCT01345929 and NCT01345955). A total of 1,083 hospitalized patients were enrolled into the combined study: 543 patients were randomized into the ceftolozane/tazobactam arm and 540 were randomized into the levofloxacin arm. Eight hundred (73.9%) subjects who received study drug and had positive baseline urine cultures were included in the microbiological modified intention to treat (ITT) and the microbiological evaluable populations (398 patients in the ceftolozane/tazobactam arm and 402 in the levofloxacin arm). All patients were older than 18 years with pyuria and clinical symptoms of complicated UTI/pyelonephritis requiring intravenous (IV) antibacterial therapy. Subject demographics were comparable in both treatment groups, and 82% of randomized patients had pyelonephritis.
Intervention: Patients were randomly selected to receive IV ceftolozane 1 g/tazobactam 0.5 g every 8 hours (n = 543) or IV levofloxacin 750 mg once daily (n = 540) for 7 days. Of the patients randomized, 800 patients (398 patients in the ceftolozane/tazobactam group and 402 patients in the levofloxacin group) who received study drug and had confirmed positive baseline urine cultures were included in the microbiological modified ITT and microbiological evaluable populations.
Results: All results listed below were measured at the test of cure visit, which was 5 to 9 days after the end of therapy.
Primary Endpoint(s)
- The proportion of subjects in the microbiological modified ITT and microbiological evaluable population demonstrating a composite cure rate (significant resolution or complete resolution of all signs and symptoms of the infection), defined as both clinical cure and microbiological eradication (reduction in colony-forming units/mL uropathogens) at the test of cure visit (5 to 9 days after the end of therapy), were used to assess noninferiority of ceftolozane/tazobactam compared with levofloxacin. The composite microbiological eradication and clinical cure rate with ceftolozane/tazobactam was higher than with levofloxacin. In the microbiological modified ITT population, the composite cure endpoint was reached in 306 of 398 patients (76.9%) with ceftolozane/tazobactam and 275 of 402 patients (68.4%) with levofloxacin; treatment difference was 8.5% (95% confidence interval [CI], 2.3 to 14.6). In the microbiological evaluable population, it was reached in 284 of 341 patients (83.3%) with ceftolozane/tazobactam and 266 of 353 patients (75.4%) with levofloxacin; treatment difference was 8 (95% CI, 2 to 14).
Secondary Endpoint(s)
- The microbiological eradication rate reached with ceftolozane/tazobactam (284 of 341 patients [84.7%]) was noninferior to the microbiological eradication rate with levofloxacin (266 of 353 [75.1%]), with a treatment difference of 9.7% (95% CI, 1.8 to 17.4).
- The clinical cure rate with ceftolozane/tazobactam occurred in 327 of 341 (95.9%) compared with 329 of 353 (93.2%) with levofloxacin; treatment difference was 2.7% (95% CI, −0.8 to 6.2).
- Microbiological eradication rate per pathogen in the Enterobacteriaceae class showed higher microbiological eradication rates with ceftolozane/tazobactam (278 of 313 [88.8%]) compared with levofloxacin (253 of 325 [77.8%]); treatment difference was 9.7% (95% CI, 1.8 to 17.4).
Endpoint(s)
- Safety was evaluated in patients that were treated with either drug.
Comments: Noninferiority of ceftolozane/tazobactam to levofloxacin for the primary endpoint was declared based on a noninferiority margin of 10% with a 1-sided alpha of 0.025. The lower bound of the 95% CI greater than zero demonstrated achievement of the primary objective of noninferiority and demonstrated statistical superiority of ceftolozane/tazobactam over levofloxacin in the study. For 1 patient in the study, the outcome differed depending on the United States versus European Union definitions of microbiological eradication and was not included in the analysis.
Indication: Complicated Intra-Abdominal Infections
Guidelines
Guideline: Diagnosis and management of complicated intra-abdominal infection in adults and children, guidelines by the Surgical Infection Society and the Infectious Diseases Society of America
Reference: Solomkin J, et al, 20108
Comments: Guideline recommends beta-lactam/beta-lactamase inhibitors, carbapenems, cephalosporins, tigecycline, fluoroquinolones, metronidazole, aminoglycosides, aztreonam, and vancomycin alone or in combination for initial empiric treatment in adults with complicated intra-abdominal infections. Therapy duration should be 4 to 7 days in adult patients.
Studies
Drug: Ceftolozane/Tazobactam plus Metronidazole vs Meropenem
Reference: Eckmann C, et al, 20149
Study Design: Two phase 3, global, multicenter, double-blind, randomized, noninferiority studies
Study Funding: Cubist Pharmaceuticals
Patients: This study pooled together data from 2 identical phase 3, double-blind, randomized, multicenter studies (NCT01445665 and NCT01445678). A total of 993 subjects were enrolled in the combined study of which 806 subjects (81.2%) were included in the modified ITT population (389 patients in the ceftolozane/tazobactam group and 417 in the meropenem group). Subjects had to be 18 years and older with a diagnosis of complicated intra-abdominal infection. The most common infections were intra-abdominal abscess or peritonitis due to perforation of a hollow viscus or abdominal infection following an intra-abdominal procedure. The most common diagnosis was appendiceal
perforation or periappendiceal abscess in 420 of 970 patients (43.3%). Demographic and infection baseline characteristics were similar at baseline. The clinically evaluable population had 375 patients (78.8%) in the ceftolozane/tazobactam arm and 393 patients (80.8%) in the meropenem arm who received an adequate amount of study drug, met the protocol definition of complicated intra-abdominal infections, adhered to study procedures, and had a test of cure visit. Two hundred seventy-five patients (57.8%) in the ceftolozane/tazobactam group and 321 patients (65%) in the meropenem arm comprised the microbiologically evaluable population, a subset of the clinically evaluable arm who had at least 1 baseline pathogen that was susceptible to study drug received. The safety population included all subjects who received any amount of study drug.
Intervention: Patients were randomly assigned to treatment with ceftolozane 1 g/tazobactam 0.5 g plus metronidazole 500 mg every 8 hours or meropenem 1 g plus placebo every 8 hours. Duration of therapy was 4 to 14 days. Intra-abdominal cultures were collected at baseline.
Results
Primary Endpoint(s)
- The primary endpoint was to demonstrate noninferiority of ceftolozane/tazobactam plus metronidazole compared with meropenem with respect to the proportion of subjects in the modified ITT population who achieved clinical cure (defined as complete resolution/significant improvement of the index infection, with no additional antibiotics or surgical procedure required). Ceftolozane/tazobactam plus metronidazole was noninferior to meropenem (clinical cure 83% vs 87.3%; treatment difference was −4.2% [95% CI, −8.9 to 0.5]) in the clinical cure at the test of cure visit (26 to 30 days after the first dose of study drug).
Secondary Endpoint(s)
- Microbiological eradication rates were 94.2% with ceftolozane/tazobactam plus metronidazole and 94.7% with meropenem (treatment difference was −0.5 [95% CI, −4.5 to 2.6]).1
- Microbiological eradication rates for the 2 most common Enterobacteriaceae were 193 of 201 (96%) versus 214 of 225 (95.1%) for E. coli and 28 of 28 (100%) versus 22 of 25 (88%) for
K. pneumonia for the ceftolozane/tazobactam plus metronidazole and meropenem groups. Against P. aeruginosa, noninferiority was shown with ceftolozane/tazobactam plus metronidazole, achieving eradication in 25 of 25 (100%) versus meropenem in 28 of 28 (100%).
Comments: Efficacy and safety were evaluated in hospitalized patients diagnosed with complicated intra-abdominal infections.
Limitations: Very few of these patients were from the United States (6.3%); 75% were from Eastern Europe.1
CONTRAINDICATIONS, WARNINGS, AND PRECAUTIONS
Contraindications
Ceftolozane/tazobactam is contraindicated in patients who have experienced serious hypersensitivity reactions to any beta-lactam, especially ceftolozane/
tazobactam or piperacillin/tazobactam.1
Warnings and Precautions
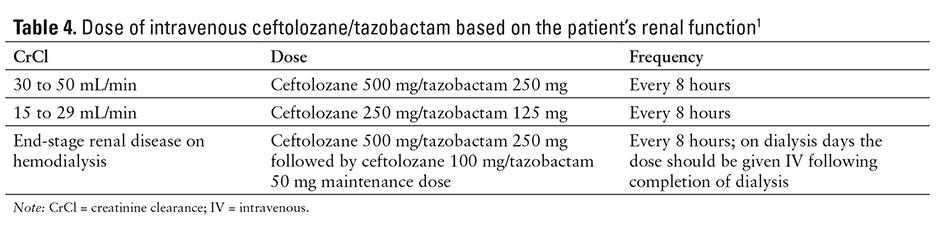
Patients with decreased kidney function (creatinine clearance [CrCl] 30 to 50 mL/h) and complicated UTI or complicated intra-abdominal infection may have decreased efficacy compared with patients with CrCl greater than 50 mL/min.1 In a phase 3 trial of complicated intra-abdominal infections, clinical cure rates were 85.2% and 87.9% with ceftolozane/tazobactam plus metronidazole and meropenem, respectively, in patients with normal renal function (CrCl at least 50 mL/min), but were 47.8% and 69.2%, respectively, in patients with moderate renal impairment. A similar trend was also observed in a phase 3 study in complicated UTIs. The reason for this observation is unknown. Monitor patients with changing renal function daily to adjust the ceftolozane/tazobactam dose accordingly.1
Hypersensitivity reactions may occur after administration of ceftolozane/tazobactam. If there is a known hypersensitivity to beta-lactams, cephalosporins, or penicillins and this product is to be administered, exercise caution because cross-sensitivity
has been established. If anaphylactic reaction occurs, discontinue the drug and administer appropriate therapy.1
Ceftolozane/tazobactam should only be used in situations with proven or strongly suspected bacterial infection. Indiscriminate use may increase the risk of developing drug-resistant bacteria.1
Clostridium difficile–associated diarrhea has been associated with systemic antimicrobials, including ceftolozane/tazobactam, and can range from mild diarrhea to fatal colitis. C. difficile–associated diarrhea should be considered in all patients who present with diarrhea during or after a course of antibiotics. If C. difficile–associated diarrhea is confirmed, then any antimicrobials that are not effective against C. difficile should be discontinued, if possible. Treatment includes monitoring electrolytes, fluids, and protein intake while treating C. difficile–associated diarrhea, and managing fluid and electrolyte levels.1
Ceftolozane/tazobactam has been classified as Pregnancy Category B. No adequate and well-controlled trials have been conducted with either ceftolozane or tazobactam; however, animal reproductive studies have not predicted any negative effects in humans.1
It is not known if ceftolozane/tazobactam is excreted in human milk. Therefore, caution should be used in women who wish to continue breast-feeding during treatment with ceftolozane/tazobactam.1
Safety and effectiveness have not been established in pediatric patients.1
Elderly patients and those with renal impairment may be predisposed to an increased risk of adverse effects. Dosage in this group of patients should be based on renal function because the drug is excreted unchanged in the urine.
ADVERSE REACTIONS
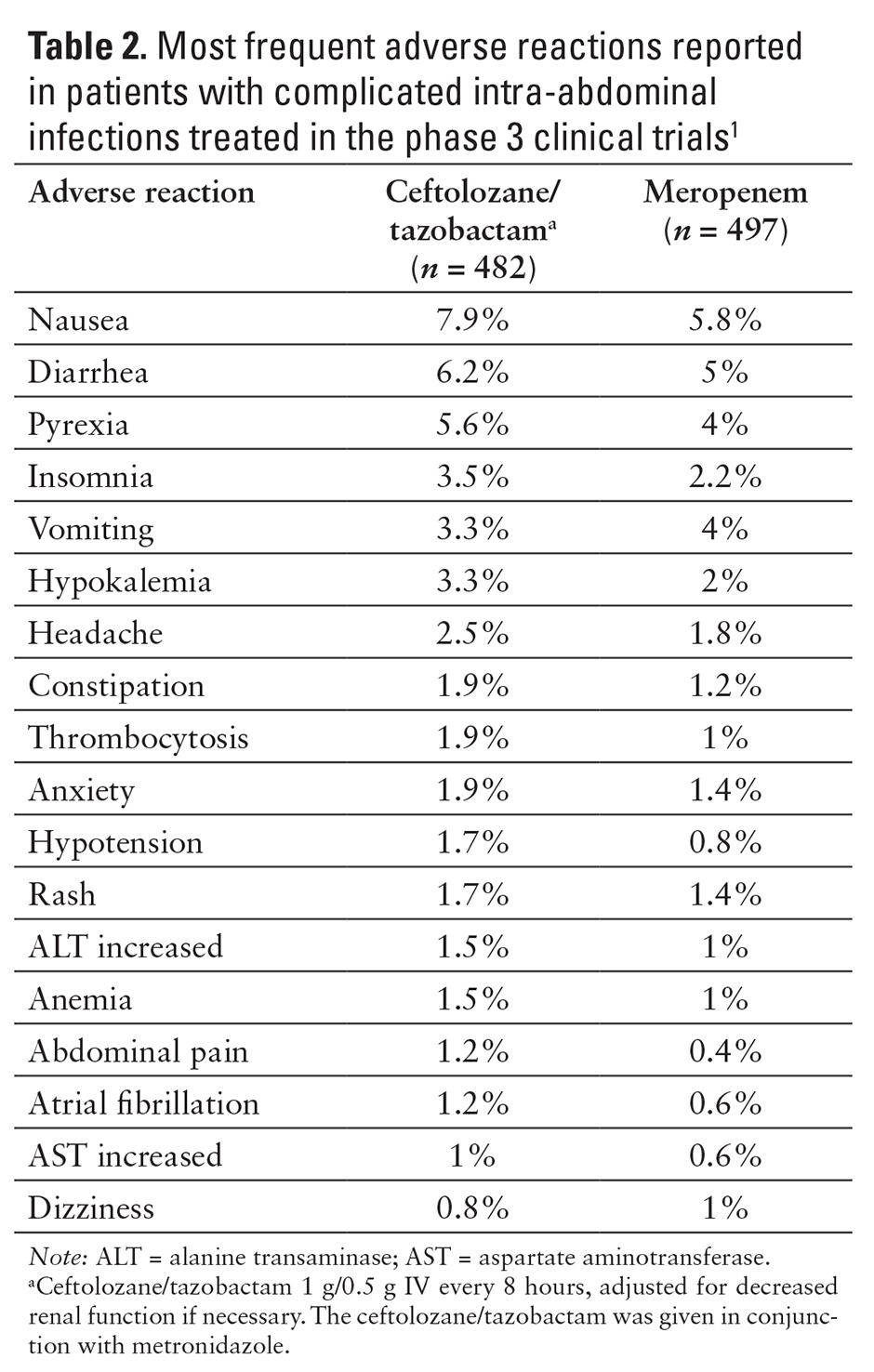
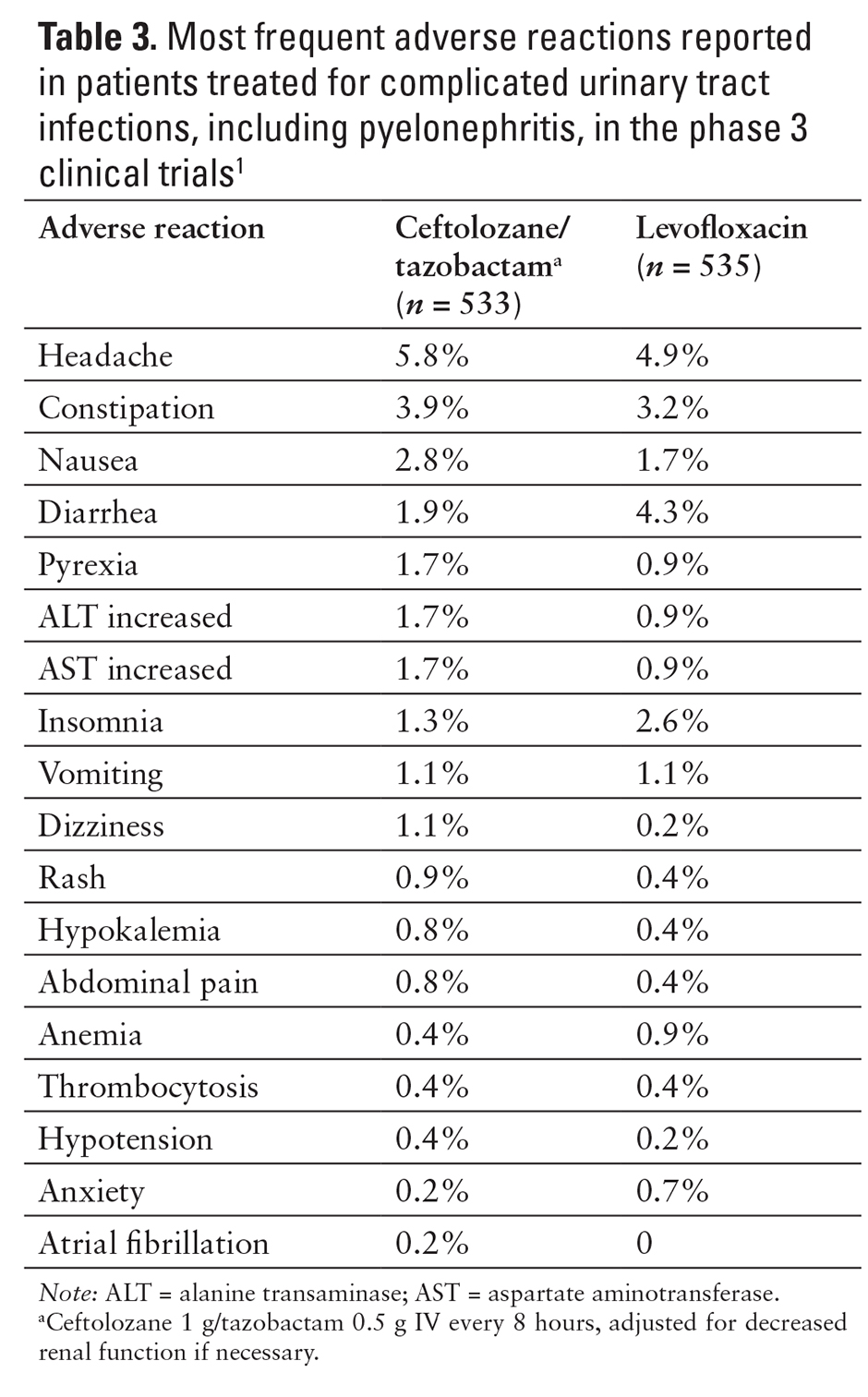
The most common adverse reactions (5% or more) reported in the clinical trials included nausea, diarrhea, headache, and pyrexia (see Tables 2 and 3).1,9,10
In patients with mild, moderate, severe, or end-stage renal disease on nondialysis days, there was no apparent difference in adverse reactions that occurred compared with patients with normal renal function; therefore, no major safety concerns were found following administration of a single dose.4
No effects on heart rate, electrocardiogram morphology, PR, QRS, or QT interval were observed.1
DRUG INTERACTIONS
No drug interactions have been noted and none are anticipated between ceftolozane/tazobactam and substrates, inducers, and inhibitors of the cytochrome P450 enzymes, P-glycoprotein, or BCRP. Tazobactam’s half-life and plasma levels can be increased with probenecid because of its inhibition of OAT1 and OAT3.1
Compatibility studies have not been conducted. Therefore, ceftolozane/tazobactam should not be mixed with other drugs or added to a solution containing other drugs.1
RECOMMENDED MONITORING
Renal function should be monitored prior to therapy to determine the need for dosing adjustment. Monitor patients with changing renal function daily to adjust the ceftolozane/tazobactam dose accordingly.1 If a patient reports diarrhea during or shortly after treatment with ceftolozane/tazobactam, they should be immediately evaluated for C. difficile infection. If the patient tests positive for C. difficile, therapy should be discontinued if possible.
DOSING
The recommended ceftolozane/tazobactam dosage for treating complicated intra-abdominal infection or complicated UTI in patients 18 years and older with normal renal function or mild renal impairment (CrCl great than 50 mL/min) is ceftolozane 1 g/tazobactam 0.5 g administered every 8 hours by IV infusion over 1 hour. The severity and site of infection should guide the duration of therapy. Complicated intra-abdominal infections should be treated for 4 to 14 days and combined with metronidazole 500 mg IV every 8 hours. Complicated UTIs, including pyelonephritis, should be treated for 7 days. Doses should be adjusted based on renal function (see Table 4).1
No adjustments are necessary in patients with hepatic impairment or because of age, gender, or race. However, older patients may require a dosage adjustment based on renal function.1
PRODUCT AVAILABILITY
Ceftolozane/tazobactam was approved in December 2014.1 It is available as a single-dose vial (ceftolozane 1 g [equivalent to ceftolozane sulfate 1.147 g] and tazobactam 0.5 g [equivalent to tazobactam sodium 0.537 g]). The glass vial consists of white to yellow sterile powder for reconstitution. Other ingredients include sodium chloride, citric acid, and L-arginine. Vials are supplied in cartons containing 10 vials.1
The unopened vials should be stored in a refrigerator (2°C to 8°C [36°F to 46°F]) and protected from light.1
After reconstitution with sterile water for injection or sodium chloride 0.9% injection, the solution may be held for 1 hour prior to transfer and dilution in an infusion bag. The solution is then diluted into 100 mL sodium chloride 0.9% or dextrose 5%. The diluted solution can be stored at room temperature for 24 hours or stored in a refrigerator (2°C to 8°C [36°F to 46°F]) for up to 7 days. Neither the reconstituted solution nor the final diluted solution should be frozen.1
DRUG SAFETY/RISK EVALUATION AND MITIGATION STRATEGY (REMS)
No REMS is required for ceftolozane/tazobactam.11
CONCLUSION
Ceftolozane/tazobactam is a combination of a new cephalosporin with an established beta-lactamase inhibitor. Ceftolozane/tazobactam is administered by the IV route every 8 hours for complicated UTIs, including pyelonephritis, and complicated intra-abdominal infections in patients without renal impairment. Ceftolozane/tazobactam has been shown to be noninferior and superior to levofloxacin in the treatment of complicated UTIs. When administered with metronidazole, noninferiority to meropenem has been demonstrated in treating complicated intra-abdominal infections. Ceftolozane/tazobactam covers gram-negative bacilli, including P. aeruginosa, extended-spectrum beta-lactamase producers, and multidrug resistant organisms. Ceftolozane/tazobactam does not show any significant drug-drug interactions or adverse reactions when compared with other novel antibiotics. The efficacy of ceftolozane/tazobactam has been established through 2 phase
3 trials for complicated intra-abdominal infections and 2 phase 3 trials for complicated UTIs.
REFERENCES
- Zerbaxa (ceftolozane/tazobactam) [prescribing information]. Lexington, MA: Cubist Pharmaceuticals; December 2014.
- Chandorkar G, Xiao A, Moukasassi M, Hershberger E, Krishna G. Population pharmacokinetics of ceftolozane/
tazobactam in healthy volunteers, subjects with varying degrees of renal function and patients with bacterial infections. J Clin Pharmacol. 2015;55(2):230-239. - Miller B, Hershberger E, Benziger D, Trinh M, Friedland I. Pharmacokinetics and safety of intravenous ceftolozane-tazobactam in healthy adult subjects in healthy adult subjects following single and multiple ascending doses. Antimicrob Agents Chemother. 2012;56(6):3086-3091.
- Wooley M, Miller B, Krishna G, Herhberger E, Chandorkar G. Impact of renal function on the pharmacokinetics and safety of ceftolozane-tazobactam. Antimicrob Agents Chemother. 2014;58(4):2249-2255.
- Zosyn (piperacillin/tazobactam) [prescribing information]. Philadelphia, PA: Pfizer; 2012.
- Grabe M, Bartoletti R, Bjerklund-Johansen HM, et al. Guidelines on urological infections. European Association of Urology. http://www.uroweb.org/gls/pdf/19%20Urological%20infections_LR.pdf. Published March 2013. Accessed January 8, 2015.
- Wagenleher F, Umeh O, Huntington J, et al. Efficacy and safety of ceftolozane/tazobactam versus levofloxacin in the treatment of complicated urinary tract infections (CUTI/pyelonephritis in hospitalised adults: Results from the phase 3 aspect–CUTI trial [abstract]. European Society of Clinical Microbiology and Infectious Diseases; May 10-13, 2014; Barcelona, Spain. Abstract eP449.
- Solomkin JS, Mazuski JE, Bradley JS, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: Guidelines by the Surgical Infection Society and the Infectious Diseases Society of America [published correction appears in Clin Infect Dis. 2010;50(12):1695]. Clin Infect Dis. 2010;50(2):133-164.
- Eckmann C, Hershberger E, Miller B, et al. Efficacy and safety of ceftolozane/tazobactam versus meropenem in the treatment of complicated intra-abdominal (cIAI) in hospitalised adults: Results from the phase 3 ASPECT-cIAI trial. 24th European Congress of Clinical Microbiology and Infectious Diseases; May 10-13, 2014; Barcelona, Spain. Poster 0266a.
- Lucasti C, Hershberger, Miller B, et al. Multicenter, double-blind, randomized phase II trial to assess the safety and efficacy of ceftolozane-tazobactam plus metronidazole compared with meropenem in adult patients with complicated intra-abdominal infections. Antimicrob Agents Chemother. 2014;58(9):5350-5357.
- Cox EM. NDA approval letter: Zerbaxa (ceftolozane/tazobactam NDA 206829). US Food and Drug Administration Web site. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2014/206829Orig1s000ltr.pdf. Published December 19, 2014. Accessed January 8, 2015.

*Founder and Contributing Editor, The Formulary; †PGY1 Resident, Kootenai Health & Medical Center, Coeur d’Alene, Idaho; ‡Director, Drug Information Center, and Professor of Pharmacy Practice, College of Pharmacy, Washington State University Spokane; §Clinical Professor, College of Pharmacy, Washington State University Spokane. The authors indicate no relationships that could be perceived as a conflict of interest.