Original Article
In Vitro Stability Evaluation of Different Pharmaceutical
Products Containing Meropenem
Cristina Tomasello, PharmD*; Anna Leggieri, PharmD*; Roberta Cavalli, PhD†; Giovanni Di Perri, MD,
DTM&H, PhD‡,§; and Antonio D’Avolio, BSc, MSc, SM§
Original Article
In Vitro Stability Evaluation of Different Pharmaceutical
Products Containing Meropenem
Cristina Tomasello, PharmD*; Anna Leggieri, PharmD*; Roberta Cavalli, PhD†; Giovanni Di Perri, MD,
DTM&H, PhD‡,§; and Antonio D’Avolio, BSc, MSc, SM§
Original Article
In Vitro Stability Evaluation of Different Pharmaceutical
Products Containing Meropenem
Cristina Tomasello, PharmD*; Anna Leggieri, PharmD*; Roberta Cavalli, PhD†; Giovanni Di Perri, MD,
DTM&H, PhD‡,§; and Antonio D’Avolio, BSc, MSc, SM§
Abstract
Background: Meropenem is a beta-lactam antibiotic for treating multidrug-resistant gram-negative bacilli infections. The expiry of the drug’s patent (Merrem) allowed the production of generics to be commercialized by a few companies, including Hospira and Hikma. The stability of these medicines after reconstitution as reported on a data sheet report is 6 hours for Merrem and 1 hour for generics.
Objectives: The aim of this work was to evaluate the stability profile of 3 products in 0.9% sodium chloride until 6 hours.
Methods: Six polyolefin bags (2 for each drug, stored in the light and in the dark) were prepared for every test run (n =10) at concentrations of 4 and 10 mg/mL. All solutions were stored at controlled room temperature (25°C ± 3°C) and sampled immediately after preparation and at every hour until 6 hours had passed. The concentrations, pH changes, and the visual clarity were used as stability and compatibility indicators.
Results: All 3 drugs retained over 95% of the initial concentration at 3 to 4 hours. At the sixth hour, all the concentrations decayed 8% to 10%. No statistical differences were observed in the percentage deviation values of the stability profile between generics and the branded drug.
Conclusion: The stability profile of the products in polyolefin bags, at 4 and 10 mg/mL, was superimposable during the period of analysis and seems to show small values of deviation (1%-2%). These data do not affect the pharmacokinetics because these variations could be attributed to the intra- and interindividual variability between patients. The products showed the same stability, and consequently they could be used interchangeably in hospital pharmacy.
Key Words—hospital pharmacy, meropenem, pharmacokinetic, stability, UPLC-PDA
Hosp Pharm—2015;50:296-303
Abstract
Background: Meropenem is a beta-lactam antibiotic for treating multidrug-resistant gram-negative bacilli infections. The expiry of the drug’s patent (Merrem) allowed the production of generics to be commercialized by a few companies, including Hospira and Hikma. The stability of these medicines after reconstitution as reported on a data sheet report is 6 hours for Merrem and 1 hour for generics.
Objectives: The aim of this work was to evaluate the stability profile of 3 products in 0.9% sodium chloride until 6 hours.
Methods: Six polyolefin bags (2 for each drug, stored in the light and in the dark) were prepared for every test run (n =10) at concentrations of 4 and 10 mg/mL. All solutions were stored at controlled room temperature (25°C ± 3°C) and sampled immediately after preparation and at every hour until 6 hours had passed. The concentrations, pH changes, and the visual clarity were used as stability and compatibility indicators.
Results: All 3 drugs retained over 95% of the initial concentration at 3 to 4 hours. At the sixth hour, all the concentrations decayed 8% to 10%. No statistical differences were observed in the percentage deviation values of the stability profile between generics and the branded drug.
Conclusion: The stability profile of the products in polyolefin bags, at 4 and 10 mg/mL, was superimposable during the period of analysis and seems to show small values of deviation (1%-2%). These data do not affect the pharmacokinetics because these variations could be attributed to the intra- and interindividual variability between patients. The products showed the same stability, and consequently they could be used interchangeably in hospital pharmacy.
Key Words—hospital pharmacy, meropenem, pharmacokinetic, stability, UPLC-PDA
Hosp Pharm—2015;50:296-303
Abstract
Background: Meropenem is a beta-lactam antibiotic for treating multidrug-resistant gram-negative bacilli infections. The expiry of the drug’s patent (Merrem) allowed the production of generics to be commercialized by a few companies, including Hospira and Hikma. The stability of these medicines after reconstitution as reported on a data sheet report is 6 hours for Merrem and 1 hour for generics.
Objectives: The aim of this work was to evaluate the stability profile of 3 products in 0.9% sodium chloride until 6 hours.
Methods: Six polyolefin bags (2 for each drug, stored in the light and in the dark) were prepared for every test run (n =10) at concentrations of 4 and 10 mg/mL. All solutions were stored at controlled room temperature (25°C ± 3°C) and sampled immediately after preparation and at every hour until 6 hours had passed. The concentrations, pH changes, and the visual clarity were used as stability and compatibility indicators.
Results: All 3 drugs retained over 95% of the initial concentration at 3 to 4 hours. At the sixth hour, all the concentrations decayed 8% to 10%. No statistical differences were observed in the percentage deviation values of the stability profile between generics and the branded drug.
Conclusion: The stability profile of the products in polyolefin bags, at 4 and 10 mg/mL, was superimposable during the period of analysis and seems to show small values of deviation (1%-2%). These data do not affect the pharmacokinetics because these variations could be attributed to the intra- and interindividual variability between patients. The products showed the same stability, and consequently they could be used interchangeably in hospital pharmacy.
Key Words—hospital pharmacy, meropenem, pharmacokinetic, stability, UPLC-PDA
Hosp Pharm—2015;50:296-303
Hosp Pharm 2015;50(4):296–303
2015 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj5004-296

Meropenem, chemically (4R,5S,6S)-3-[[3S,5S)-5-dimethylcarbamoyl pyrrolidin-3-yl]thio]-6-[(1R1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3,2,0] hept-2-ene-2-carboxylic acid, is a recent carbapenem antibiotic with bactericidal activity. The molecular formula is C17H25N3O5S and the molecular weight is 383.4625 g/mol.1 This antibiotic is stable to ring opening by human renal dehydropeptidase I (DHP-I), and consequently it does not require concomitant administration of a DHP-1 inhibitor. Meropenem is a white crystalline powder, and its pKa values are 2.9 and 7.4. It has limited aqueous solubility. Meropenem has a beta-lactam ring, which makes it susceptible to hydrolytic degradation, and its formulation is in powder for injection.
Meropenem for injection is available as 0.5 g or 1 g sterile lyophilized powder for either intravenous (IV) injection or intramuscular (IM) injection after reconstitution with appropriate infusion solutions.2 The recommended routes of administration and diluents for meropenem are IV bolus injection reconstituted with sterile water for injection (5 mL for 250 mg of meropenem) and IV infusion reconstituted directly with an infusion solution of sodium chloride 0.9% or glucose 5%. The reconstituted solution is a clear and colorless to pale yellow solution. Aseptic technique must be used to prepare the final IV solution.
The stability of reconstituted meropenem in solution is usually influenced by storage temperature and type of reconstitution solution. The drug is stable for a longer time in solutions stored at 4°C to 5°C than in solutions stored at 2°C to 26°C. The drug reconstituted in normal saline solution is stable for a longer time than the drug reconstituted in 5% dextrose in water.3,4 Mendez et al4 showed that for a reconstituted sample, meropenem degrades extensively after reconstitution in saline solution. Almost 80% of drug degradation was observed after exposure to heat at 45°C for 36 hours, and it had developed a yellowish color. Degradation products were derived by the enzymatic hydrolysis.
The efficacy of an antibiotic agent is dependent on its pharmacokinetic and pharmacodynamic properties. The elimination half-life of meropenem is around 1 hour, and the clinical effect is time dependent. Free drug concentrations, higher than the minimum inhibitory concentration (MIC), have to be maintained for a long time in a dosing interval (%T > MIC) for efficacy. For these reasons, administration in prolonged infusion may be a convenient strategy for obtaining higher efficiency.5 In clinical practice, meropenem is often administered by slow IV infusion (3-4 hours), because of the pharmacokinetic characteristics previously described.5
The reported stability in a data sheet, a document created by the manufacturer that summarizes the performance and other chemical characteristics of the product, is different for various products containing meropenem. In Italy, different times of stability have been reported in the data sheet for the branded drug (Merrem; AstraZeneca-Milan, Italy) and generic drugs (meropenem; Hospira, [Naples, Italy] and meropenem; Hikma [Pavia, Italy]): 6 hours for Merrem and 1 hour for generic products, after reconstitution.
We contacted the manufacturers of the generic products used in this study to get more information about data stability. They confirmed that the only available European studies on the reconstituted meropenem product are those reported in the “AIC” Italian dossier.6 These studies were performed in plastic bags at concentrations of 1 mg/mL and 20 mg/mL, demonstrating a stability of 4 hours at 25°C and 24 hours at 4°C. Due to the very rapid degradation of known reconstituted solutions, and to be in accordance with Article 30-Procedures (Directive 2001/83/EC) for meropenem powder for solution for injection or infusion 500 mg and 1 g, it was confirmed that meropenem could be reconstituted with isotonic sodium chloride solution 0.9% and glucose 5% solution and that it should be used within 1 hour.
There are many published studies about meropenem stability,3,4,7,8 but this study compares the chemical and physical stability among the 3 different products present on the Italian market. The aim of this work was to evaluate the stability profile of these 3 different medicines to investigate the possibility of using them interchangeably in the hospital pharmacy.
METHODS
Chemicals and Reagents
Merrem 1000 mg [AstraZeneca-Milan, Italy] (A), meropenem 500 mg [Hospira, Naples, Italy] (H), and meropenem 1000 mg [Hikma, Pavia, Italy] (X) were purchased from each pharmaceutical company. These pharmaceutical products contain, respectively: (A) 1000 mg, as anhydrous base, of the drug and 208 mg of the anhydrous sodium carbonate as excipient (corresponding to 4 mEq of sodium); (H and X) 500 mg, as anhydrous base, of the drug and 104 mg of the anhydrous sodium carbonate as excipient (corresponding to 2 mEq of sodium).
Saline solution 0.9% for dilution was purchased from Bioindustria L.I.M. (Novi Ligure [AL], Italy). Meropenem powder (as reference material) was purchased from Sigma-Aldrich, and the polyolefin bags Viaflo were purchased from Baxter (Trieste, Italy). Acetonitrile HPLC grade was purchased from J.T. Baker (Deventer, Holland). HPLC grade water was produced with a Milli-DI system coupled with a Synergy 185 system by Millipore (Milan, Italy). Orthophosphoric acid and potassium dihydrogen phosphate were purchased from Sigma–Aldrich (Milan, Italy).
Sample Preparation
The study simulated the real-life preparation of the IV mixtures. Twelve solutions (bags) of meropenem were prepared: 2 solutions (light protected and not light protected) were prepared at different concentrations (10 mg/mL and 4 mg/mL) for each pharmaceutical product. Merrem 1000 mg was reconstituted with 20 mL of sodium chloride 0.9% and diluted in polyolefin bags (100 and 250 mL) to obtain final concentrations of 10 mg/mL and 4 mg/mL, respectively. A sterile syringe was used to withdraw 0.5 mL of these solutions and put it in the vials for the injection of chromatographic system. The same procedure was performed for the other drugs: meropenem 500 mg (H) and meropenem 1000 mg (X). Ten series of tests (5 for each concentration) were performed for each drug formulation. The samples were withdrawn from each bag immediately after reconstitution (time 0) and every hour throughout the process until time 6. Injections into the chromatographic system were performed in duplicate (and results averaged) to eliminate the possible differences due to the injection system. The solutions were visually inspected for precipitate and color change and were tested for pH. The pH value of each sample was measured from time 0 to 6 for every test run, using an Orion model SA520 pH meter (Milan, Italy). The solutions were assayed using an new ultra-performance liquid chromatography (UPLC-PDA) method similar to the previously adopted method by Mendez et al.4
UPLC Assay and Chromatographic Condition
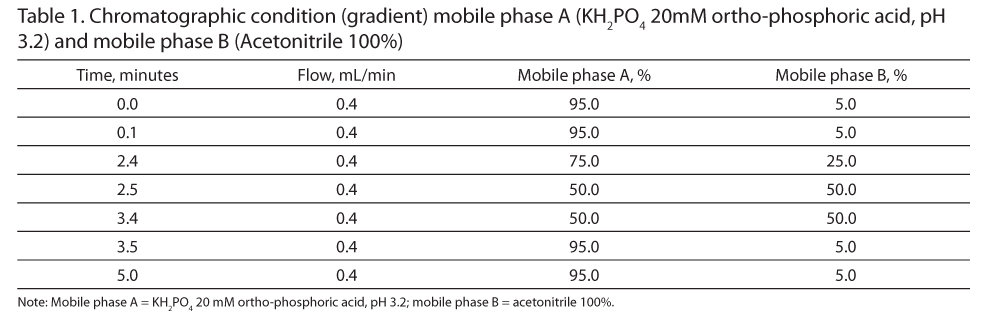
The chromatographic separations were performed using an AQUITY UPLC H-Class System (Waters-Milan, Italy) that included a quaternary solvent manager (QSM), sample manager (SM), and a photo-diode array (PDA) detector. The gradient run has been performed as shown in Table 1. In according with robustness criteria,9 the mobile phase, composed of Solvent A (KH2PO4 20 mM with orto-phosphoric acid, pH 3.23) and Solvent B (acetonitrile 100%), was freshly prepared and pH was measured at the beginning of each chromatographic session. Autosampler temperature (SM) was set at 25°C. The temperature of the reversed-phase column C18 (ACQUITY UPLC HSS T3 1.8 µm 2.1x150 mm with pre-column) was heated at 40°C, and the flow rate was set at 0.4 mL/min. The PDA detector was set at 300 nm and the injection volume for each sample was 0.1 µL. Gradient run time was 5 minutes (Table 1). Each run was also monitored with a scan wavelength (range, 200-600 nm) to identify possible degradation products.
Calibration Curve
The calibration curve for meropenem was generated with meropenem reference powder (Sigma – SZBA323XV) with known purity (reference material), diluted with MES [2-(N-morpholino) ethanesulfonic acid] buffer. The calibration curve and the linearity assessment of the method were evaluated at 9 concentrations (STD1, 0.047 mg/mL; STD2, 0.094 mg/mL; STD3, 0.188 mg/mL; STD4, 0.375 mg/mL; STD5, 0.75 mg/mL; STD6, 1.5 mg/mL; STD7, 3 mg/mL; STD8, 6 mg/mL; and STD9,12 mg/mL).
Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) was carried out using a Perkin-Elmer DSC/7 (Perkin-Elmer, CT, USA) equipped with a TAC 7/DX instrument controller. The instrument was calibrated with indium for melting point and heat of fusion. A heating rate of 10°C/min was employed in the 25°C to 160°C temperature range. Standard aluminium sample pans (Perkin-Elmer) were used; an empty pan was used as reference standard. Thermal analyses of the 3 products were performed in triplicate on 5 mg samples under nitrogen purge.
Stability
The stability profile of this drug in 3 different commercial products was investigated until hour 6. The bags, prepared as described in detail previously, were stored in darkness and light at controlled room temperature (25°C ± 3°C).The stability of the drug concentrations was analyzed between the samples and the quality controls (QCs) that were freshly prepared from the same bags at time 0. Data were expressed in percent (%) as ratio between the drug concentrations considered at different times of analysis and the concentrations at time zero (QCs). Stability was defined according to the International Conference on Harmonization (ICH) guidelines.9,10
Another stability indicator was evaluated for all pharmaceutical products; this was visual inspection against a dark and a light background.
Selection of Batches
This stability study was carried out using 3 different batches for each medicinal product, as requested by the European guidelines on stability studies.9,10
Stress Testing
In accordance with the European guidelines on stability studies, stress testing is necessary to examine the degradation of products under different stress conditions.9 Stress tests can be considered adequate if they show a degradation of the drug by 30% to 40%, as reported by ICH guidelines.9 Degradation of meropenem in aqueous solution is influenced by pH, temperature, initial concentration, and type of infusion solution.8
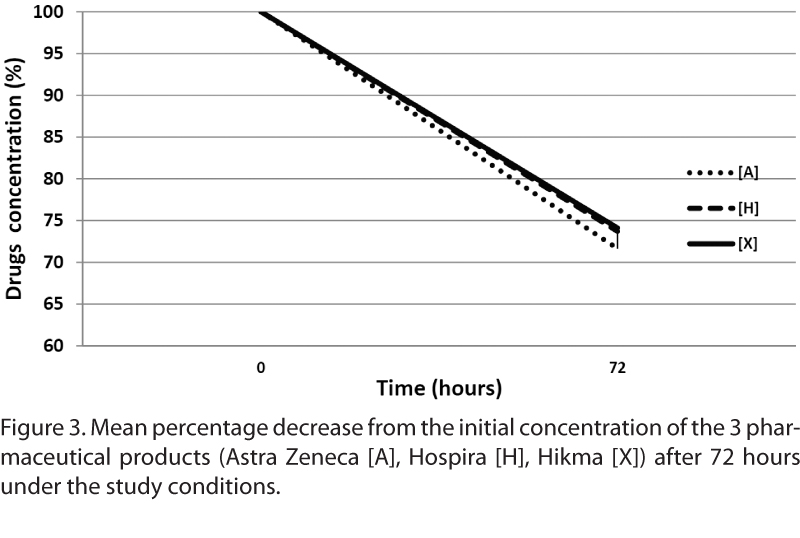
Stress experiments have been performed in glass vials to study the intrinsic stability of the solution. For this study, the stress component for meropenem was the time. This drug is stable for a short time as demonstrated by Berthoin et al7 who showed that carbapenems are quite unstable in concentrated (>10 mg/mL) aqueous solutions (≥10% degradation in ≤5-6 hours at 25°C). The samples were withdrawn from each bag after 72 hours and were injected in UPLC-PDA.
Statistics
We evaluated the percentage differences in degradation between the different pharmaceutical products. Values have been expressed as mean, and we have considered at time 0 each nominal value as 100% for each product.
RESULTS
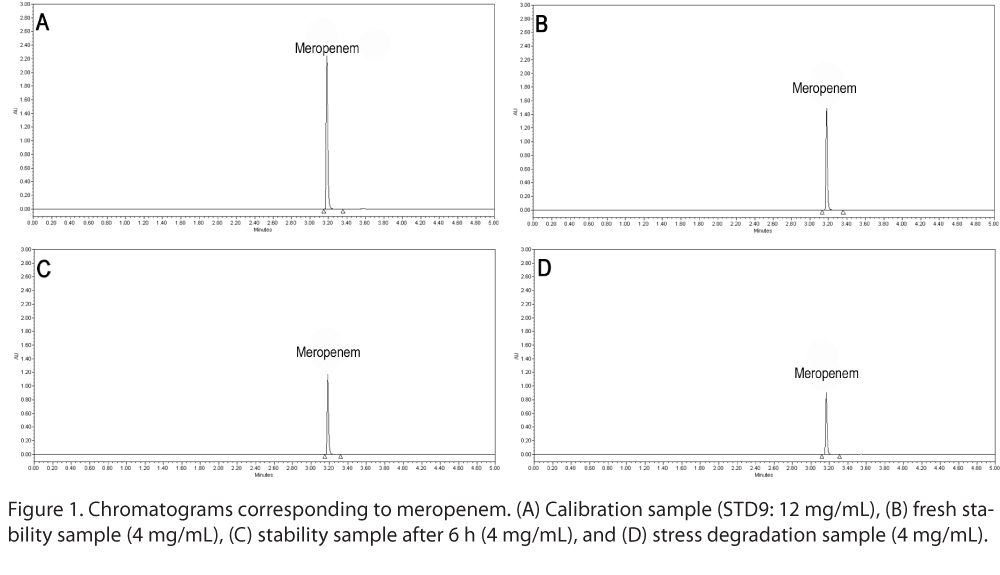
The retention time of meropenem showed by our UPLC-PDA method was 3.2 minutes. Figure 1 shows the different chromatograms of the (A) calibration sample, (B) fresh stability sample, (C) stability sample after 6 hours, and (D) stress degradation sample. The method showed a good linearity response: the mean results of the linear regression analysis showed r2 ≥ 0.998 (n = 10). The small volume (0.1 µL) of injection was chosen to avoid saturation of the PDA detector. Moreover, the manufacturer’s guide to the autosampler (Waters), in particular in the specifications of the Waters sample manager, indicates 0.1 to 10.0 µL as standard for the injection volume range with a relative standard error less than 0.15% for 6 replicate injections. These data were supported by our continuous repeated injections results.
Due to the short duration of the study (6 hours), the time spent in the UPLC autosampler is negligible; the overall duration of a complete cycle of injections for each time period was less than an hour, because each chromatographic run was 5 minutes. Assay precision of our method, using QC solutions at 4 mg/mL and 10 mg/mL, showed a standard deviation less than 1%. Assay accuracy, in according with ICH Q2 (R1)10 criteria, was evaluated with the application of an analytical procedure to an analyte of known purity (reference material), as reported in Material and Methods section. No appreciable pH change occurred over the 6 hours; all the solutions showed a pH of about 7.3 to 8.2, as suggested by Trissel,11 and there was no visual evidence of incompatibility in all drug solutions.
The mean of initial meropenem concentrations for all 3 pharmaceutical products that were studied, compared to calibration curve, showed no differences between each other. The mean relative percentage deviation with respect to the nominal values was +7.1%, -2.8%, and +0.2% for Merrem (A), meropenem (H), and meropenem (X), respectively.
Stability
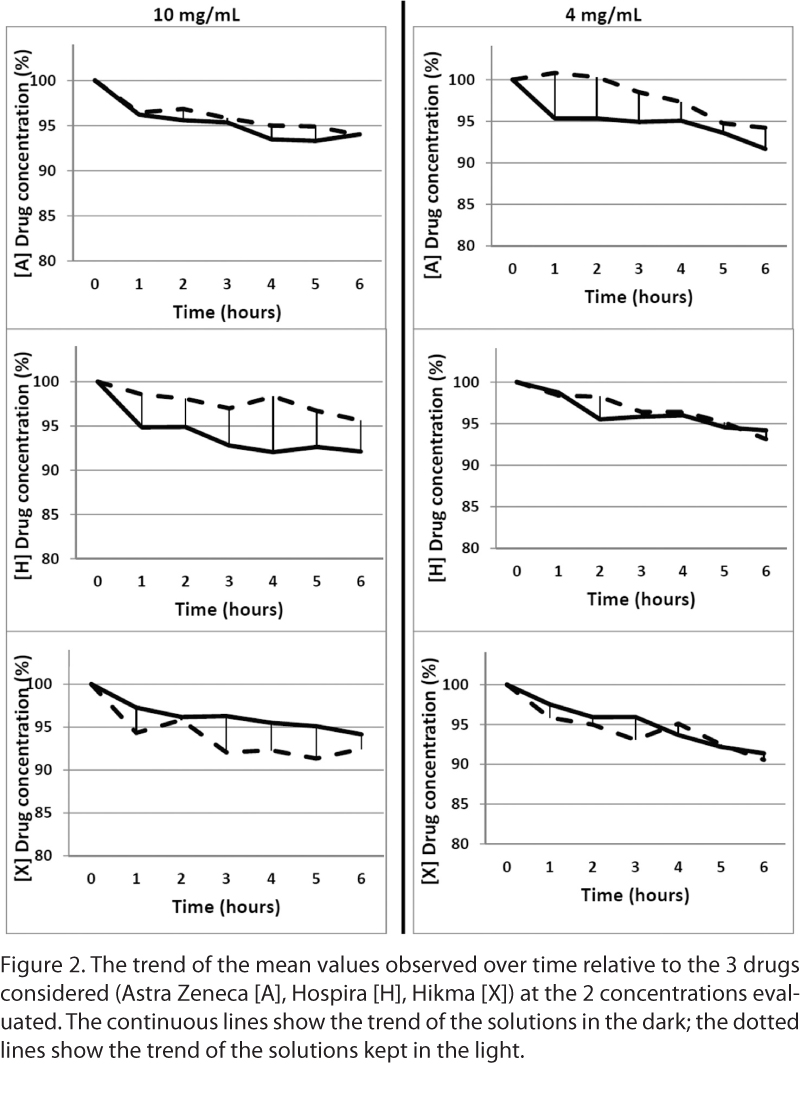
An overview of the results (mean observed values) is shown in the Figure 2. Merrem(A), at the concentration of 10 mg/mL, seems to have a loss in initial concentration of around 5% after 4 hours and around 6% after 6 hours. At the concentration of 4 mg/mL, the loss observed after 4 hours is the same; after 6 hours, the decrease is approximately 8% for the bags stored in the light and 6% for bags stored in the darkness.
Meropenem (H) (10 mg/mL) appears to have little difference in stability between the light-protected bags and those not light protected, with a decrease from initial concentration of approximately 8% and only 2%, respectively. For the lowest concentration (4 mg/mL), we observed no differences between the bags stored in the light and in the dark.
Meropenem (X) at the concentration of 10 mg/mL showed stability until 6 hours, with a degradation of 7% to 8% from initial concentration. For the other concentration (4 mg/mL), the decrease observed was around 4% after 3 to 4 hours and around 10% after 6 hours. The mean percentage of meropenem concentration observed from time 0 to 4 hours was greater than 95% for all studied pharmaceutical products when stored in polyolefin bags at room temperature. No differences among the 3 batches were observed.
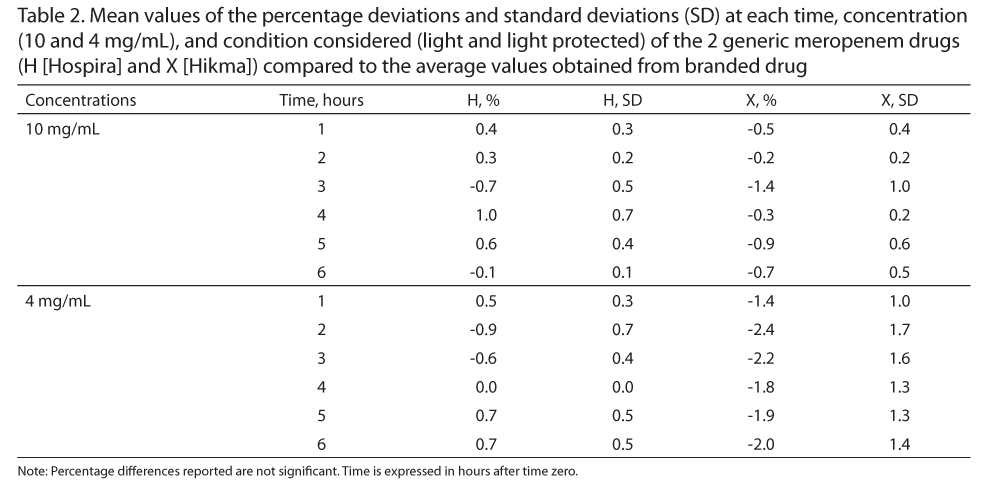
The deviation values (%) of the stability profile between generics (meropenem [H] and [X]) and the branded drug (Merrem [A]) were less than 2.4% (Table 2). These values are very low, and the stability profiles of generic products are similar to the branded drug.
Stress Testing
The assay to highlight the degradation products of meropenem after 72 hours at room temperature is shown in Figure 3. The initial concentration of all 3 products experienced the same effect: the drug degrades to 30% at the sixth hour and the color of the solutions becomes yellow. Degradation of the products was due to hydrolysis of the active substance.
Differential Scanning Calorimetry
The solid state of the 3 products was evaluated by thermal analysis. The differential scanning calorimetry thermograms demonstrated the same thermal behavior.
DISCUSSION
Stability of meropenem stock solution at room temperature was previously investigated by Berthoin et al7 and, recently, by Mollà-Cantavella et al.12 No data on in vitro stability of generic meropenem drugs are available. The hospital pharmacist may have difficulty in stability data management for many injectable drugs, especially if the drugs have to be infused for long time. Often, data sheets of the drugs do not report sufficient data to establish the full stability profile. For these reasons, we conducted this stability evaluation for these 3 different pharmaceutical products.
This stability study was conducted in accordance with the guidelines for stability studies. The EMEA guidelines are not applicable to this work from the statistical point of view, because we have not performed a “true” assessment of the stability of a single pharmaceutical product, but a simple comparison. As compared to these previously methods,6,12 our chromatographic assay provides some technical and cost advantages, such as the extraction simple dilution and injection in UPLC-PDA method.
The concentration values studied about meropenem of 10 mg/mL (maximum drug concentration) and 4 mg/mL (minimum drug concentration) were considered therapeutic. Degradation products of this molecule are characterized asbeta-lactam ring–opened meropenem and the drug dimer, which results from intermolecular aminolysis.13,14 The degradation follows pseudo first-order decomposition13 without any toxic products. The concentration-time profile of all meropenem pharmaceutical products evaluated in this study remained above 95% of the initial concentrations until the fourth hour and above 90% until the sixth hour, then the stability profile within the 3 products was shown to be comparable after reconstitution in 0.9% sodium chloride in polyolefin bags stored at room controlled temperature (25°C ± 3°C). Moreover, as shown in the studies of Patel et al,13 the material of the containers used for gravity infusion do not have any impact on the stability of meropenem.
We have considered the same stability profile of the drug at intermediate concentration values on the basis of the linear response using the calibration curve. The chemical and physical stability of meropenem (as previously demonstrated) is especially temperature sensitive4,7 (≤5 hours at 32°C -37°C), as this study highlights.4 Our observation in relation to the initial concentration (time 0) detected in each kind of drug is interesting. If the generic formulations showed content amount as expected (from - 2.8% to +0.2%), the concentrations found for the branded product showed an average amount more than 7% in respect to our calibration curve, but this could be considered negligible.
Meropenem has time-dependent bactericidal activity, so a continuous infusion is preferred to increase the efficiency.6,16 Therefore, considering both the pharmacokinetic and pharmacodynamic (PK/PD) properties of meropenem, the small percentages of deviation between the stability profiles of the products (about 1%-2%) (Table 2) do not affect the pharmacokinetic trend. These variations of PK/PD can be detected in the values of individual patients (intra-individual variability) and between patients (interindividual variability). In addition, genetic, physiologic, and pathologic factors are more important for the patients and require accurate dose adjustments.17,18
The results of our study showed negligible differences between meropenem A, H, and X. Considering the UPLC data, it is possible to conclude that no differences were identified among the reconstituted samples of the 3 products containing meropenem. For these reasons, we conclude that the stability profiles of generics products are similar to the branded one after 6 hours in NaCl 0.9% in polyolefin bags. Moreover, the possibility to choose generic products instead of the branded product allows important cost saving for the hospitals.
ACKNOWLEDGMENTS
The authors declare no conflicts of interest.
UNI EN ISO 9001:2008 Certificate Laboratory; Certificate No. IT-64386; Certification for: “Design, Development and Application of Determination Methods for Anti-Infective Drugs. Pharmacogenetic Analyses.” www.tdm-torino.org
REFERENCES
- Meropenem. PubChem open chemistry database. pubchem.ncbi.nlm.nih.gov/compound/meropenem
- Mendez A, Chagastelles P, Palma E, Nardi N, Schapoval E. Thermal and alkaline stability of meropenem: Degradation products and cytotoxicity. Int J Pharm. 2008;350:95-102.
- Jaruratanasirikul S, Sriwiriyajan S. Stability of meropenem in normal saline solution after storage at room temperature. Southeast Asian J Trop Med Public Health. 2003;34:627-629.
- Mendez AS, Dalomo J, Steppe M, Schapoval EE. Stability and degradation kinetics of meropenem in powder for injection and reconstituted sample. J Pharm Biomed Anal. 2006;41:1363-1366.
- Nicolau DP. Pharmacokinetic and pharmacodynamic properties of meropenem. Clin Infect Dis. 2008;47(suppl 1):S32-40.
- AIC Italian dossier. www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Meronem_30/WC500018555.pdf.
- Berthoin K, Le Duff CS, Marchand-Brynaert J, Carryn S, Tulkens PM. Stability of meropenem and doripenem solutions for administration by continuous infusion. J Antimicrob Chemother. 2010;65:1073-1075.
- Kramer, I. Stability of meropenem in elastomeric portable infusion device. Eur J Hosp Pharm. 1997;3:168-171.
- International Conference on Harmonisation on Technical Requirments for Registration for Pharmaceuticals for Human Use. ICH Harmonised Tripartate Guidelines. Stability testing of new drug substances and products. Q1A (R2). February 2003. www.ich.org/products/guidelines/quality.html.
- International Conference on Harmonisation on Technical Requirments for Registration for Pharmaceuticals for Human Use. ICH Harmonised Tripartate Guidelines. Validation of analytical procedures: Text and methodology. Q2 (R1). November 2005. www.ich.org/products/guidelines/quality.html
- Trissel LA. Handbook on Injectable Drugs. 11th ed. Bethesda, MD: American Society of Health-System Pharmacists; 2007:835-836.
- Mollà-Cantavella S, Ferriols-Lisart R, Torrecilla-Junyent T, Alós-Almiñana M. Intravenous meropenem stability in physiological saline at room temperature. Eur J Hosp Pharm. 2014;21:202-207.
- Patel PR, Cook SE. Stability of meropenem in intravenous solutions. Am J Health Syst Pharm. 1997;54:412-421.
- Takeuchi Y, Sunagawa M, Isobe Y, Hamazume Y, Noguchi T. Stability of a 1 beta-methylcarbapenem antibiotic, meropenem (SM-7338) in aqueous solution. Chem Pharm Bull (Tokyo). 1995;43:689-692.
- Takeuchi Y, Inoue T, Sunagawa M. Studies on the structures of meropenem (SM-7338) and it’s primary metabolite. J Antibiot (Tokyo). 1993;46:827-832.
- Craig WA. The pharmacology of meropenem, a new carbapenem antibiotic. Clin Infect Dis. 1997;24(suppl 2):
S266-275. - Robatel C, Decosterd LA, Biollaz J, Eckert P, Schaller MD, Buclin T. Pharmacokinetics and dosage adaptation of meropenem during continuous venovenous hemodiafiltration in critically ill patients. J Clin Pharmacol. 2003;43:1329-1340.
- Thalhammer F, Horl WH. Pharmacokinetics of meropenem in patients with renal failure and patients receiving renal replacement therapy. Clin Pharmacokinet. 2000;39:271-279.

*Hospital Pharmacy, Maria Vittoria, S.G. Bosco, and Amedeo di Savoia Hospitals, ASL TO2, Turin, Italy; ?Department of Medicine Science and Technology, University of Turin, Italy; ‡Departmental Director, Unit of Infectious Diseases, University of Turin; §Department of Medical Sciences, Amedeo di Savoia Hospital, Turin, Italy. Corresponding author: Cristina Tomasello, PharmD, Hospital Pharmacy, Maria Vittoria, S.G. Bosco, and Amedeo di Savoia Hospitals, ASL TO2, Corso Svizzera 164, 10149 Turin, Italy; phone: +39011/4393674, +393486625761; fax: +39011/4393654; e-mail: cristina.tomasello@aslto2.piemonte.it; cri-pharm@libero.it