The Role of Estrogen Modulators in Male Hypogonadism and Infertility
Amarnath Rambhatla, MD, Jesse N. Mills, MD, Jacob Rajfer, MD
Department of Urology, David Geffen School of Medicine at UCLA, Los Angeles, CA
Estradiol, normally considered a female hormone, appears to play a significant role in men in a variety of physiologic functions, such as bone metabolism, cardiovascular health, and testicular function. As such, estradiol has been targeted by male reproductive and sexual medicine specialists to help treat conditions such as infertility and hypogonadism. The compounds that modulate estradiol levels in these clinical conditions are referred to as selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs). In a certain subset of infertile men, particularly those with hypogonadism, or those who have a low serum testosterone to estradiol ratio, there is some evidence suggesting that SERMs and AIs can reverse the low serum testosterone levels or the testosterone to estradiol imbalance and occasionally improve any associated infertile or subfertile state. This review focuses on the role these SERMs and AIs play in the aforementioned reproductive conditions.
[Rev Urol. 2016;18(2):66-72 doi: 10.3909/riu0711]
© 2016 MedReviews®, LLC
The Role of Estrogen Modulators in Male Hypogonadism and Infertility
Amarnath Rambhatla, MD, Jesse N. Mills, MD, Jacob Rajfer, MD
Department of Urology, David Geffen School of Medicine at UCLA, Los Angeles, CA
Estradiol, normally considered a female hormone, appears to play a significant role in men in a variety of physiologic functions, such as bone metabolism, cardiovascular health, and testicular function. As such, estradiol has been targeted by male reproductive and sexual medicine specialists to help treat conditions such as infertility and hypogonadism. The compounds that modulate estradiol levels in these clinical conditions are referred to as selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs). In a certain subset of infertile men, particularly those with hypogonadism, or those who have a low serum testosterone to estradiol ratio, there is some evidence suggesting that SERMs and AIs can reverse the low serum testosterone levels or the testosterone to estradiol imbalance and occasionally improve any associated infertile or subfertile state. This review focuses on the role these SERMs and AIs play in the aforementioned reproductive conditions.
[Rev Urol. 2016;18(2):66-72 doi: 10.3909/riu0711]
© 2016 MedReviews®, LLC
The Role of Estrogen Modulators in Male Hypogonadism and Infertility
Amarnath Rambhatla, MD, Jesse N. Mills, MD, Jacob Rajfer, MD
Department of Urology, David Geffen School of Medicine at UCLA, Los Angeles, CA
Estradiol, normally considered a female hormone, appears to play a significant role in men in a variety of physiologic functions, such as bone metabolism, cardiovascular health, and testicular function. As such, estradiol has been targeted by male reproductive and sexual medicine specialists to help treat conditions such as infertility and hypogonadism. The compounds that modulate estradiol levels in these clinical conditions are referred to as selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs). In a certain subset of infertile men, particularly those with hypogonadism, or those who have a low serum testosterone to estradiol ratio, there is some evidence suggesting that SERMs and AIs can reverse the low serum testosterone levels or the testosterone to estradiol imbalance and occasionally improve any associated infertile or subfertile state. This review focuses on the role these SERMs and AIs play in the aforementioned reproductive conditions.
[Rev Urol. 2016;18(2):66-72 doi: 10.3909/riu0711]
© 2016 MedReviews®, LLC
Key words
Selective estrogen receptor modulator • Aromatase inhibitor • Male infertility • Hypogonadism
Key words
Selective estrogen receptor modulator • Aromatase inhibitor • Male infertility • Hypogonadism
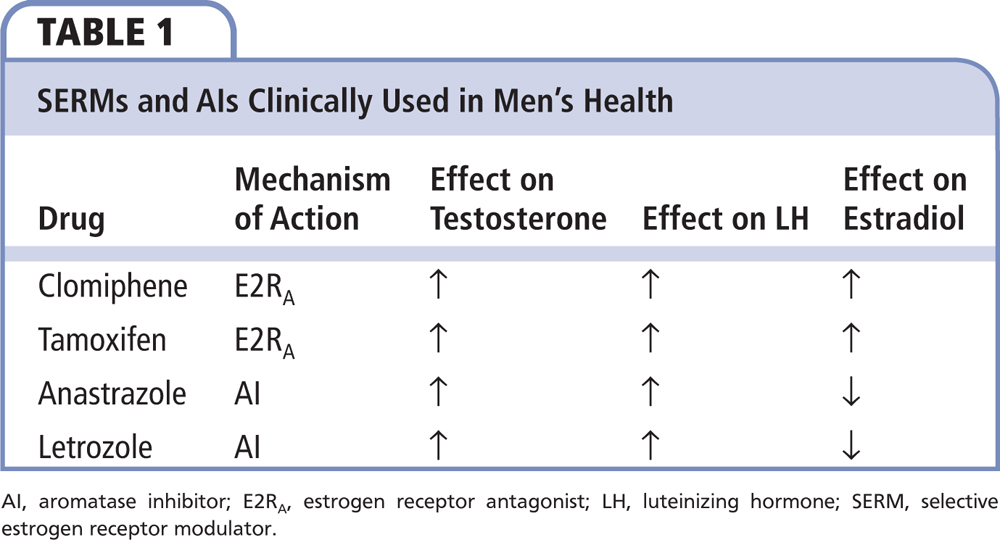

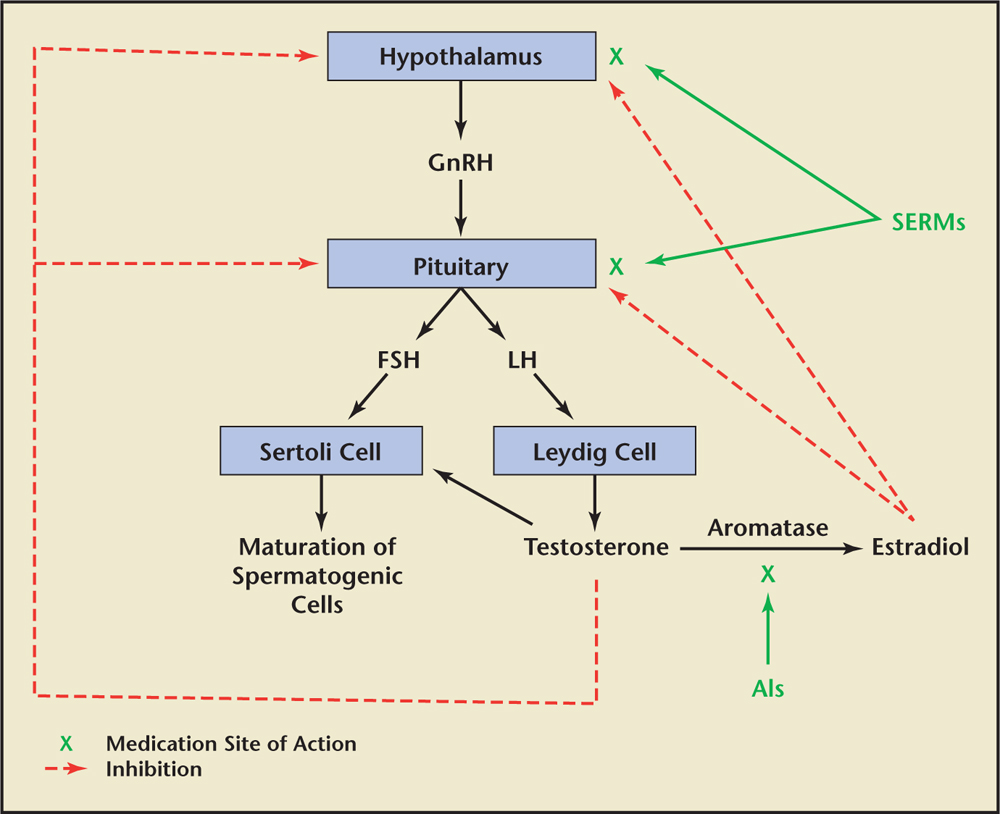
Figure 1. AI, aromatase inhibitor; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; SERM, selective estrogen receptor modulator.

Figure 1. AI, aromatase inhibitor; FSH, follicle-stimulating hormone; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; SERM, selective estrogen receptor modulator.
… clomiphene, unlike exogenous testosterone therapy, should not have a negative effect on spermatogenesis and consequently it does not impact the size of the testes.
Due to its antiestrogen effect on the pituitary, which causes an increase in the serum levels of FSH and LH, clomiphene appears to be an ideal product to treat the patient who has both a low testosterone level and a subnormal sperm count in the setting of low or normal gonadotropin levels.
… tamoxifen results in the inhibition of the negative feedback of estrogen at the hypothalamus and pituitary gland, and results in the release of LH and FSH, which in turn increases testosterone biosynthesis and “stimulates” spermatogenesis.
AIs are thought to inhibit the conversion of testosterone to estradiol peripherally inside adipocytes and also within the testes.
Despite their success, there are no long-term data evaluating the efficacy of AIs and, therefore, their use for hypogonadism cannot be routinely recommended at this time.
There is evidence that SERMs and AIs can be used in combination with human chorionic gonadotropin (hCG) for spermatogenesis recovery in azoospermia or severe oligospermia that is due to exogenous testosterone therapy.
Main Points
• Estradiol has been targeted by male reproductive and sexual medicine specialists to help treat conditions such as infertility and hypogonadism. In some infertile men, particularly those with hypogonadism, or those who have a low serum testosterone to estradiol ratio, there is some evidence suggesting that selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs) can reverse the low serum testosterone levels or the testosterone to estradiol imbalance and occasionally improve any associated infertile or subfertile state.
• SERMs exhibit tissue-specific estrogen receptor agonist or antagonist activity. Clomiphene citrate (clomiphene) and tamoxifen are two SERMs that are commonly used in men’s health. They work as estrogen antagonists at the level of the pituitary gland and thus stimulate the release of luteinizing hormone and follicle-stimulating hormone, which in turn drive both the steroidogenic and spermatogenic functions of the testes.
• Although the studies on tamoxifen use in men have largely focused on those men who have infertility, its mechanism of action suggests it can also be used to raise testosterone levels in men with low testosterone who possibly may have relatively elevated serum estradiol levels.
• AIs lower estrogen levels by blocking the aromatase enzyme, which converts testosterone to estradiol. Their use has broadened to include conditions in which the desire is to either lower serum estradiol and/or increase serum testosterone levels.
Main Points
• Estradiol has been targeted by male reproductive and sexual medicine specialists to help treat conditions such as infertility and hypogonadism. In some infertile men, particularly those with hypogonadism, or those who have a low serum testosterone to estradiol ratio, there is some evidence suggesting that selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs) can reverse the low serum testosterone levels or the testosterone to estradiol imbalance and occasionally improve any associated infertile or subfertile state.
• SERMs exhibit tissue-specific estrogen receptor agonist or antagonist activity. Clomiphene citrate (clomiphene) and tamoxifen are two SERMs that are commonly used in men’s health. They work as estrogen antagonists at the level of the pituitary gland and thus stimulate the release of luteinizing hormone and follicle-stimulating hormone, which in turn drive both the steroidogenic and spermatogenic functions of the testes.
• Although the studies on tamoxifen use in men have largely focused on those men who have infertility, its mechanism of action suggests it can also be used to raise testosterone levels in men with low testosterone who possibly may have relatively elevated serum estradiol levels.
• AIs lower estrogen levels by blocking the aromatase enzyme, which converts testosterone to estradiol. Their use has broadened to include conditions in which the desire is to either lower serum estradiol and/or increase serum testosterone levels.
It is well recognized that estradiol also plays a crucial role in a number of physiologic functions in men. The most recognized and well researched role of estradiol is its effect on bone metabolism.1 However, lesser known is the effect that estradiol has on the regulation of testosterone biosynthesis and spermatogenesis.2
In men, the majority of circulating estradiol is primarily derived from the peripheral aromatization of circulating testosterone by adipocytes located throughout the body.3 Estradiol can also be synthesized within certain tissues that require the hormone for its normal tissue homeostasis (eg, bone, brain, hypothalamus).4 Similar to what is found in the peripheral adipocytes, the bone, brain, and hypothalamus are known to contain an aromatase enzyme and/or an estrogen receptor site, suggesting a role for estrogen within these tissues.4 As such, it is believed that, in men, circulating testosterone—the majority of which is produced by the testes—is taken up by these nontesticular tissues via passive diffusion and is then converted within those specific tissues to estradiol, thereby allowing the newly formed estradiol to exert its effect on those specific tissues. Alternatively, and similar to what occurs with the circulating testosterone, it is believed that the circulating estradiol that is produced by those peripheral adipocytes and secreted into the circulation is also passively taken up by certain tissues and exerts its effect on the tissue via estrogen receptor sites located on the surface membranes of these tissues. An example of this similar effect by both testosterone and estradiol in men is seen within the hypothalamus, where either of these two hormones can be used to modulate the negative feedback inhibition of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary gland.5 It is primarily the modulation by selective estrogen receptor modulators (SERMs) and aromatase inhibitors (AIs) of this mechanism of action of testosterone and estradiol on the hypothalamic-pituitary-testicular axis that forms the clinical basis for trying to increase testosterone production and/or increase spermatogenesis within the testes (Table 1).6
It is well established that high levels of intratesticular testosterone are necessary for normal spermatogenesis.7 A common misconception by urologists is that exogenously administered testosterone can increase this intratesticular concentration of testosterone and result in an improvement in both the hypogonadism and infertility. This is evidenced by a recent American Urological Association (AUA) survey in which 25% of practicing urologists admitted administering testosterone to infertile men with hypogonadism in an effort to treat their infertility.8 Most of the time the prescriber in this setting is unaware of the inhibitory effect that exogenously administered testosterone has on spermatogenesis via its inhibition of both FSH and LH secretion by the pituitary gland. To circumvent the inhibition of LH and FSH from the pituitary by exogenous testosterone, a common strategy used by male reproductive and sexual medicine specialists, in which the goal is to increase testosterone levels while maintaining spermatogenesis, is to use SERMs and/or AIs instead of exogenous testosterone (Figure 1).
Selective Estrogen Receptor Modulators
SERMs are compounds that exhibit tissue-specific estrogen receptor agonist or antagonist activity.9 Although many SERMs have been developed, clomiphene citrate (clomiphene) and tamoxifen are the ones that are commonly used in men’s health. Clomiphene is a SERM that exists as two isomers, zuclomiphene and enclomiphene. It has been used since the 1960s, initially to treat oligomenorrhea but then adapted to be used in anovulation and ovulation induction in women.10 A decade later Paulson and colleagues11 first reported on the use of clomiphene in men and demonstrated a 40% pregnancy rate in partners of a group of subfertile men. Clomiphene citrate works as an estrogen antagonist at the level of the pituitary gland and thus stimulates the release of LH and FSH, which in turn drives both the steroidogenic and spermatogenic functions of the testes. When the goal is to increase circulating testosterone levels in a patient with low testosterone levels, there are a number of reasons that make clomiphene therapy a more practical choice than conventional testosterone replacement therapy. Most important is that oral clomiphene does not suppress endogenous gonadotropin secretion that is universally seen with practically all forms of exogenous testosterone therapy; as a result, clomiphene, unlike exogenous testosterone therapy, should not have a negative effect on spermatogenesis and consequently it does not impact the size of the testes. Due to its mechanism of action of increasing gonadotropin levels, clomiphene is less effective in raising testosterone levels in men who already have elevated LH levels prior to the initiation of treatment. Mazzola and colleagues12 reported that men with hypogonadism and LH values ≤ 6 IU/mL are the ideal candidates for treatment with clomiphene.
Although clomiphene was initially and exclusively used in men with infertility, it has recently taken on a secondary role as a sole therapeutic option for men with hypogonadism. Several studies have evaluated clomiphene as a non–US Food and Drug Administration-approved alternative to exogenous testosterone therapy. In elderly men with erectile dysfunction, Guay and colleagues13 found that the use of clomiphene citrate for 2 months raised LH, FSH, and testosterone levels; however, despite the increase in serum testosterone, sexual function in these elderly men did not significantly improve. However, a follow-up study with an extended 4-month duration of treatment with clomiphene did demonstrate an improvement in sexual function in 75% of men with secondary hypogonadism and erectile dysfunction, confirming that improvement in hypogonadal symptoms may require at least 3 months of eugonadal levels before symptoms improve.14-16 In a large prospective trial, 125 men with testosterone levels < 400 ng/dL and low sexual desire were selected to receive 25 mg of clomiphene citrate daily. There was a statistically significant improvement in testosterone levels, from 309 ng/dL to 642 ng/dL 3 months after initiation of therapy. Moreover, all men reported improvements in post-treatment quality of life scores.17 Other studies have also found that aside from improvement in serum testosterone levels, clomiphene therapy also leads to significant improvement in bone mineral density, as well as androgen deficiency in the aging male (ADAM) scores without any significant adverse events.18,19
Since the studies of Paulson and associates11 were published in the 1970s, clomiphene citrate has been used as an off-label management of infertility in men. Due to its antiestrogen effect on the pituitary, which causes an increase in the serum levels of FSH and LH, clomiphene appears to be an ideal product to treat the patient who has both a low testosterone level and a subnormal sperm count in the setting of low or normal gonadotropin levels. However, despite the lack of solid evidence to support its use in this setting, the drug is being used by many urologists for simple idiopathic infertility, in which patients have both a normal testosterone level and a normal sperm count. This is evidenced by a recent survey from the AUA that demonstrated that 60% of respondents use it for idiopathic infertility.8 The evidence that some clinicians use to support prescribing clomiphene for unexplained or idiopathic infertility emanates from case reports or studies lacking a control group.20,21 In contrast, a study by the World Health Organization of 190 men with normal testosterone levels and sperm counts who were randomized to placebo versus clomiphene found no significant improvement in semen analysis parameters or pregnancy rates in the treatment group compared with the placebo group.22 Therefore, it is safe to state that because the side-effect profile of clomiphene is minimal, the drug appears to be a safe and effective option for improving sperm counts and, hence, fertility in those men with low sperm counts who are hypogonadal. Its use in idiopathic infertility, however, is controversial, and further evidence-based studies are needed before it can be recommended for this indication.
Tamoxifen citrate is another oral SERM that was approved in the 1970s for the treatment of breast cancer. It has tissue specific action and acts as an estrogen receptor blocker in breast tissue and exhibits agonistic properties in the bone and uterus.9 Although its use is primarily in women, in men’s health it is used off-label and acts as an estrogen antagonist in the hypothalamus and pituitary gland.23 Because of its mechanism of action, tamoxifen results in the inhibition of the negative feedback of estrogen at the hypothalamus and pituitary gland, and results in the release of LH and FSH, which in turn increases testosterone biosynthesis and “stimulates” spermatogenesis. Although the studies on tamoxifen use in men have largely focused on those with infertility, its mechanism of action suggests it can also be used to raise testosterone levels in men with low testosterone who possibly may have relatively elevated serum estradiol levels. Tsourdi and colleagues23 looked at the effects of three SERMs—tamoxifen, toremifine, and raloxifene—on the hypothalamic-pituitary axis in men with oligospermia. They found that after 3 months of treatment with each of these SERMs, there was a statistically significant increase in serum gonadotropins, testosterone, and semen parameters. However, as is the case with clomiphene, it is controversial whether tamoxifen should be used in the setting of idiopathic infertility because much of the data are from case reports or uncontrolled studies. In the few randomized placebo-controlled studies for idiopathic infertility that have been reported with use of this SERM, the results are mixed and contradictory.24-26
Aromatase Inhibitors
AIs are drugs that lower estrogen levels by blocking the aromatase enzyme, the enzyme that converts testosterone to estradiol.27 AIs were initially used for the treatment of metastatic breast cancer, but over the years their scope of use has broadened to include conditions in which the desire is to either lower serum estradiol and/or increase serum testosterone levels.28 In men, this use has been adapted in certain subsets of patients with infertility and possibly in those in whom there is an imbalance between serum estradiol and testosterone. AIs are thought to inhibit the conversion of testosterone to estradiol peripherally inside adipocytes and also within the testes. In addition, they lower or remove the inhibitory effect of both the circulating estradiol level and the endogenous tissue level of estradiol at the site of the hypothalamic-pituitary axis, which results physiologically in the increased release of both LH and FSH.29 Multiple AIs have been developed, but the ones most commonly studied in men’s health are all oral: testolactone, anastrazole, and letrozole. Testolactone is a steroidal AI that is no longer commercially available in the United States but has been studied in multiple trials for use in male infertility.28 Anastrazole is a selective nonsteroidal AI that is approved for the treatment of breast cancer and has been used off-label to treat a certain subset of infertile patients. In addition, it is also used off-label to treat men with relatively high levels of serum estradiol compared with their serum testosterone levels.28 Letrozole is a more potent selective AI and, similar to anastrozole, has been used to treat those subsets of infertile men with abnormal testosterone to estradiol (T/E) ratios.28
Like clomiphene, AIs have also been used in hypogonadal men as an off-label alternative to testosterone replacement therapy. Zumoff and colleagues29 performed a prospective trial looking at obese men with hypogonadotropic hypogonadism. Administering 1 g of testolactone daily for 6 weeks resulted in an increase of testosterone and LH and a decrease in estradiol. Two prospective studies investigated whether letrozole could successfully treat obesity-related hypogonadotropic hypogonadism.27,30 Both studies demonstrated normalization of testosterone levels after treatment with letrozole. The majority of studies evaluating AIs as an alternative to testosterone replacement therapy involved trials with anastrazole. A randomized placebo-controlled study by Burnett-Bowie and colleagues31 looked at 88 hypogonadal men. They were treated with anastrazole or placebo. There was a significant increase in testosterone levels with a decrease in estradiol levels after 3 months of treatment. However, patients did not achieve significant symptomatic improvement in their hypogonadal symptoms, as evidenced by their ADAM questionnaire, or improvement in body habitus and strength. A follow-up study from the authors demonstrated a decrease in bone mineral density in those patients treated with anastrozole.32 Nonetheless, other studies have demonstrated that a short-term decrease in estradiol has no adverse effects on bone metabolism or adverse effects on lipid profiles or insulin resistance over a 3-month period.33,34 This discrepancy may be due to the fact that the first study was over a 1-year time period. Perhaps with prolonged exposure to lower estrogen levels bone mineral density may decrease over time or there may be estrogen receptor polymorphisms that make some men susceptible to lower bone mineral density.35 Despite their success, there are no long-term data evaluating the efficacy of AIs and, therefore, their use for hypogonadism cannot be routinely recommended at this time.
AIs have also gained popularity as off-label options in the treatment of male infertility. As agents that can alter the hypothalamic-pituitary-gonadal axis, they are sometimes used in men when hormonal manipulation is needed. Animal models suggest that high intratesticular estrogen levels have been associated with impaired steroidogenesis and spermatogenesis.36 AIs can alter the T/E ratio and their use has been validated in men with low ratios. Pavlovich and colleagues37 studied 63 infertile men and fertile control subjects and found that those men with infertility had a T/E ratio of < 10:1. After these men were treated with testolactone, semen parameters and T/E ratios improved.
Other studies have evaluated anastrazole for preserving fertility in men. Raman and Schlegel38 looked at 140 men who were infertile and had abnormal T/E ratios. They were treated with anastrazole or testolactone and were noted to have significant improvements in semen volume, motility, concentration, and an increase in the T/E ratio. Letrozole has also been evaluated in the setting of male infertility. Saylam and associates39 treated 27 infertile patients with a T/E ratio of < 10 with letrozole. There was an increase in testosterone for all patients; 20% of the oligospermic patients conceived naturally and 24% of azoospermic men had return of semen to their ejaculate. Another study by Gregoriou and associates40 treated 29 men with infertility and low T/E ratios (< 10) and normal gonadotropin levels with either anastrozole or letrozole daily. They found an improvement in both hormonal and semen parameters after treatment with AIs.
There is evidence that SERMs and AIs can be used in combination with human chorionic gonadotropin (hCG) for spermatogenesis recovery in azoospermia or severe oligospermia that is due to exogenous testosterone therapy.41 Wenker and coworkers41 used a combination of 3000 IU hCG every other day with either SERMs, AIs, or recombinant FSH and found that there was a return of spermatogenesis in azoospermic men or improved counts in men with severe oligospermia in 95.9% of the participants.
It is hypothesized that gynecomastia occurs from an alteration in the estrogen to androgen ratio at the level of the breast.42 Tamoxifen, given its estrogen antagonist properties in the breast, as well as AIs, has also been used in the treatment of gynecomastia. Much of the data stem from boys with pubertal gynecomastia and in men with prostate cancer on antiandrogen therapy; there is evidence that these medications are effective in the treatment of gynecomastia.43-45 Their use in the treatment of gynecomastia induced by exogenous testosterone therapy is largely anecdotal and not evidence based.46 However, the manipulation of estrogen levels in men may not be without consequences. We know that estrogen receptors are present throughout the body and play a role in bone health, body composition, cardiovascular well being, libido and sexual function, and testicular steroidogenesis and spermatogenesis.47-49 Although a high estradiol level may have a negative impact on fertility, a level that is excessively low may not be desirable either.
Estrogen receptor polymorphisms and gene mutations have been implicated in male infertility.50,51 These polymorphisms may be the key in truly identifying a population of men that may benefit from estrogen modulation. Until then, AIs have a role in the treatment of male infertility in those men with low T/E ratios. Further research on the use of AIs in these conditions is required in order to fully establish their benefit in these aforementioned conditions.
Conclusions
There have been significant advancements in understanding the interaction between testosterone and estrogen and how these two hormones impact multiple physiologic functions in men. SERMs and AIs have been used off-label in men to increase testosterone levels while maintaining spermatogenesis. Though their use in idiopathic infertility is questionable, there are data to support their use in men with both hypogonadism and low sperm counts, and also in men who are symptomatic from low testosterone levels. AIs can also be used in these same subsets of men who have an imbalance between testosterone and estradiol. However, because estrogen is also an important player in many physiologic functions in men including bone metabolism and cardiovascular health, caution must always be exercised and follow-up is mandatory in those men who receive long-term treatment with SERMs or AIs. ![]()
References
- Smith EP, Boyd J, Frank GR, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331:1056-1061.
- Hess RA, Bunick D, Lee KH, et al. A role for oestrogens in the male reproductive system. Nature. 1997;390:509-512.
- Lakshman KM, Kaplan B, Travison TG, et al. The effects of injected testosterone dose and age on the conversion of testosterone to estradiol and dihydrotestosterone in young and older men. J Clin Endocrinol Metab. 2010;95:3955-3964.
- Arevalo MA, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci. 2015;16:17-29.
- Gooren LJ, van der Veen EA, van Kessel H, Harmsen-Louman W. Estrogens in the feedback regulation of gonadotropin secretion in men: effects of administration of estrogen to agonadal subjects and the antiestrogen tamoxifen and the aromatase inhibitor delta’-testolactone to eugonadal subjects. Andrologia. 1984;16:568-577.
- Spitzer M, Huang G, Basaria S, et al. Risks and benefits of testosterone therapy in older men. Nat Rev Endocrinol. 2013;9:414-424.
- Jarow JP, Zirkin BR. The androgen microenvironment of the human testis and hormonal control of spermatogenesis. Ann N Y Acad Sci. 2005;1061:208-220.
- Ko EY, Siddiqi K, Brannigan RE, Sabanegh ES Jr. Empirical medical therapy for idiopathic male infertility: a survey of the American Urological Association. J Urol. 2012;187:973-978.
- Komm BS, Mirkin S. An overview of current and emerging SERMs. J Steroid Biochem Mol Biol. 2014;143:207-222.
- Holtkamp DE, Greslin JG, Root CA, Lerner LJ. Gonadotrophin inhibiting and anti-fecundity effects of chloramiphene. Proc Soc Exp Biol Med. 1960;105:197-201.
- Paulson DF, Hammond CB, de Vere White R, Wiebe RH. Clomiphene citrate: pharmacologic treatment of hypofertile male. Urology. 1977;9:419-421.
- Mazzola CR, Katz DJ, Loghmanieh N, et al. Predicting biochemical response to clomiphene citrate in men with hypogonadism. J Sex Med. 2014;11:2302-2307.
- Guay AT, Bansal S, Heatley GJ. Effect of raising endogenous testosterone levels in impotent men with secondary hypogonadism: double blind placebo-controlled trial with clomiphene citrate. J Clin Endocrinol Metab. 1995;80:3546-3552.
- Guay AT, Jacobson J, Perez JB, et al. Clomiphene increases free testosterone levels in men with both secondary hypogonadism and erectile dysfunction: who does and does not benefit? Int J Impot Res. 2003;15:156-165.
- Wang C, Cunningham G, Dobs A, et al. Long–term testosterone gel (Androgel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 2004;89:2085-2098.
- Amory JK, Watts NB, Easley KA, et al. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab. 2004;89:503-510.
- Da Ros CT, Averbeck MA. Twenty-five milligrams of clomiphene citrate presents positive effect on treatment of male testosterone deficiency–a prospective study. Int Braz J Urol. 2012;38:512-518.
- Moskovic DJ, Katz DJ, Akhavan A, et al. Clomiphene citrate is safe and effective for long-term management of hypogonadism. BJU Int. 2012;110:1524-1528.
- Taylor F, Levine L. Clomiphene citrate and testosterone gel replacement therapy for male hypogonadism: efficacy and treatment cost. J Sex Med. 2010;7:269-276.
- Ghanem H, Shaeer O, El-Segini A. Combination clomiphene citrate and antioxidant therapy for idiopathic male infertility: a randomized controlled trial. Fertil Steril. 2010;93:2232-2235.
- Moradi M, Moradi A, Alemi M, et al. Safety and efficacy of clomiphene citrate and L-carnitine in idiopathic male infertility: a comparative study. Urol J. 2010;7:188-193.
- A double-blind trial of clomiphene citrate for the treatment of idiopathic male infertility. World Health Organization. Int J Androl. 1992;15:299-307.
- Tsourdi E, Kourtis A, Farmakiotis D, et al. The effect of selective estrogen receptor modulator administration on the hypothalamic-pituitary-testicular axis in men with idiopathic oligozoospermia. Fertil Steril. 2009;91(4 suppl):1427-1430.
- Kotoulas IG, Cardamakis E, Michopoulos J, et al. Tamoxifen treatment in male infertility. I. Effect on spermatozoa. Fertil Steril. 1994;61:911-914.
- Krause W, Holland-Moritz H, Schramm P. Treatment of idiopathic oligozoospermia with tamoxifen–a randomized controlled study. Int J Androl. 1992;15:14-18.
- AinMelk Y, Belisle S, Carmel M, Jean-Pierre T. Tamoxifen citrate therapy in male infertility. Fertil Steril. 1987;48:113-117.
- de Boer H, Verschoor L, Ruinemans-Koerts J, Jansen M. Letrozole normalizes serum testosterone in severely obese men with hypogonadotropic hypogonadism. Diabetes Obes Metab. 2005;7:211-215.
- Schlegel PN. Aromatase inhibitors for male infertility. Fertil Steril. 2012;98:1359-1362.
- Zumoff B, Miller LK, Strain GW. Reversal of the hypogonadotropic hypogonadism of obese men by administration of the aromatase inhibitor testolactone. Metabolism. 2003;52:1126-1128.
- Loves S, Ruinemans-Koerts J, de Boer H. Letrozole once a week normalizes serum testosterone in obesity-related male hypogonadism. Eur J Endocrinol. 2008;158:741-747.
- Burnett-Bowie SA, Roupenian KC, Dere ME, et al. Effects of aromatase inhibition in hypogonadal older men: a randomized, double-blind, placebo-controlled trial. Clin Endocrinol (Oxf). 2009;70:116-123.
- Burnett-Bowie SA, McKay EA, Lee H, Leder BZ. Effects of aromatase inhibition on bone mineral density and bone turnover in older men with low testosterone levels. J Clin Endocrinol Metab. 2009;94:4785-4792.
- Leder BZ, Finkelstein JS. Effect of aromatase inhibition on bone metabolism in elderly hypogonadal men. Osteoporos Int. 2005;16:1487-1494.
- Dougherty RH, Rohrer JL, Hayden D, et al. Effect of aromatase inhibition on lipids and inflammatory markers of cardiovascular disease in elderly men with low testosterone levels. Clin Endocrinol (Oxf). 2005;62:228-235.
- Lorentzon M, Lorentzon R, Bäckström T, Nordström P. Estrogen receptor gene polymorphism, but not estradiol levels, is related to bone density in healthy adolescent boys: a cross-sectional and longitudinal study. J Clin Endocrinol Metab. 1999;84:4597-4601.
- Bharti S, Misro MM, Rai U. Clomiphene citrate potentiates the adverse effects of estrogen on rat testis and down-regulates the expression of steroidogenic enzyme genes. Fertil Steril. 2013;99:140-148.
- Pavlovich CP, King P, Goldstein M, Schlegel PN. Evidence of a treatable endocrinopathy in infertile men. J Urol. 2001;165:837-841.
- Raman JD, Schlegel PN. Aromatase inhibitors for male infertility. J Urol. 2002;167(2 Pt 1):624-629.
- Saylam B, Efesoy O, Cayan S. The effect of aromatase inhibitor letrozole on body mass index, serum hormones, and sperm parameters in infertile men. Fertil Steril. 2011;95:809-811.
- Gregoriou O, Bakas P, Grigoriadis C, et al. Changes in hormonal profile and seminal parameters with use of aromatase inhibitors in management of infertile men with low testosterone to estradiol ratios. Fertil Steril. 2012;98:48-51.
- Wenker EP, Dupree JM, Langille GM, et al. The use of HCG-based combination therapy for recovery of spermatogenesis after testosterone use. J Sex Med. 2015;12:1334-1337.
- Eckman A, Dobs A. Drug-induced gynecomastia. Expert Opin Drug Saf. 2008;7:691-702.
- Lapid O, van Wingerden JJ, Perlemuter L. Tamoxifen therapy for the management of pubertal gynecomastia: a systematic review. J Pediatr Endocrinol Metab. 2013;26:803-807.
- Kunath F, Keck B, Antes G, et al. Tamoxifen for the management of breast events induced by non-steriodal antiandrogens in patients with prostate cancer: a systematic review. BMC Med. 2012;10:96.
- Riepe FG, Baus I, Wiest S, et al. Treatment of pubertal gynecomastia with the specific aromatase inhibitor anastrazole. Horm Res. 2004;62:113-118.
- Rahnema CD, Lipshultz LI, Crosnoe LE, et al. Anabolic steroid-induced hypogonadism: diagnosis and treatment. Fertil Steril. 2014;101:1271-1279.
- Vaucher L, Funaro MG, Mehta A, et al. Activation of GPER-1 estradiol receptor downregulates production of testosterone in isolated rat Leydig cells and adult human testis. PLoS One. 2014;9:e92425.
- Kumar A, Dumasia K, Gaonkar R, et al. Estrogen and androgen regulate actin-remodeling and endocytosis-related genes during rat spermiation. Mol Cell Endocrinol. 2015;404:91-101.
- Finkelstein JS, Lee H, Burnett-Bowie SA, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369:1011-1022.
- Guarducci E, Nuti F, Becherini L, et al. Estrogen receptor alpha promoter polymorphism: stronger estrogen action is coupled with lower sperm count. Hum Reprod. 2006;21:994-1001.
- Su MT, Chen CH, Kuo PH, et al. Polymorphisms of estrogen-related genes jointly confer susceptibility to human spermatogenic defect. Fertil Steril. 2010;93:141-149.