Case Report
Glacial Acetic Acid Adverse Events: Case Reports and
Review of the Literature
William Doles, PharmD*; Garrett Wilkerson, PharmD†; Samantha Morrison, BS, CPhT‡; and Rodney G. Richmond, MS, CGP, FASCP§
Case Report
Glacial Acetic Acid Adverse Events: Case Reports and
Review of the Literature
William Doles, PharmD*; Garrett Wilkerson, PharmD†; Samantha Morrison, BS, CPhT‡; and Rodney G. Richmond, MS, CGP, FASCP§
Case Report
Glacial Acetic Acid Adverse Events: Case Reports and
Review of the Literature
William Doles, PharmD*; Garrett Wilkerson, PharmD†; Samantha Morrison, BS, CPhT‡; and Rodney G. Richmond, MS, CGP, FASCP§
Abstract
Glacial acetic acid is a dangerous chemical that has been associated with several adverse drug events involving patients over recent years. When diluted to the proper concentration, acetic acid solutions have a variety of medicinal uses. Unfortunately, despite warnings, the improper dilution of concentrated glacial acetic acid has resulted in severe burns and other related morbidities. We report on 2 additional case reports of adverse drug events involving glacial acetic acid as well as a review of the literature. A summary of published case reports is provided, including the intended and actual concentration of glacial acetic acid involved, the indication for use, degree of exposure, and resultant outcome. Strategies that have been recommended to improve patient safety are summarized within the context of the key elements of the medication use process.
Key Words—adverse events, glacial acetic acid, medication errors
Hosp Pharm—2015;50:304–309
Abstract
Glacial acetic acid is a dangerous chemical that has been associated with several adverse drug events involving patients over recent years. When diluted to the proper concentration, acetic acid solutions have a variety of medicinal uses. Unfortunately, despite warnings, the improper dilution of concentrated glacial acetic acid has resulted in severe burns and other related morbidities. We report on 2 additional case reports of adverse drug events involving glacial acetic acid as well as a review of the literature. A summary of published case reports is provided, including the intended and actual concentration of glacial acetic acid involved, the indication for use, degree of exposure, and resultant outcome. Strategies that have been recommended to improve patient safety are summarized within the context of the key elements of the medication use process.
Key Words—adverse events, glacial acetic acid, medication errors
Hosp Pharm—2015;50:304–309
Abstract
Glacial acetic acid is a dangerous chemical that has been associated with several adverse drug events involving patients over recent years. When diluted to the proper concentration, acetic acid solutions have a variety of medicinal uses. Unfortunately, despite warnings, the improper dilution of concentrated glacial acetic acid has resulted in severe burns and other related morbidities. We report on 2 additional case reports of adverse drug events involving glacial acetic acid as well as a review of the literature. A summary of published case reports is provided, including the intended and actual concentration of glacial acetic acid involved, the indication for use, degree of exposure, and resultant outcome. Strategies that have been recommended to improve patient safety are summarized within the context of the key elements of the medication use process.
Key Words—adverse events, glacial acetic acid, medication errors
Hosp Pharm—2015;50:304–309
Hosp Pharm 2015;50(4):304–309
2015 © Thomas Land Publishers, Inc.
doi: 10.1310/hpj5004-304

Glacial acetic acid is the trivial name used to refer to pure acetic acid in an anhydrous state. It is a colorless, hygroscopic, weak acid that is available in concentrations of 99.5% to 100%. Similar to the German name Eisessig (ice-vine-gar), the word “glacial” is derived from the ice-like crystals that form at 16.6°C (61.9°F), slightly below room temperature.1 Although classified as a weak acid, glacial acetic acid is a corrosive poison that can cause injury or death when human tissue is exposed to it. At concentrations of 10% to 25% (1.67-4.16 mol/L), it acts as an irritant; but at concentrations greater than 25% (>4.16 mol/L), it is corrosive and should be handled in a fume hood.2 Skin contact may produce blistering or burns, while liquid or spray mist may produce tissue damage particularly on mucous membranes of the eyes, mouth, and respiratory tract.3
Acetic acid has been used for centuries in food production, manufacturing, cleaning, and even medical purposes. Generally, undiluted glacial acetic acid has no medical use, but a review of the literature reveals that acetic acid in diluted concentrations has been used for a variety of indications. Diagnostically, such indications include oral screening and lesion identification4 (1%, mouth rinse) and Barrett’s esophagus5 (1.5%-2.5%, spray). As treatment, it has been used for bladder and wound irrigation6 (0.25%-0.5%, irrigation solution). Slightly higher concentrations have been used for otitis externa,7 iontophoresis,8 ear wax removal,9 and cervicoscopy after an abnormal Pap smear10 (1%-5%, topical solution). Controversially, it has also been used for wound infections,11 vaginal douching,12 and as a neutralizing diluting agent for alkali skin burns.13 Yet even more concentrated, acetic acid has a place in renal cyst sclerotherapy14 and hepatocellular carcinoma15 (50%, injection).
A number of acetic acid products are commercially available. Prediluted, ready-to-use USP formulations include products such as acetic acid/aluminum acetate otic and premixed irrigation solutions. Dietary vinegar (5%) has also been used medically for irrigation and topical application. Sometimes, however, concentrated chemical-grade glacial acetic acid is used, but it requires a pharmacist’s skill to compound and dispense before it is acceptable for human use. Despite warning labels on the container, repeated incidents of concentrated glacial acetic acid being dispensed instead of a diluted form have caused patient injury. We describe 2 reports of injuries due to glacial acetic acid and a review of the literature of similar cases with recommendations for patient safety.
CASE REPORTS
Patient Case 1
A 59-year-old female was admitted to the hospital to undergo a wide local excision of a lesion on her vulva. Acetic acid 4% was to be used in the procedure to demarcate the abnormal epithelium. Although there was confusion over whether the strength ordered electronically was 0.25% or 4%, ultimately the order that was delivered to the operating room was prepared by the pharmacist as acetic acid 80%. No explanation was given regarding how this difference occurred. The acetic acid solution was poured onto the indicated area; within a minute of application, a strong acetic acid smell was noted. The surgeon requested the strength of the solution be verified, and an inspection of the label visible on the bottle indicated acetic acid 80%. The procedure was terminated and 4 L of normal saline was used to irrigate the area, followed by an injection of bupivacaine 0.25% with epinephrine 1:200,000 and the application of silver sulfadiazine cream. The patient experienced second-degree partial thickness chemical burns on her labia minora and majora, perineum, rectum, and sacrum extending to the lower lumbar back. Subsequent treatment included topical lidocaine, triple antibiotic cream, and oral opiate analgesia and antibiotics.
Patient Case 2
A 50-year-old male paraplegic who had an indwelling suprapubic catheter developed problems with the build-up of particulate matter in the catheter. Acetic acid solution 0.25% was ordered for catheter clearance, but an error occurred when the telephone order was placed resulting in a prescription for acetic acid 25%. Because the pharmacist had never compounded an acetic acid solution at that -concentration, he declined to dispense the prescription and referred the patient back to his physician. Because the prescribing physician was unavailable, a covering physician recommended the patient wait to speak with the prescriber because there was confusion over the order. However, the patient was not advised that a 25% acetic acid solution posed a danger. In the interim, the patient went to a different pharmacy that agreed to compound the prescription. The pharmacist filling the prescription did not question the strength, even though they later stated that they knew it was inappropriate for the intended purpose. Use of the 25% solution caused severe burns to the urethra and bladder and “melted” the catheter around the patient’s penis. The patient required inpatient treatment, after which he chronically complained of burning sensations in his penis and bladder.
DISCUSSION
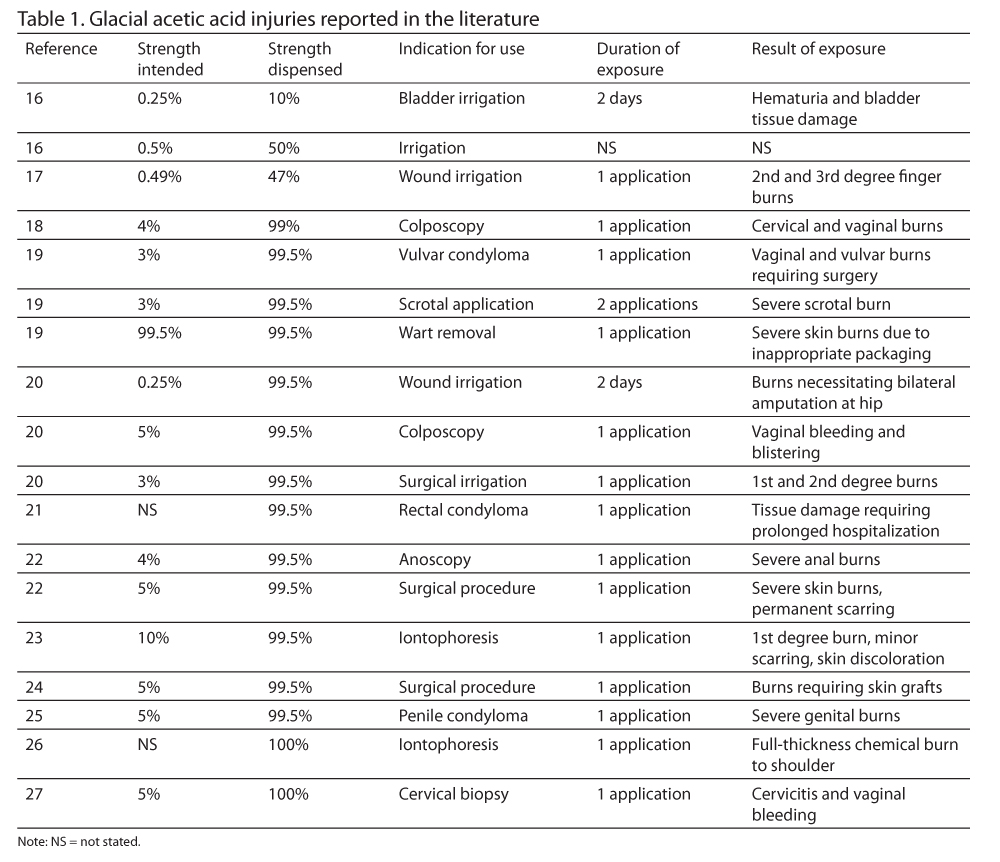
Multiple reports have been published advising of the dangers associated with the use of glacial acetic acid in clinical patient care. Table 1 summarizes 18 cases of injury that have been reported in the literature.16-27 An additional report appears in the literature of 5 women who were treated in a colposcopy clinic with glacial acetic acid instead of acetic acid 5% as a result of a computer entry error, although no detectable harm was reported.28
As can be seen from these cases, serious patient harm has resulted from the use of concentrated glacial acetic acid. Root cause analyses have revealed that problems associated with inaccurate medication reconciliation; improper prescribing, dilution, and labeling; inappropriate packaging; an absence of checks and balances; inadequate drug information resources; and lack of familiarity regarding the properties of glacial acetic acid are -common causes of errors among health care providers. Other causes have been attributed to the fact that glacial acetic acid is not a drug and therefore not regulated by the US Food and Drug Administration (FDA). Thus, precautionary labels applied to product packaging by the chemical industry (Figure 1)29
are often inconsistent, inconspicuous, or even absent when compared to those customarily seen on FDA-approved drugs.
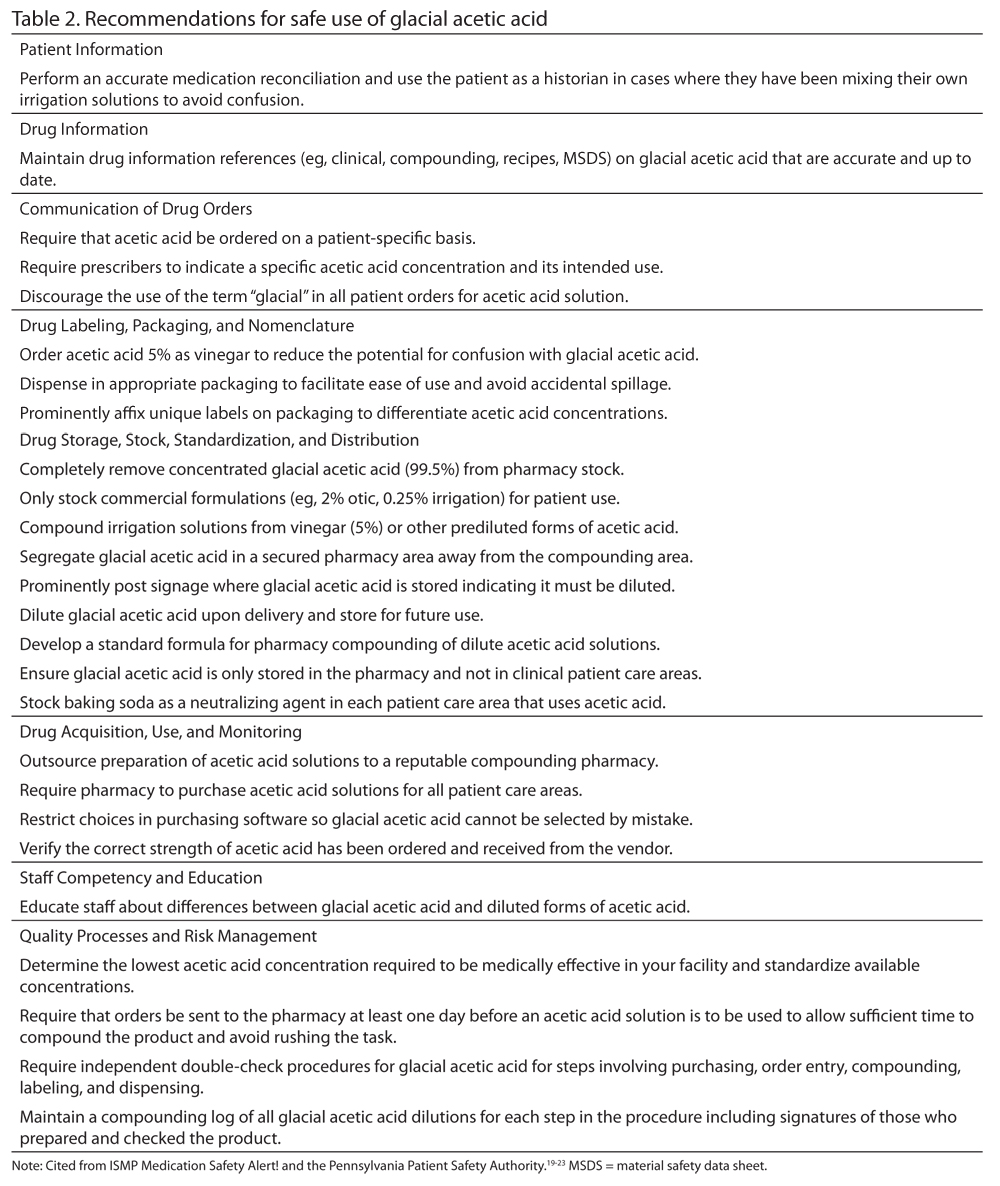
Based on these findings, several patient safety strategies have been recommended in an effort to avoid incidents with glacial acetic acid. These recommendations are gathered from previous ISMP Medication Safety Alert! publications and other case reports19-23,30 and have been organized according to ISMP’s Key Elements of the Medication Use System31 (Table 2). This set of recommenda-tions can serve as a tool for managing this recurring problem.
CONCLUSIONS
The storage and use of glacial acetic acid inherently poses a potential danger and can cause patient injury when not properly diluted. Medication safety experts have advocated for several strategies that can mitigate the risk, for both patients and health care providers, involved in the use of this chemical. The -application of these strategies to the medication use process can work to establish a system with fail-safes that prevent glacial acetic acid from reaching the patient.
ACKNOWLEDGMENTS
The authors declare no conflicts of interest. Mr. Richmond has provided expert testimony as a pharmacist through The Mackenzie Group, LLC.
REFERENCES
- Armarego WLF, Chai CLL. Purification of Laboratory Chemicals. 6th ed. Waltham, MA: Butterworth-Heinemann (Elsevier); 2009.
- Yee A. HSIS consolidated list – Alphabetical index. Updated May 10, 2013. http://hsis.safeworkaustralia.gov.au. Accessed June 26, 2014
- ScienceLab.com. Glacial acetic acid MSDS. Updated October 9, 2005. http://www.sciencelab.com/msds.php?msdsId=9922769. Accessed June 26, 2014.
- Farah CS, McCullough MJ. A pilot case control study on the efficacy of acetic acid wash and chemiluminescent illumination (ViziLite) in the visualisation of oral mucosal white lesions. Oral Oncol. 2007; 43(8): 820-824.
- Vázquez-Iglesias JL, Alonso-Aguirre P, Diz-Lois MT, Vázquez-Millán MA, Alvarez A, Lorenzo MJ. Acetic acid allows effective selection of areas for obtaining biopsy samples in Barrett’s esophagus. Eur J Gastroenterol Hepatol. 2007;19(3):187-193.
- Hospira, Inc. Acetic acid 0.25% irrigation solution, USP product labeling. Updated October 2004. http://www.hospira.com/Images/EN-0098_32-5378_1.pdf. Accessed June 26, 2014.
- Facts & Comparisons eAnswers. Acetic acid/aluminum acetate otic solution monograph. http://online.factsandcomparisons.com. Accessed June 26, 2014.
- Shetty S, Moore TL, Jackson S, Brettle D, Herrick AL. A pilot study of acetic acid iontophoresis and ultrasound in the treatment of systemic sclerosis-related calcinosis. Rheumatology (Oxford). 2005;44(4):536-538.
- First Databank, Inc. Auralgan with acetic acid otic monograph. March 2013. http://www.webmd.com/drugs/drug-149029. Accessed June 26, 2014.
- Gaffikin L, Lauterbach M, Blumenthal PD. Performance of visual inspection with acetic acid for cervical cancer screening: A qualitative summary of evidence to date. Obstet Gynecol Surv. 2003;58(8):543-550.
- Johnston CS, Gaas CA. Vinegar: Medicinal uses and antiglycemic effect. MedGenMed. 2006;8(2): 61.
- Martino JL, Vermund SH. Vaginal douching: Evidence for risks or benefits to women’s health. Epidemiol Rev. 2002;24(2):109-124.
- Andrews K, Mowlavi A, Milner SM. The treatment of alkaline burns of the skin by neutralization. Plast Reconstr Surg. 2003;111(6):1918-1921.
- Yoo KH, Lee SJ, Jeon SH. Simple renal cyst sclerotherapy with acetic acid: Our 10-year experience. J Endourol. 2008;22(11):2559-2563.
- Ma H, Liu J, Liu F. CT-guided single high-dose percutaneous acetic acid injection for small hepatocellular carcinoma: A long-term follow-up study. Eur J Radiol. 2012;81(6):1184-1186.
- Institute for Safe Medication Practices. Messages in our mailbox-in response to our May 5, 2005 article. End the ice age-is glacial acetic acid really needed? ISMP Medication Safety Alert. 2005;10(13):3.
- Brown v. Southern Baptist Hospital. No. 96-CA-1990. 715 So. 2d 423; 1998 La. App. LEXIS 556.
- Johnson v. Settle, MD. No. M1999-01237-COA-R3-CV. 2001 Tenn. App. LEXIS 412.
- O’Donnell J. From the courtroom: Pharmacists get burned by acid. Pharm Pract News. 2004;31(2).
- Institute for Safe Medication Practices. End the ice age- – Is glacial acetic acid really needed? ISMP Medication Safety Alert. 2005;10(9):1-2.
- Institute for Safe Medication Practices. Worth repeating: Is glacial acetic acid really needed at your hospital? ISMP Medication Safety Alert. 2012;17(19):1-2.
- National Alert Network. Warning! Severe burns and permanent scarring after glacial acetic acid (>99.5%) mistakenly applied topically. NAN Alert. 2013:1-2.
- Pennsylvania Patient Safety Authority. Glacial acetic acid: Doing more harm than good? Patient Safety Advisory. 2006;3(1):26-28.
- New Mexico Board of Pharmacy. Significant adverse drug events. New Mexico Board of Pharmacy News. 2012;14(3):1.
- Avery JK. Genital injuries–expensive. Tenn Med. 2004;97(8):353-354.
- Hines v. Meyer. No. 6444/10. 2011 N.Y. Misc. LEXIS 6804; 2011 NY Slip Op 33737(U).
- Ou KY, Chen YC, Hsu SC, Tsai EM. Topical vainal oestrogen cream used for treatment of burn injury of vaginal mucosa after misapplication of 100% acetic acid in a perimenopausal woman: A case report. Aust N Z J Obstet Gynaecol. 2007;47(4):345-346.
- Patients erroneously treated with concentrated acid [editorial]. Pharm J. 1987;238:129.
- SmartSign. Glacial acetic acid warning label. http://www.smartsign.com/SMT/QS/acetic_acid.aspx. Accessed June 26, 2014.
- Institute for Safe Medication Practices. 2014-15 targeted medication safety best practices for hospitals. http://www.ismp.org/tools/bestpractices/TMSBP-for-Hospitals.pdf. Accessed September 15, 2014.
- Institute for Safe Medication Practices. Pathways for medication safety: Looking collectively at risk. 2002. http://www.ismp.org/tools/pathwaysection2.pdf. Accessed June 26, 2014.

*Hickory Hill Pharmacy, Helena, Arkansas; ?Walgreens, Middletown, Kentucky; ‡The Mackenzie Group, LLC, Searcy, Arkansas; §Harding University College of Pharmacy, Searcy, Arkansas. Drs. Doles and Wilkerson and Ms. Morrison completed this work while serving as a doctor of pharmacy candidates at Harding University College of Pharmacy. Corresponding author: Rodney G. Richmond, MS, CGP, FASCP, Associate Professor, Pharmacy Practice, Harding University College of Pharmacy, 915 East Market Street, Box 12230, Searcy, AR 72149; phone: 501-279-4858; e-mail: rrichmond@harding.edu