Original Article
Near-Miss Transcription Errors: A Comparison of Reporting Rates Between a Novel Error-Reporting Mechanism and a Current Formal Reporting System
David A. South, PharmD*; Jessica W. Skelley, PharmD, BCACP†; Mary Dang, PharmD‡; and Thomas Woolley, PhD§
Original Article
Near-Miss Transcription Errors: A Comparison of Reporting Rates Between a Novel Error-Reporting Mechanism and a Current Formal Reporting System
David A. South, PharmD*; Jessica W. Skelley, PharmD, BCACP†; Mary Dang, PharmD‡; and Thomas Woolley, PhD§
Original Article
Near-Miss Transcription Errors: A Comparison of Reporting Rates Between a Novel Error-Reporting Mechanism and a Current Formal Reporting System
David A. South, PharmD*; Jessica W. Skelley, PharmD, BCACP†; Mary Dang, PharmD‡; and Thomas Woolley, PhD§
Abstract
Purpose: The medication use process comprises several steps. In institutions without full implementation of computerized prescriber order entry (CPOE), transcription is a critical step in this process. As focus is increasingly placed on identifying near-miss errors, this study aimed to compare near-miss transcription error (NMTE) reporting rates between an institution’s formal reporting system and an NMTE reporting mechanism.
Methods: Two NMTE reporting mechanisms were assessed for 3 months. These mechanisms included the institution’s formal error-reporting system and a specific transcription error queue within the institution’s order imaging software. Date, patient-care unit, and type of transcription error were recorded for each order image in the transcription error queue and for each transcription error reported formally. Following data collection, reporting rates for both systems were compared.
Results: Data collection spanned 92 days and an estimated 460,000 medication orders. In total, 1,563 NMTEs were reported using the transcription error queue and 12 errors were reported via the formal reporting mechanism. Of the 1,563 errors identified via the transcription error queue, 325 (20.79%) were of an unknown type. Reporting rates (with unknown errors removed) were 0.27% and 0.0026% for the novel system and formal reporting system, respectively (P < .001).
Conclusion: Significantly more NMTEs were reported utilizing the novel system compared with the formal reporting system.
Key Words—medication safety, near miss, transcription error
Hosp Pharm—2015;50:118–124
Abstract
Purpose: The medication use process comprises several steps. In institutions without full implementation of computerized prescriber order entry (CPOE), transcription is a critical step in this process. As focus is increasingly placed on identifying near-miss errors, this study aimed to compare near-miss transcription error (NMTE) reporting rates between an institution’s formal reporting system and an NMTE reporting mechanism.
Methods: Two NMTE reporting mechanisms were assessed for 3 months. These mechanisms included the institution’s formal error-reporting system and a specific transcription error queue within the institution’s order imaging software. Date, patient-care unit, and type of transcription error were recorded for each order image in the transcription error queue and for each transcription error reported formally. Following data collection, reporting rates for both systems were compared.
Results: Data collection spanned 92 days and an estimated 460,000 medication orders. In total, 1,563 NMTEs were reported using the transcription error queue and 12 errors were reported via the formal reporting mechanism. Of the 1,563 errors identified via the transcription error queue, 325 (20.79%) were of an unknown type. Reporting rates (with unknown errors removed) were 0.27% and 0.0026% for the novel system and formal reporting system, respectively (P < .001).
Conclusion: Significantly more NMTEs were reported utilizing the novel system compared with the formal reporting system.
Key Words—medication safety, near miss, transcription error
Hosp Pharm—2015;50:118–124
Abstract
Purpose: The medication use process comprises several steps. In institutions without full implementation of computerized prescriber order entry (CPOE), transcription is a critical step in this process. As focus is increasingly placed on identifying near-miss errors, this study aimed to compare near-miss transcription error (NMTE) reporting rates between an institution’s formal reporting system and an NMTE reporting mechanism.
Methods: Two NMTE reporting mechanisms were assessed for 3 months. These mechanisms included the institution’s formal error-reporting system and a specific transcription error queue within the institution’s order imaging software. Date, patient-care unit, and type of transcription error were recorded for each order image in the transcription error queue and for each transcription error reported formally. Following data collection, reporting rates for both systems were compared.
Results: Data collection spanned 92 days and an estimated 460,000 medication orders. In total, 1,563 NMTEs were reported using the transcription error queue and 12 errors were reported via the formal reporting mechanism. Of the 1,563 errors identified via the transcription error queue, 325 (20.79%) were of an unknown type. Reporting rates (with unknown errors removed) were 0.27% and 0.0026% for the novel system and formal reporting system, respectively (P < .001).
Conclusion: Significantly more NMTEs were reported utilizing the novel system compared with the formal reporting system.
Key Words—medication safety, near miss, transcription error
Hosp Pharm—2015;50:118–124
Hosp Pharm 2015;50(2):118–124
2015 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj5002-118
Medication errors have been a center of attention for the medical and pharmacy communities since the Institute of Medicine (IOM) published To Err is Human: Building a Safer Health System in 2000. The comprehensive review of available data reported that up to 3.7% of hospitalizations were associated with adverse events and from 44,000 to 98,000 Americans died annually as the result of medical errors.1 This overwhelming incidence of medication errors led the IOM, along with the Centers for Medicare and Medicaid Services (CMS), to publish Preventing Medication Errors in 2007. This publication reported that 450,000 preventable adverse drug events occur in hospitals each year and put forth a national agenda for reducing and preventing medication errors.2
A cornerstone for the prevention of medication errors is vigilant surveillance and effective reporting.3 Through the study of reported errors, health systems are able to identify problems within the medication use process (MUP) and work to implement process changes that help prevent similar errors from happening in the future. More recently, there has been an increasing focus on the reporting of near-miss medication errors, a trend that has lagged behind that of other industries such as aviation, nuclear energy, and steel production.4 A near miss has been defined by the Institute for Safe Medication Practices (ISMP) as an event, situation, or error that occurred but was captured before reaching the patient.5 It is important to studynear misses even though there is no patient harm because of their shared pathways and causation with actual medication errors. Furthermore, near misses occur in greater frequency than actual errors, providing a tremendous opportunity to detect these precursors of patient harm. This has obvious positive implications for patient safety.6
Near misses can occur at any point during the MUP, a process consisting of prescribing, transcribing, dispensing, administering, and monitoring.7 Transcription is the act of taking a handwritten order and inputting that order into an electronic medical record (EMR) system. Following transcription, pharmacists are often responsible for “verifying” that the transcribed electronic order matches the handwritten order of the prescriber. Because of this crucial role in order verification, pharmacists are in a prime position to intercept errors in prescribing and transcription before they reach the patient.8
Available data tend to show higher rates of errors within the prescribing node of the MUP and lower rates in the transcription node.9 In one study, 74% of reported errors were attributed to inaccurate prescription whereas just 10% were attributed to inaccurate transcription.10 A study examining 10-fold medication errors at a university-affiliated pediatric hospital demonstrated that transcription errors accounted for only 4% of all reported errors.11 These low numbers may indicate the possibility of the disproportionate underreporting of transcription errors. Obvious errors in transcription are often corrected by pharmacists without hesitation and with minimal documentation during order verification. This may be especially true when those responsible for transcription (ward-clerks, unit secretaries, etc) have minimal medical training.
Full reporting of near misses, in every node of the MUP, translates into more robust data and the opportunity for more quantitative analysis of the current state of medication safety.4 Unfortunately, near misses are infrequently documented and many times go unnoticed in daily clinical practice.12 Reasons for underreporting include a lack of access to or knowledge of the reporting form, the belief that near misses are unimportant, a lack of feedback on submitted reports, or a fear of some type of punishment or repercussion.13-15 For these reasons, attempts should continually be made to improve the culture of reporting at the institution and the ease with which individuals can report near misses.
This study was conducted at a large, community-owned, not-for-profit hospital that serves as a major referral center for its area. The pharmacy department employs 74 pharmacist full-time equivalents (FTEs), 8 pharmacy resident FTEs, and 68 technician FTEs and utilizes a unit-based pharmacist model to verify orders. Because the hospital utilizes paper charting for physician orders, health unit assistants (HUAs) are relied upon to complete the actual transcription of written orders into the hospital’s EMR. (It is important to note that there is no requirement for HUAs to have formal medical training.) Following entry of the order into the EMR, an order imaging software designed by Health Care Systems (HCS, Montgomery, AL) is used to send an electronic image of the order to a pharmacist for review. The pharmacist then verifies the accuracy of transcription from the written chart into the EMR by viewing the scanned image of the written order. Following verification of the order, the medication can be dispensed and administered to the patient.
Previously, the institution actively tracked medication errors via an internally developed formal, comprehensive, anonymous, Web-based, error-reporting form. Institution policy encouraged the reporting of all medication errors, including near misses. Upon completion of an error report by an individual, data from the error report are entered into a Microsoft Access database and are given a series of “tags” and other identifiers that allow different types of errors to be grouped together and trended. In the 3 months preceding data collection for the current study, a total of 294 events were reported through this pathway. Of these 294 events, 68 stemmed from the transcription node and 24 of which could be classified as near misses.
As previously stated, pharmacists are in a prime position to intercept and correct errors of transcription. Because the error is corrected before the medication is administered, this transcription error can be classified as a near-miss transcription error (NMTE). Furthermore, it is likely that these NMTEs are vastly underreported, perhaps due to the inconvenience of stopping verification in order to complete an error report. Therefore, in an attempt to increase reporting, a novel method that allowed for the reporting of NMTEs using the same software used for order verification was created. To test the new method, we compared the rate of NMTE reporting (defined as number of reports per number of total medication orders) using the new method with those rates seen in the existing reporting system.
METHODS
In an attempt to make the reporting of NMTEs more convenient for pharmacists, a new “queue” was developed within the institution’s order imaging software (HCS) to quarantine order images associated with transcription errors. Queues are different collections of scanned order images. Each image corresponds with a particular patient’s orders in the EMR, and images are grouped into different queues according to patient care unit, urgency, etc. Pharmacists responsible for verifying orders work from assigned, position-specific queues and verify the accuracy of transcription by the HUA along with appropriateness of therapy. Following creation of a transcription error queue within the order imaging software, an e-mail alerting pharmacists to the presence and functionality of the new queue was distributed. Pharmacists were instructed to copy images associated with transcription errors from their position-specific verification queue into the transcription error queue. During this time, the request for pharmacists to report all medication errors, including near misses, via the institution’s formal reporting process was unchanged. Because it would not be feasible to determine what type of transcription error was made by viewing an order image alone, pharmacists were given the ability to highlight the part of the order image associated with a transcription error and create comments to describe the nature of the error (ie, metformin 500 mg twice daily entered as metformin 500 mg daily).
A classification taxonomy was developed to encompass all types of foreseeable errors (see Appendix A). The taxonomy was revised over the course of the first week of data collection to include some classifications that were not part of the original scheme. After the first week of data collection, the taxonomy was not revised further, and any error that did not fall into a specific classification was classified as an overt error not otherwise classified.Comments were recorded for each overt error not otherwise classified and for various other errors of note.
Transcription error data were evaluated for 3 months. The primary investigator reviewed all new images placed into the transcription error queue at regular intervals (once every 1-2 business days). Upon review of order images and associated pharmacist comments, NMTEs were classified according to the presented classification taxonomy. Patient care unit, date, and type of error were recorded for each image utilizing a Microsoft Excel spreadsheet. Order images placed in the queue without a pharmacist comment were classified as unknownNMTEs. Following evaluation of the transcription error, order images were deleted from the queue, thus keeping the queue up to date.
To draw the comparison of order images placed in the transcription error queue with the number of NMTEs reported via the formal reporting system,
all NMTEs formally reported during the data collection period were given the tag “NM HUA transcription error” (near-miss Health Unit Assistant transcription error) when initially reviewed by the institution’s medication safety pharmacist. This allowed the investigators to query the database in order to review all errors associated with the tag “NM HUA transcription error.” The data from this query were reviewed and similarly put into a Microsoft Excel spreadsheet that tracked the patient care unit, date, and type of error for each NMTE formally reported.
Following the conclusion of data collection, the results from the 2 systems of reporting NMTEs were compiled and stratified based on type of NMTE captured. An overall error-reporting rate (as a percentage of all estimated medication orders) was calculated and compared between the 2 methods using Fisher’s exact test at a significance level of .05. Further comparisons were made of the percentages of each type of error reported between the 2 systems.
Our study did not collect any identifiable patient information. Institutional review board approval was sought at both the hospital and our academic institution, with both boards granting an exempt status.
RESULTS
Data collection began on August 1, 2013 and ended on October 31, 2013. The collection period spanned 92 days and an estimated 460,000 medication orders. Over 3 months of data collection, 1,563 NMTEs identified by pharmacists were reported in the HCS transcription error queue, corresponding to an overall error detection rate of 0.34%. During that time, only 12 NMTEs identified by pharmacists were reported through the institution’s formal reporting system, corresponded to an overall error detection rate of 0.0026%.
Because unknown errors do not provide actionable information, we removed these from our analysis when determining a difference in reporting rate. With these errors removed, there is a significant difference (P = .001) in reporting rates between the formal reporting system (0.0026%) and the transcription queue (0.27%).
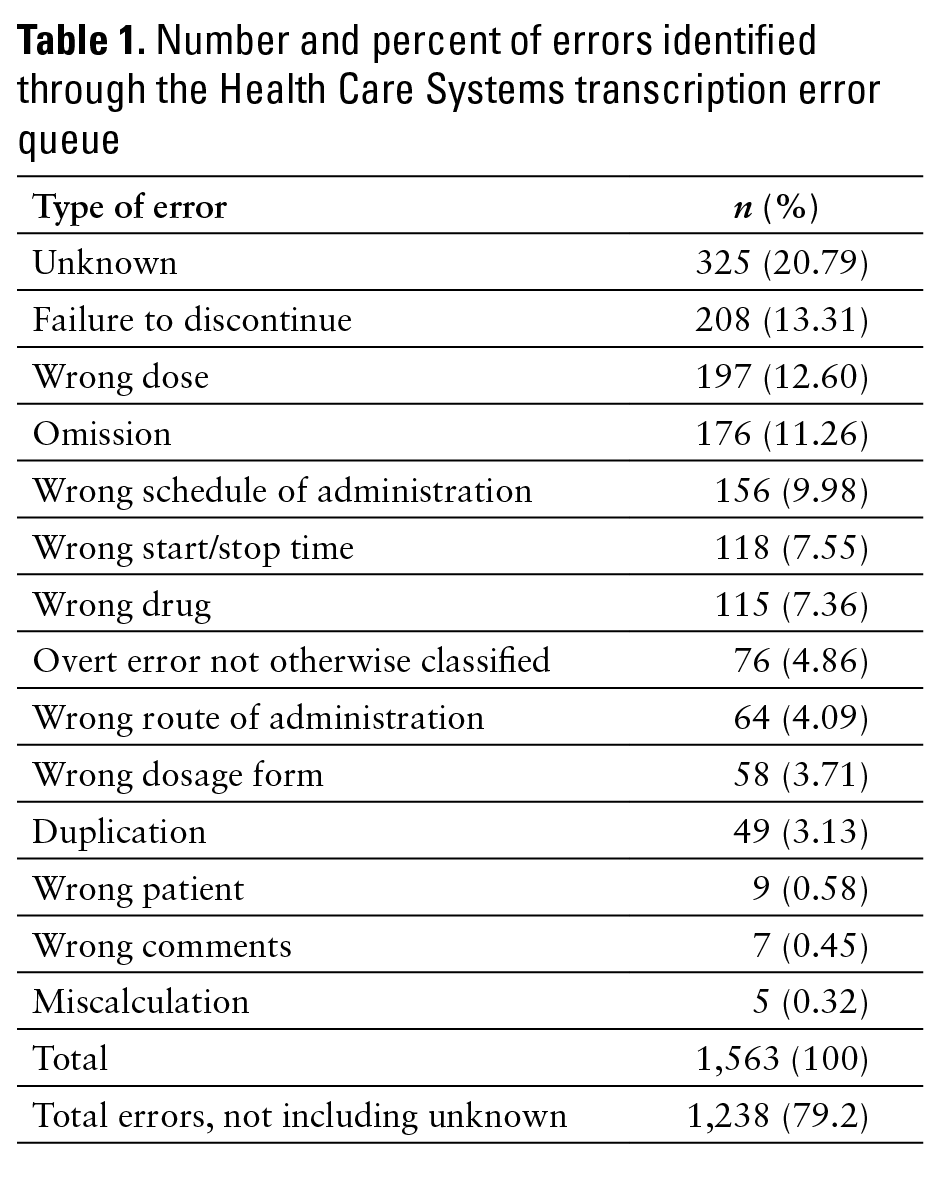
For the transcription error queue, unknown NMTEs made up the largest percentage (20.79%) of errors identified (see Table 1). Conversely, miscalculation errors made up the smallest percentage, with only 5 (0.32%) identified. Seventy-six (4.86%) NMTEs were classified as overt error not otherwise classified.
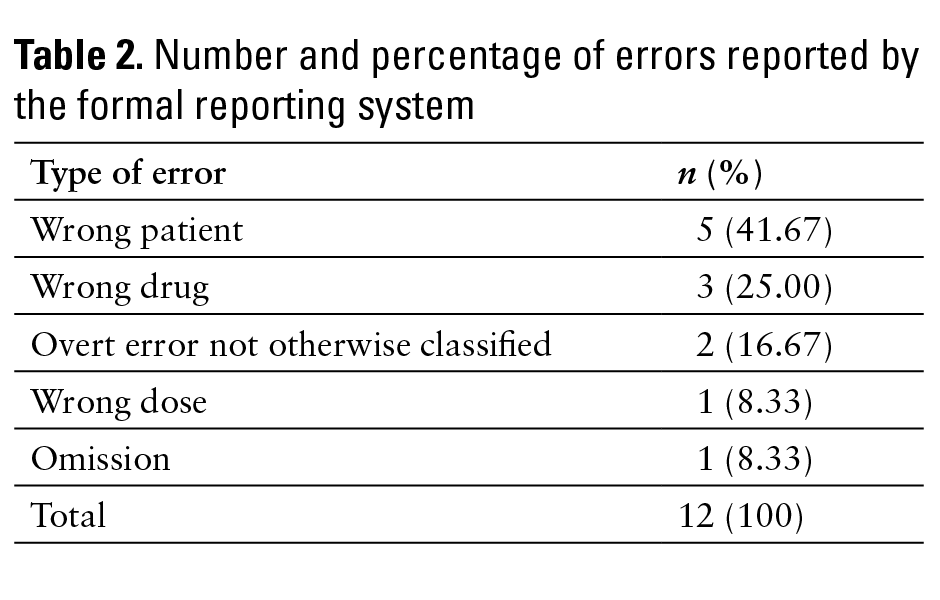
Twelve NMTEs caught by pharmacists were formally reported via the hospital’s formal reporting system. Of these, 5 (41.67%) were orders entered for the wrong patient (see Table 2). This is in contrast to the 9 wrong patient errors (0.58%) identified through the HCS transcription error queue. Only 4 other types of errors (wrong drug, wrong dose, omission, and overt error not otherwise classified) were formally reported.
Various contributing factors were identified in conjunction with certain types of NMTEs. In each (100%) of the NMTEs classified as miscalculation, the error was attributed to an order written in mg/kg dosing. Three of the 5 miscalculation errors (60%) were attributed to an order for “Lovenox 1 mg/kg” transcribed as “Lovenox 1 mg.” Similarly, of 115 NMTEs classified as wrong drug, 19 (16.52%) were associated with look-alike/sound-alike drug names. Appendix B gives a list of drug names associated with look-alike/sound-alike wrong drug NMTEs identified via the novel system.
DISCUSSION
This study chiefly demonstrated that NMTEs caught by pharmacists are vastly underreported at the institution studied. While 1,563 NMTEs were identified through the HCS transcription error queue, only 12 NMTEs were reported via the institution’s formal reporting system. The underreporting of these errors can have far-reaching consequences. When a problem within the transcription node goes unrecognized, efforts aimed at improving the entire MUP may not focus on this important step. Because a chain is only as strong as its weakest link, system-wide analysis and improvements that fail to recognize a particular weakness will be less effective than intended. Furthermore, the vast underreporting of these transcription errors presents unrealized and undocumented evidence of the important role of pharmacists within the MUP. Although the study did not attempt to qualify, quantify, or assign value or severity to the avoided errors, one can easily draw conclusions about the far-reaching consequences these errors could have caused had they reached the patient.
Because this study demonstrated a vast underreporting of NMTEs, it further demonstrated that the use of a transcription error queue within an order imaging software can be a viable means of capturing data related to transcription errors caught by pharmacists. It is not possible to know how many transcription errors were corrected by pharmacists and not placed into the transcription error queue, but this study demonstrated that significantly more errors were identified using this methodology compared with the formal reporting system. We hypothesize that the significantly higher detection rate is due to a quicker and easier means for pharmacists to “report” the transcription errors. Anecdotally, pharmacists stated that they were more willing to report via the transcription error queue rather than a formal reporting form because of the ease of reporting in the queue (simply moving the image to another queue).
Although the use of a transcription error queue within the order imaging software led to more data on NMTEs, the data generated were far from comprehensive. Unknown errors were the most frequent (325, 20.79%) type of NMTE identified. This was due to the placement of an order image into the transcription error queue with either absent or inadequate comments, detail, or documentation of the type of error contained. For example, many images of orders were placed in the transcription error queue with comments simply stating “transcription error.” Because many order images contained multiple medication orders, it was not feasible to determine specifically which medication order was associated with the transcription error, let alone the type of transcription error committed. We did not attempt to ascertain why pharmacists placed images into the queue without commenting about the error, but a potential cause could be insufficient education regarding proper use of the queue.
It is also interesting to note the difference in the major type of error reported between the 2 reporting mechanisms studied. Of 1,563 NMTEs identified through the HCS transcription error queue, the majority of reported errors (325 errors, 20.79%) were of unknowntranscription error type. This is in contrast to zero (0%) errors of unknown transcription error type formally reported. This major difference demonstrates that the formal reporting system captures more meaningful data on reported errors, allowing the institution to make targeted intervention within the transcription node of the MUP. It is difficult to make the same targeted intervention based on data generated by the transcription error queue because this method does not always identify the major type of transcription error committed. Therefore, it is imperative that the transcription error queue have a hard requirement of identifying exactly which part of the order was associated with the transcription error as well as identifying the error itself.
Another interesting finding is that errors of the wrong patient type accounted for 5 (41.67%) of all NMTEs formally reported. This is in contrast to the 9 (0.58%) wrong patient NMTEs identified through the HCS transcription error queue. The second highest rates of formal reporting were for wrong drug errors and overt error not otherwise classified errors at 2.62% and 2.63%, respectively. Thus, it appears as though wrong patient errors are reported at higher rates via formal mechanisms compared with other types of errors. Again, although the study did not attempt to classify severity of transcription errors, we postulate that transcription errors in which an order was entered under the wrong patient’s name are perceived as more severe (and thus more important to formally report) than other types of NMTEs (eg, failure to discontinue a medication following an order for discontinuation).
With a focus on improving the MUP, efforts should necessarily shift to how to prevent errors. Our novel method of detecting transcription errors has led to some process changes and systematic improvements. For example, a particular problem with a pre-printed order set was identified and has subsequently been corrected. Furthermore, plans are in place to collate data and provide feedback to specific units regarding HUA errors.
Our method can be particularly useful for institutions in which CPOE is not yet fully implemented. Although CPOE is the primary method that is associated with reducing transcription errors,2 it has not yet been fully adopted nationwide. In the meantime, institutions still need to continually make process improvements, and our method details a potential way to positively affect the transcription node of
the MUP.
This study was not without limitations. Our results may have been biased by the fact that errors were seemingly underreported via the traditional system once the new system was initiated. In the 3 months prior to data collection, 24 NMTEs were formally reported versus 12 during the study period. Although institution policy on reporting errors and near misses was unchanged, pharmacists were not directly instructed to report via both mechanisms. Another potential limitation is the subjectivity of our transcription error classification taxonomy. Only one investigator completed the classification (thus contributing to consistency of classification); our taxonomy was designed solely for this study and does not constitute a validated system for error classification. Our novel method was time intensive. On average, almost 17 transcription errors were detected per day, and each of these errors and associated images had to be reviewed. This process of reviewing the detected errors required about 1 to 2 hours per day.
CONCLUSION
The objectives for this study were to test a novel method for the reporting of NMTEs and to compare rates of reported NMTEs utilizing this new method with those rates seen in the existing formal reporting mechanism. Upon review of the data, it is evident that significantly more NMTEs were reported utilizing the novel system compared with the formal error-reporting system. However, this novel method was far from perfect. Needed improvements include a method for required notation identifying the specific order associated with the transcription error as well as documentation of the type of transcription error committed.
ACKNOWLEDGMENTS
At the time of writing, David South was a PharmD candidate at Samford University McWhorter School of Pharmacy, Birmingham, Alabama; he graduated in May 2014.
Disclosures: The authors of this paper have nothing to disclose concerning possible financial or personal relationships with commercial entities.
Additional contributions: The authors would like to thank Michele Durda, PharmD, Medication Safety Pharmacist at the institution studied, for her expertise in medication safety and her help with compiling data from the institution’s formal error-reporting system.
REFERENCES
- Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academy Press; 2000.
- Aspden P, Wolcott JA, Bootman JL, Cronenwett LR, eds. Preventing Medication Errors. Washington, DC: National Academies Press; 2007.
- Leape LL. Reporting of adverse events. N Engl J Med. 2002;347:1633-1638.
- Barach P, Small S. Reporting and preventing medical mishaps: Lessons from non-medical near miss reporting systems. BMJ. 2000;320:759-763.
- ISMP survey helps define near miss and close call. ISMP Medication Safety Alert! September 24, 2009. http://www.ismp.org/newsletters/acutecare/articles/20090924.asp. Accessed April 11, 2013.
- Guffey P, Szolnoki J, Caldwell J, Polaner D. Design and implementation of a near miss reporting system at a large, academic pediatric anesthesia department. Pediatr Anaesth. 2011;21(7):810-814.
- Cheng CM. Hospital systems for the detection and prevention of adverse drug events. Clin Pharmacol Ther. 2011;89(6):779-781.
- Hooper R, Adam A, Kheir N. Pharmacist-documented interventions during the dispensing process in a primary health care facility in Qatar. Drug Healthc Patient Saf. 2009;1:73-80.
- Kaushal R, Shojania KG, Bates DW. Effects of computerized order entry and clinical decision support systems on medication safety. Arch Intern Med. 2003;163(12):1409-1416.
- Kaushal R, Bates D, Landrigan C, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA. 2001;285(16):2114-2120.
- Doherty C, McDonnell C. Tenfold medication errors: 5 years’ experience at a university affiliated pediatric hospital. Pediatrics.2012;129:916-924.
- Jeffs L, Berta W, Lingard L, et al. Learning from near misses: From quick fixes to closing off the Swiss-cheese holes. BMJ Qual Saf. 2012;21:287-294.
- Raymond CB, Woloschuk DMM, Honcharik N. Attitudes and behaviors of hospital pharmacy staff toward near misses. Healthc Q. 2011;14(3):48-56.
- Evans SM, Berry JG, Smith BJ, et al. Attitudes and barriers to incident reporting: A collaborative hospital study. Qual Saf Health Care.2006;(15):39-43.
- Lawton R, Parker D. Barriers to incident reporting in a health-care system. Qual Saf Health Care. 2002;(11):15-18.
APPENDIX A
Types of Transcription Errors
- Miscalculation: A transcription error resulting from a miscalculation in dose or rate when a specified concentration (5 mg/2.5 mL) or rate (2 mL/kg/h) are given and occurring on the part of the person entering the order, not on the part of the prescriber.
- Wrong dose: The right drug is selected, but the dose entered is incorrect. If this dose is incorrect due to miscalculation, the error will be classified as type 1. This type of error also includes transcription errors in which the wrong rate for an intravenous fluid is entered.
- Wrong start or stop time: The right drug is selected along with the right dose, but the wrong start or end time has been entered.
- Wrong drug: The wrong drug has been entered. This type also includes errors in which the wrong intravenous fluid has been entered.
- Duplicate medication: A medication is wrongly entered twice on a patient’s profile. This is not a duplication in therapy, as that is possibly another type of medication error.
- Omission: A medication is ordered by a prescriber but is not entered on a patient’s profile.
- Failure to discontinue medication: A medication is not discontinued from a patient’s profile despite an order for discontinuation.
- Wrong patient: A medication/medications is/are entered for the wrong patient.
- Wrong frequency: The frequency of administration is entered incorrectly. This includes medication orders in which the medication is ordered as needed (PRN), but is not entered as PRN or vice versa.
- Wrong comments: This is a transcription error in which a provider’s comments are entered incorrectly or not at all.
- Wrong route of administration: The route of administration is entered incorrectly.
- Wrong dosage form: The dosage form is entered incorrectly. This type of error included drugs ordered to be given IV piggyback being entered as IV push, or vice versa.
- Overt error not otherwise classified: This is a category for any error that does not fit another category, but it excludes orders that are placed in the queue without any comments or other information showing the type of transcription error committed.
- Unknown: This is a category that includes all images placed into the queue without any comments or markups alluding to the type of transcription error present.
APPENDIX B
Look-Alike/Sound-Alike Drug Names Identified
Amiloride/Amlodipine
Cardene/Cardizem
Ceftriaxone/Ceftaroline
Cytotec/Cytomel
Diflucan/Dificid
Dobutamine/Dopamine
Fioricet/Lorcet
Hydrochlorothiazide/Hydralazine
Hydroxyzine/Hydralazine
Norvasc/Norco
Propranolol/Propofol
Protonix/Prednisone
Ranexa/Xanax
Riboflavin/Ribavirin
Solumedrol/Solucortef
Symbicort/Synthroid
*PGY1 Health-System Pharmacy Administration Resident, UNC Hospitals, Chapel Hill, North Carolina; †Assistant Professor Pharmacy Practice, ‡Clinical Assistant Professor, Samford University McWhorter School of Pharmacy, Birmingham, Alabama; §Professor of Statistics, Samford University Brock School of Business, Birmingham, Alabama. Corresponding author: David A. South, PharmD, Department of Pharmacy, 101 Manning Drive, Campus Box 7600, Chapel Hill, NC 27514; e-mail: david.south@unchealth.unc.edu