Calyceal Diverticula: A Comprehensive Review
Nikhil Waingankar, MD,1 Samih Hayek, MD,2 Arthur D. Smith, MD,1 Zeph Okeke, MD1
1The Arthur Smith Institute for Urology, Hofstra North Shore-LIJ School of Medicine, New Hyde Park, NY; 2University of Pittsburgh Medical Center, Pittsburgh, PA
Calyceal diverticula are rare outpouchings of the upper collecting system that likely have a congenital origin. Stones can be found in up to 50% of calyceal diverticula, although, over the combined reported series, 96% of patients presented with stones. Diagnosis is best made by intravenous urography or computed tomography urogram. Shock wave lithotripsy (SWL) is an option for first-line therapy in patients with stone-bearing diverticula that have radiologically patent necks in mid- to upper-pole diverticula and small stone burdens. Stone-free rates are the lowest with SWL, although patients report being asymptomatic following therapy in up to 75% of cases with extended follow-up. Ureteroscopy (URS) is best suited for management of anteriorly located mid- to upper-pole diverticular stones. Drawbacks to URS include difficulty in identifying the ostium and low rate of obliteration. Percutaneous management is best used in posteriorly located mid- to lower-pole stones, and offers the ability to directly ablate the diverticulum. Percutaneous nephrolithotomy remains effective in the management of upper-pole diverticula, but carries the risk of pulmonary complications unless subcostal access strategies such as triangulation or renal displacement are used. Laparoscopic surgery provides definitive management, but should be reserved for cases with large stones in anteriorly located diverticula with thin overlying parenchyma, and cases that are refractory to other treatment. This article reviews the current theories on the pathogenesis of calyceal diverticula. The current classification is examined in addition to the current diagnostic methods. Here we summarize an extensive review of the literature on the outcomes of the different treatment approaches.
[ Rev Urol. 2014;16(1):29-43 doi: 10.3909/riu0581]
© 2014 MedReviews®, LLC
Key words
Calyceal diverticula • Percutaneous nephrostolithotomy • Laparoscopic surgery • Shock wave lithotripsy • Ureterorenoscopy
There is no consensus regarding the cause of calyceal diverticula, although the majority of investigators have favored congenital over acquired origins. Furthermore, the similarity in incidence in children and adults is consistent with an embryologic cause.
Calyceal diverticula are classified as type I, those communicating with a minor calyx or an infundibulum, or type II, those emanating from the renal pelvis or a major calyx. Type II diverticula are larger, tend to be symptomatic, and are located in the central part of the kidney.
The vast majority of patients with calyceal diverticula are asymptomatic. Indications for operative intervention include chronic pain, recurrent urinary tract infection, gross hematuria, or decline in renal function.
Main Points
•Calyceal diverticula are rare outpouchings of the upper collecting system that have a congenital origin. Stones can be found in up to 50% of cases, although over the combined series reported here, 96% of patients presented with stones. Adequate imaging is essential to diagnosis of calyceal diverticula, which are radiolucent and cannot be seen on a plain radiograph. Diagnosis is primarily made by intravenous urogram or computed tomography urogram.
•The vast majority of patients are asymptomatic. Indications for operative intervention include chronic pain, recurrent urinary tract infection, gross hematuria, or decline in renal function.
•Shock wave lithotripsy (SWL) is an option for first-line therapy in patients with stone-bearing diverticula that have radiologically patent necks in mid- to upper-pole diverticula and small stone burdens. Stone-free rates are the lowest with SWL, although patients report being asymptomatic following therapy in up to 75% of cases with extended follow-up.
•Ureteroscopy (URS) is best suited for management of mid- to upper-pole anteriorly located diverticular stones. Drawbacks to URS include difficulty of identifying the ostium and low rate of obliteration.
•Percutaneous management is best used in posteriorly located mid- to lower-pole stones, and offers the ability to directly ablate the diverticulum. PCNL remains effective in the management of upper-pole diverticula, but carries the risk of pulmonary complications unless subcostal access strategies such as triangulation or renal displacement are used.
•Laparoscopic surgery should be reserved for cases with large stones in anteriorly located diverticula with thin overlying parenchyma, and cases that are refractory to other treatment.
PCNL has provided surgeons with the opportunity to directly treat the underlying disorder in patients with calyceal diverticular stones, thus improving stone-free rates over SWL while also minimizing the risk of recurrence. From the patient’s standpoint, symptom-free rates and quality-of-life mental- and emotional-health subscores have also been shown to improve following PCNL. However, the efficacy of PCNL must be weighed against its invasiveness, complication rates, as well as its limited role and poor results in anteriorly located diverticula.
Calyceal diverticula are eventrations of the upper collecting system lying within the renal parenchyma.1 These nonsecretory outpouchings are lined by transitional cell epithelium and communicate with the main collecting system via a narrow channel, allowing for passive filling with urine. They were first described in 1841 by Rayer in “Traitements des maladies des reins.”2 Thought to be either cysts or localized hydronephrosis, he used the term kyste urinaire to describe his finding of intrarenal urine-containing cavities that communicate with calyces. Other investigators reported similar findings and—depending on location and postulated etiology—described them as pelvic cysts,3 peripelvic cysts,4 pyelorenal cysts,5 pyelosynaptic cysts,6 pyelogenous cysts,7 hydrocalicosis,8 cystic dilatations of the calyx,9 congenital cortical cysts,10 congenital cystic dysplasia,4,11 calyceal pseudocysts,12 juxta-calyceal cysts,13 pelvic diverticula,14 congenital diverticula of the calyx,15 and finally, calyceal diverticula.16-18 Prather is credited with coining the term and the definition of calyceal diverticulum that we use today.
Epidemiology
Calyceal diverticula are found in 0.21% to 0.6% of intravenous urograms (IVU) performed on adults, with a similar prevalence in children.1,19-21 From a meta-analysis of the combined series we examined, they are found in the upper pole calyces 48.9% of the time versus 29.7% and 21.4% in the middle and lower poles, respectively. This is, of course, a striking difference compared with the analysis by Abeshouse and Abeshouse, who showed a 12:3:2 pattern dominated by the upper pole.17 The disease process affects women (63%) more commonly than men (37%), and has no predilection toward a particular side of the body. Average diverticulum size across the series is 1.72 cm and ranges from 0.5 to 7.5 cm. Stones have reportedly been found in 9.5% to 50% of cases1,20; in the combined series, this number reaches 96% and average stone size is 12.1 mm and ranges from 1 to 30 mm (Table 1).
Cause
There is no consensus regarding the cause of calyceal diverticula, although the majority of investigators have favored congenital over acquired origins.17,18,22,23 Furthermore, the similarity in incidence in children and adults is consistent with an embryologic cause.17,20,22,24 One proposed etiology is the formation of a diverticulum during branching of the ureteral bud into the metanephric blastema; if one of the branchings fails to stimulate an appropriate section of the metanephros, a diverticulum results.19,20 A second proposed etiology centered on disordered branching describes the future renal pelvis as having first-order branches that become major calyces, second-order branches that become minor calyces, and further branching to the 15th order. In this schema, the higher orders persist as collecting tubules whereas the lower orders degenerate; calyceal diverticula, then, are thought to be branches that persist because of failed degeneration.25 Even among the proponents of an embryologic cause of calyceal diverticula, there is no consensus about the timing of the anomaly relative to birth. Schwartz and colleagues postulated that a malformation occurs early in development. This was supported by the discovery of the association of calyceal diverticula with butterfly vertebrae, or the result of the faulty union of two halves of the cartilaginous vertebral bodies. Butterfly vertebrae form at approximately 35 days of development, which is essentially the same time as the development of the ureteric bud.26 Other authors have supported a timeline that places the formation of calyceal diverticula just before birth.27
Potentially acquired causes of calyceal diverticula can be broadly classified as obstructive, neuromuscular, traumatic, or fibrotic. Obstruction has been proposed as a factor secondary to stone formation28 or infection either within the calyx or from a localized cortical abscess draining into a calyx.29 An alternative potential acquired cause is derived from dysfunction within sphincters surrounding the calyces that facilitate synchronized filling and emptying. Such calyceal achalasia results in chronic inefficient emptying, progressive dilatation proximal to the sphincter, and subsequent formation of a diverticulum.8,30-32 Flank trauma has also been reported as a presenting factor in patients found to have calyceal diverticula.8 Finally, progressive fibrosis of an infundibulum is an alternative theoretical cause. Examination of surgical specimens has failed to reveal pathological findings that would support any of the aforementioned as causes rather than concurrent findings.
Classification
Calyceal diverticula are classified as type I, those communicating with a minor calyx or an infundibulum, or type II, those emanating from the renal pelvis or a major calyx. Type II diverticula are larger, tend to be symptomatic, and are located in the central part of the kidney.19 Dretler proposed an alternative classification scheme that includes both anatomical description as well as his recommended treatment for each. In this system, a type I diverticulum has an open mouth and short neck, type II has a closed mouth and short neck, type III has a closed mouth and long neck, and type IV has an obliterated neck; shock-wave lithotripsy (SWL) was recommended for type I, ureterorenoscopic management for type II, and percutaneous treatment for types III and IV.33
Diagnosis
The majority of patients with calyceal diverticula are asymptomatic and the diagnosis is made on imaging performed for other reasons. One-third to one-half of patients, however, present with flank pain, urinary tract infection, and/or hematuria.1There is no history, physical examination, or laboratory findings that are specific to the diagnosis of calyceal diverticula. Although urinary stasis and increased particle retention time play a role in the pathogenesis of diverticular stones,34 there is no consensus regarding the role of metabolic abnormalities. Hsu and Streem reported 50% of the patients in their series with urinary excretion abnormalities, including hypercalciuria and hyperoxaluria, both with or without hyperuricosuria; furthermore, they reported 64% of their patients with synchronous or metachronous distal stones, of which 56% had definable metabolic abnormalities.35 Similarly, Auge and colleagues found that all diverticula patients in their series receiving a complete metabolic workup were found to have at least one metabolic abnormality, with hypercalciuria and hyperuricosuria being the most common among them. However, there was no statistically significant difference between the numbers of metabolic abnormalities in diverticulum patients versus those in a group of randomly selected stone-forming patients.36 Matlaga and colleagues reported that diverticular stone patients have a urinary calcium excretion similar to that of calcium oxolate stone formers, suggesting a metabolic component to the pathogenesis of diverticular stones; however, urine aspirated directly from the diverticula in this study had a lower supersaturation of calcium oxolate compared with ipsi- and contralateral renal pelves, thus also supporting urinary stasis as a contributing factor.37 Liatsikos and associates found a threefold greater incidence of metabolic abnormalities in patients with simple renal stones compared with those with calyceal diverticular stones, and concluded that metabolic abnormalities do not promote calyceal diverticular calculous formation.38
Adequate imaging is essential to the diagnosis of calyceal diverticula, which are radiolucent and therefore cannot be seen on a plain radiograph. A diverticulum containing milk of calcium appears as a semilunar density with a fluid-calcium level at the upper margin that changes position on upright or lateral decubitus radiographs.39 On IVU, calyceal diverticula have the appearance of opacified cystic cavities, which communicate with the renal collecting system.20,40 Note, however, that the diverticulum itself is nonsecretory, and relies on retrograde flow from the collecting system through the ostium to fill the cavity. Therefore, the filling of a diverticulum with contrast on IVU relies on a patent neck, and opacification of the cavity may be delayed or nonexistent. Wulfsohn’s two types of diverticula can be differentiated based on the filling pattern of contrast in an IVU: type I diverticula take on a bulbous form with a narrow infundibulum, whereas type II varieties appear more spherical and have shorter necks.40
On ultrasound, calyceal diverticula appear to have similar appearance and echotexture as cysts unless filled with stones. In this case, the hyperechoic stones appear as mobile, position dependent, and with acoustic shadowing emanating from within the contrasting radiolucent cavities.41-43 On early phase contrast computed tomography (CT), calyceal diverticula appear as small, round, low--attenuation areas adjacent to the calyces. Delayed contrast images can show filling of this area with minimal overlying cortex.44 Retrograde pyelogram can be used to confirm the diagnosis or to further investigate questionable cases, although it is often unnecessary.
Differential diagnoses, which must be distinguished from calyceal diverticula on imaging, include hydrocalyx, simple cyst, parapelvic cyst, tubercular cavity, papillary necrosis, and renal tumor. Hydrocalycosis is simply hydronephrosis of a calyx secondary to infundibular obstruction. Simple cysts are unilocular and do not connect with the pelvicalyceal system. Furthermore, cysts are lined with cuboidal epithelium, whereas calyceal diverticula have a transitional cell lining. Parapelvic cysts are found adjacent to the renal pelvis; like simple cysts, they do not communicate with the collecting system. Tubercular cavities demonstrate irregular borders and enlarge progressively. Papillary necrosis is found in the renal medulla and is associated with nonsteroidal anti-inflammatory drug abuse and systemic conditions, such as sickle cell disease or diabetes mellitus. On CT urogram (CTU), findings in cases of papillary necrosis can range from blunted to eroded calyces with varying degrees of filling defects. Finally, neoplasm must be ruled out when only limited opacification of a calyceal diverticulum is seen on contrast-enhanced CT.39,44,45
Treatment
The vast majority of patients with calyceal diverticula are asymptomatic. Indications for operative intervention include chronic pain, recurrent urinary tract infection, gross hematuria, or decline in renal function.1 Historically, the treatment for symptomatic patients with calyceal diverticulectomy has involved open excision or marsupialization of the diverticulum with closure of the neck. Since the mid-1980s, minimally invasive approaches began to gain momentum,46,47 including SWL, ureteroscopic and percutaneous methods, and laparoscopic surgery. Treatment modality should be selected according to such factors as diverticulum location and stone burden and size.48
SWL
Extracorporeal SWL has been studied as a first-line treatment for symptomatic patients with calyceal diverticula because it is the least invasive treatment modality.49 Results from published case series are mixed, with the majority of authors concluding that SWL monotherapy produces suboptimal stone-free and recurrence rates.
Garcia Reboll and colleagues described 13 patients with calculi in calyceal diverticula who were all treated by SWL and found that none of the stones were completely removed. In three patients (23%), the stones were reduced to half of the original size. Two of the patients (15%) had stones that were reduced to 75% of their original size. The remaining eight patients had stones that fragmented, but without any elimination of debris. Of those patients who were symptomatic prior to treatment, only 36.6% became asymptomatic.50
Ritchie and colleagues used extracorporeal piezoelectric (EPL) lithotripsy in 20 patients with stone-bearing calyceal diverticula, of which 16 were symptomatic. Twelve of these patients (75%) were rendered symptom free. Five (25%) became stone free, and six (30%) had residual fragments < 2 mm. The authors concluded that, although endourological approaches may provide more durable success in terms of stone-free rates, EPL should be provided as an option for those patients who wish to avoid an invasive intervention.51
Psihramis and Dretler treated 10 patients with SWL monotherapy. Of these, all had stone-bearing diverticula and were symptomatic. None of the patients were rendered stone free following treatment, although all had fragments that were believed to be small enough for spontaneous passage (< 3 mm). On follow-up at 3 months, only two (20%) were stone free; of the remaining eight patients, five were asymptomatic (62.5%), and five had fragments larger than 50% of the size of the original stones.52
Streem and Yost reported more favorable results in their series of 19 patients treated with SWL monotherapy.53 Their selection criteria required radiographic evidence of a functionally patent diverticular neck, as evidenced by early filling with contrast on intravenous pyelogram (IVP) or retrograde pyelography. In addition, patients with stones > 1.5 cm were excluded from the study. Eleven patients (58%) were stone free at initial follow-up following a single session of SWL; 6 of these patients had extended -follow-up (included in a group of 13 patients with a mean follow-up of 23.8 months), of which 5 remained stone-free (38.5% of all patients with long-term follow-up, 83.3% of initially stone-free patients with long-term follow-up). Fourteen patients reported having symptoms prior to SWL, and of these, 12 (86%) were rendered symptom free at initial follow-up; 8 had extended follow-up; and 6 remained symptom free (75%). The authors concluded that, in select patients, SWL is an acceptable form of primary management for patients with calyceal diverticular stones.53
Diverticular stones may be well fragmented following SWL; this is demonstrated with the finding of layering within the diverticulum on supine and erect radiographs.54 However, passage of these fragments is prohibited by the same anatomic abnormality that caused urinary stasis and stone formation in the first place—a long and narrow diverticular neck. Stones in a calyx with little or no communication with the renal pelvis should therefore be excluded from SWL as they do not address this underlying anomaly, and an alternate treatment modality should be considered (Table 2).55
Percutaneous Nephro-stolithotomy: Technique
Percutaneous nephrostolithotomy (PCNL) has been shown to have high success rates in calyceal diverticular stone treatment and has produced universally better results than those achieved by SWL monotherapy as it provides greater access to larger, more complex, and posteriorly located stones. Moreover, it allows the surgeon to manage the diverticulum with fulguration or incision of the diverticular neck.56,57
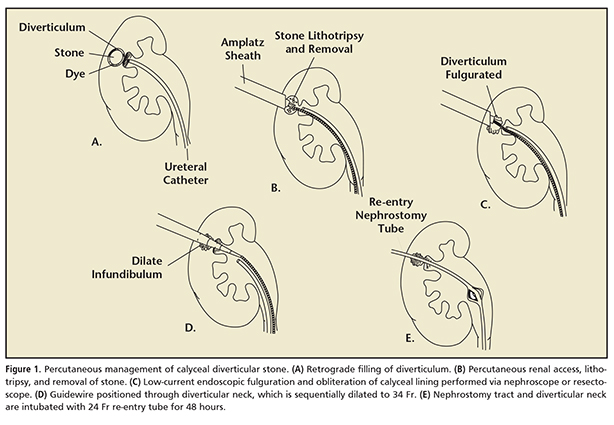
The percutaneous approach to diverticular stones begins with preoperative imaging with plain films and retrograde pyelogram in the operating room prior to obtaining renal access. A staged procedure can also be performed, where imaging and access are obtained by an interventional radiologist prior to stone removal in the operating room. For patients with radiolucent stones, or in those where the diverticulum does not opacify with retrograde contrast or on IVU, contrast can be directly instilled into the cavity with CT or with ultrasound guidance.58 It is our standard practice to begin surgery with placement of a ureteral catheter with instillation of contrast, then place the patient in the prone position and, when possible, puncture the diverticulum directly with an 18-ga diamond-tipped needle under fluoroscopic guidance. For patients with upper-pole calyceal diverticula, renal displacement or triangulation can be used to allow subcostal direct access.59 A 0.038-in J-tip guidewire is fed through the nephrostomy needle, and then a 10 Fr Amplatz dilator is placed over the wire. A second wire is then placed through the dilator and coiled into the diverticulum; when the diameter of the ostium permits, the wire can be placed into the main collecting system. The nephrostomy tract is then sequentially dilated to 34 Fr, at which point the nephroscope can be placed. Diverticular stones are then fragmented, if necessary, and removed with a grasper or basket. The diverticular neck is then sought; this can be aided with retrograde infusion of indigo carmine or carbon dioxide through the ureteral catheter. The cavity walls are then fulgurated with low-current electrocautery. Finally, the ostium is dilated and a nephrostomy tube is placed across the neck into the main collecting system (Figure 1).60 Alternatively, if intubation of the diverticular neck is impossible, some have advocated the creation of a neoinfundibulum through the diverticular wall.61-63 After 48 hours, a nephrostogram is performed, and barring any evidence of retained stones, obstruction, or extravasation, the nephrostomy tube is removed.
Anterior calyces present an added challenge in patients with calyceal diverticular stones. The acute angle required for direct puncture prevents complete visualization and instrumentation within the cavity. One option for management includes direct puncture with stone removal and fulguration, but without management of the diverticular neck.60 Alternatively, indirect puncture can be performed through a posteriorly located calyx. Rigid or flexible nephroscopy is then used to navigate the collecting system until the ostium is reached, at which point a 0.035-in Bentson guidewire can be passed into and coiled within the cavity. The diverticular neck is then balloon-dilated or endoinfundibulotomy is performed with an electrosurgical probe or Ho:YAG laser. The nephroscope is then advanced into the diverticulum and stones are fragmented and removed.64,65
PCNL: Results
One of the first published studies on percutaneous management of calyceal diverticula was by Eshghi and colleagues in 1987. In their mixed series of 14 patients with either infundibular stenosis or calyceal diverticula, 11 patients had a direct puncture into the target calyx or diverticulum. Eight patients were managed with incision of the infundibulum, four with balloon dilation, and two with direct-vision dissection. None of the diverticuli were fulgurated as the authors believed that endoinfundibulotomy with dilatation of the neck would traumatize the lining, and subsequent placement of a nephrostomy tube would allow granulation and re-epithelialization to take place, leading to eventual obliteration of the cavity. On follow-up ranging from 4 to 12 months, all patients were stone free, and 12 had a reduction in diverticulum size, whereas 2 remained unchanged.66
In their series of 17 patients, Hulbert and colleagues had a similar approach to the management of diverticula once access and stone removal were achieved: they intubated across the diverticular neck rather than fulgurating in all but one case. The lone patient who was managed with fulguration presented with a 7.5-cm diverticulum, which the authors thought would require further promotion of granulation tissue formation. All but three patients (80%) who were followed over a mean of 10.3 months had complete obliteration of their diverticula; of note, the diverticula in each of these three patients was approached indirectly.47
Hedelin and colleagues described a series of 13 patients with calyceal diverticula, of which 7 were managed via direct puncture and 6 were treated following indirect access of a nearby calyx. Similar to the series described by Eshghi and associates, none of the diverticula were fulgerated. On follow-up at 24 months, one patient (8%) had complete obliteration, and nine (69%) were both stone and symptom free.67
Ellis and colleagues reported on 12 patients, of whom 10 had stone-bearing calyceal diverticula and 2 presented with recurrent infection. A direct approach was used in 11 cases (92%) and the ostia were dilated in 9 patients, each of whom had a large Malecot catheter placed in either the diverticulum or renal pelvis; following a period of 2 to 4 days, seven of these patients (58%) returned for electrode obliteration. One patient had tetracycline infused via the nephrostomy tube. On follow-up, 75% of the diverticula were obliterated, all of the patients were stone free, and 88% were symptom free.68
In an analysis of the impact of varying approaches in their series of 30 patients with long-term follow-up, Shalhav and colleagues found a 79% success rate with a direct approach versus 50% with indirect access. Incision of the diverticular neck resulted in an 83% success rate, compared with 67% success with dilatation. Management of the wall with fulguration led to complete obliteration on follow-up in 86% of their cases, whereas those cases in which the diverticula were intubated without fulguration were successful in only 50%. Overall, the authors reported obliteration in 76% of the cases with a stone-free rate of 93% over a mean objective follow-up of 21 months, and symptomatic resolution in 85% over a mean subjective follow-up of 42 months.69
Monga and colleagues described their percutaneous technique that involved electrocautery ablation without cannulation or dilatation of the infundibulum. Once access was achieved within the cavity, stones were fragmented and/or extracted, and a transurethral resectoscope was then introduced to fulgurate the diverticular lining. A Foley catheter was then placed into but not across the fulgurated diverticulum. With this method, 100% stone- and symptom-free rates were achieved with complete obliteration of all diverticula at a mean follow-up of 38 months.70
Two reports of novel single-stage percutaneous approaches for radiopaque stones were described, first by Donnellan and associates71 and then by Kim and colleagues.72 Donnellan and colleagues reported a series of 21 patients in whom access was achieved using a single-pass dilator to expose the stone-bearing calyceal diverticula. Fulguration was not attempted, and the diverticular necks were incised in 7 cases and dilated in 13 cases. At a mean follow-up of 74.4 months, the authors reported a 30% obliteration rate and 81% stone-and symptom-free rates.71 Kim and colleagues described a technique for radiopaque stones that avoided ureteral catheter placement and diverticular neck manipulation. In this series of 22 calyceal diverticula, the procedures began with the patients in the prone position and access obtained with C-arm guidance, followed by lithotripsy, stone removal, and rollerball electrode fulguration of the cavity. With no dilatation of the ostium, a 20 Fr red rubber catheter or 8.5 Fr Cope loop catheter was placed in the diverticulum at the end of the case. With this technique, the authors reported 87.5% obliteration on follow-up at 3 months. Of note, mean operative time was under 1 hour, and 20 of the 21 patients were discharged home tubeless on postoperative day 1.72,73
Three groups specifically compared the roles of SWL and percutaneous management in calyceal diverticula. Hendrikx and colleagues compared 15 patients treated with SWL versus 16 patients treated percutaneously. In the SWL group, 13% were stone free and 60% were symptom free at 3 months. In the percutaneous group, puncture failed in three patients who subsequently underwent lumbotomy. Of the remaining patients, 77% were stone and symptom free at a mean follow-up of 18 months. However, because of a 54% complication rate (includes failed PCNL cases), the authors concluded that SWL should be first-line therapy, with PCNL reserved for cases that fail SWL.74 Jones and coauthors reached the opposite conclusion in their description of 40 diverticula managed with SWL alone (16 renal units), SWL followed by PCNL (10 renal units), or percutaneous treatment alone (14 renal units).75 Those patients managed percutaneously, regardless of prior SWL, achieved a 100% symptom-free rate compared with 56% in those who received SWL monotherapy. Similarly, the PCNL groups had 90% and 86% stone-free rates in those with and without prior SWL, respectively, whereas only 6% of the SWL monotherapy group was stone free. Although the SWL group was discharged home more expediently (2.8 days vs 9.8 days in the combined group vs 7.2 days in the percutaneous monotherapy group), the number of complications was similar across all groups, and the SWL monotherapy group had the highest-grade complication in a patient who developed a perinephric abscess requiring nephrectomy. The authors concluded that SWL is not a cost-effective solution—as a single or combined treatment modality—to patients with stone-bearing calyceal diverticula.75 Turna and colleagues compared 38 patients managed with SWL and 18 by PCNL. In the SWL group, 18% and 61% were stone and symptom free, respectively, over a mean 23-month follow-up. The PCNL group demonstrated a higher stone-free rate at 72% whereas 94% were asymptomatic.76
PCNL has provided surgeons with the opportunity to directly treat the underlying disorder in patients with calyceal diverticular stones, thus improving stone-free rates over SWL while also minimizing the risk of recurrence. From the patient’s standpoint, symptom-free rates and quality-of-life mental- and emotional-health subscores have also been shown to improve following PCNL.77 However, the efficacy of PCNL must be weighed against its invasiveness, complication rates, as well as its limited role and poor results in anteriorly located diverticula (Table 3).
Ureterorenoscopy
Technique. Ureteroscopic (URS) management of diverticular stones has a greater efficacy than SWL monotherapy, and avoids the higher complication rates and discomfort levels of the more invasive therapies such as percutaneous or laparoscopic techniques. Such management is best suited for patients with small diverticular stones located in the upper or interpolar regions of the kidney. Lower pole stones are often at an acute angle that precludes retrograde management.
Surgery begins with routine cystoscopy and flexible ureteroscopy. The ostium is identified as a small dimple in some patients. In those patients where visualization is difficult, injection of contrast can be used to identify the ostium; alternatively, the Blue Spritz technique can be used, where methylene blue is instilled into the collecting system and then suctioned out. Once saline irrigant is reintroduced, residual blue dye in the diverticulum would escape, aiding the surgeon in identifying the ostium.78 A guidewire is then passed into the cavity and the infundibulum can be dilated or incised, followed by stone fragmentation and extraction.
Through the application of a Ho:YAG laser energy source, a flexible ureteroscope, and a Nitinol tipless stone basket, the success rates of ureteroscopy for treating low to moderate stone burdens is favorable.
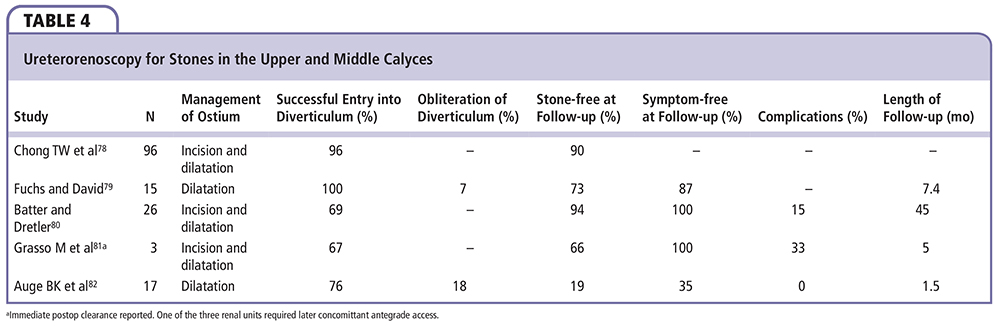
Results. In the largest series of patients managed in retrograde fashion, Chong and associates reported on 96 patients with diverticular stones, of which identification and incision of the diverticular neck was successful in all but 4 cases (96%); each of these was located in a lower pole calyx. Management of the neck was with balloon dilatation, or incision with a Bugbee electrode or Ho:YAG laser. Stones were fragmented with electrohydraulic lithotripsy (EHL), holmium, and SWL. Over a follow-up period of 8 years, only 8% of patients had recurrence of stones or symptoms.78
Fuchs and David reported the first series of calyceal diverticula stones managed with ureteroscopy. Fifteen patients with diverticular stones underwent URS for dilatation of the ostium, followed by SWL under the same anesthesia to fragment the stones. Using this combined approach, the authors reported a 73% stone-free and 87% symptom-free rate over a mean follow-up of 7.4 months.79
Batter and Dretler described a series of 26 patients who were managed ureteroscopically, of which 18 had failed prior SWL. The authors were successful in entering the cavity in 18 cases. Of the eight failures, five were in lower pole diverticula and the remaining three were at acute angles in upper pole calyces; three of these patients went on to percutaneous management, one had SWL, and one underwent laparoscopic partial nephrectomy. In those cases where the ostium was found and incised or dilated, stone removal was successful in all but three cases (83%), which required SWL to clear the remaining stone burden. At a mean follow-up of 45 months, 94% were stone free and all were asymptomatic.80
Grasso and associates described a series of four patients with five calyceal diverticular stones, of which two were managed purely endoscopically and the remaining three were managed with combined ureteroscopic and percutaneous techniques. One patient managed in a retrograde fashion had bilateral midpole diverticula; on the left side, the authors were successful in accessing the cavity and removing the stone burden, whereas the contralateral side was complicated by bleeding and required a subsequent combined retrograde/antegrade procedure to remove the stones. The second patient was successfully managed in a purely ureteroscopic procedure. The final two patients in the series had retrograde dilatation of the neck, followed by percutaneous placement of a guidewire for through-and-through access. The diverticula were fulgurated in both cases. All patients in this series were asymptomatic at 5 months.81
In their series of 39 patients, Auge and colleagues compared 22 cases of PCNL with 17 URS cases. Stone burdens were similar between the two cohorts. After 6-week follow-up, 35% of the URS group was symptom free versus 86% in the PCNL group. Stone-free rates also favored PCNL (78%) over URS (19%). When comparing the two groups for stone-free rates stratified by stone size, PCNL was significantly better than URS only in stones , 11 mm in diameter. PCNL was universally better than URS for all stone locations as well, but this was only statistically significant for upper pole stones. The authors concluded that, despite the increased rate of complications seen in PCNL, it should be the primary modality used to treat calyceal diverticular stones.82
URS for stones in upper and middle calyces with identifiable ostia produces durable results with low morbidity. However, the ostium cannot be identified during a retrograde approach in up to 30% of patients.83 For difficult cases, a percutaneous or laparoscopic approach can be applied under the same anesthesia if ureteroscopy is unsuccessful (Table 4).78
Laparoscopic Surgery
Technique. Laparoscopic surgery is a promising option for calyceal diverticula that are anteriorly located, have unidentifiable ostia that preclude endoscopic management, carry a large stone burden, or have thin overlying parenchyma. As it is the most invasive option compared with SWL, percutaneous, and ureteroscopic management, laparoscopic surgery should be considered only when other alternatives are not feasible.84,85
It is our preference to position the patient supine with the ipsilateral flank bumped 30° to 45°. Following establishment of pneumoperitoneum, the white line of Toldt is incised to permit medial mobilization of the bowel. Once the kidney is visualized, intraoperative ultrasound can be used to assist in locating the diverticulum. Alternatively, methylene blue can be injected through a preoperatively placed externalized ureteral catheter. The parenchyma overlying the lesion is incised with electrocautery scissors, revealing the diverticula cavity. Stones can then be removed with graspers and placed in an endoscopy bag. The cavity is then obliterated with Argon beam coagulation and the renal defect is sutured closed. A drain is placed and maintained until output and/or creatinine levels are low.
Results. The first case reports of laparoscopic management for calyceal diverticula came in 1993. In these early experiences, Gluckman and colleagues used five ports and located the cavity with the assistance of methylene blue injected retrograde through an externalized ureteral catheter. The cavity was unroofed, stones were removed, and the lining was ablated with argon. Operative time for the laparoscopic portion of the case was 3 hours and blood loss was 25 mL.86 Winfield and colleagues reported on a patient who underwent laparoscopic partial nephrectomy after failing prior percutaneous management. Using six ports, the authors completed a partial nephrectomy with a purpose-made renal tourniquet and argon beam coagulation to control bleeding and fulgurate the cavity. Operative time was 6 hours and 10 minutes, and estimated blood loss was 300 mL.87
Wong and Zimmerman reported a case in which laparoscopic-assisted transperitoneal PCNL was used in a patient with branched stones in an anterior upper-pole diverticulum. Using three ports, the authors dissected down to the diverticulum before introducing the nephroscope through an additionally placed 12-mm trocar; laparoscopic and nephroscopic visualization were made possible with the use of adjacent video towers. Holmium laser was passed through the nephroscope for stone fragmentation, and graspers were used for stone removal.88 Advantages of this combined technique include visualization and retraction to avoid bowel injury during PCNL (although the bowel can also be injured during trocar placement); direct puncture into the target diverticulum, which results in decreased risk of bleeding; and improved access to the diverticular neck.89
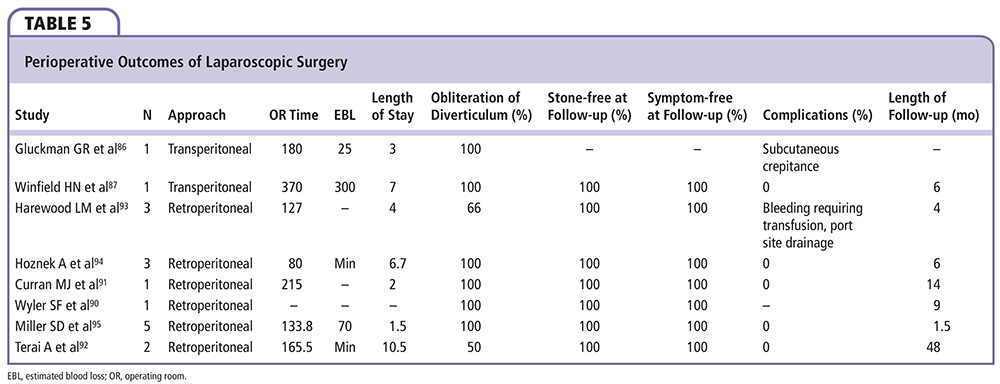
A number of authors have also reported cases in which a retroperitoneoscopic approach was taken, citing the advantages of avoiding bowel injury and intraperitoneal urine leakage, the ability to maneuver in obese patients, the low risk of hemorrhage and other intraoperative morbidity, and the opportunity for monotherapy with definitive results.90-92 Harewood and colleagues described three patients with anterior diverticula who underwent laparoscopic partial nephrectomy through a flank approach. The diverticula were identified by a depression in the surface of the kidney in two cases. In the third, an adjacent calyx was entered and the diverticulum was then located with fluoroscopic guidance. The cavities were then unroofed, the stones were removed, and the linings were fulgurated. In the second and third patients, a flap of Gerota’s fascia and perirenal fat were sutured to close the renal defect. Mean operative time was 127 minutes. One case was complicated by bleeding, which required transfusion, and another was complicated by drainage from a port site that spontaneously resolved. The patients were discharged after a median of 4 days, and on median follow-up of 4 months, all were stone and symptom free, and two patients had complete obliteration of the diverticulum.93
Hoznek and colleagues also reported a series of three patients managed with retroperitoneoscopic surgery, of which two had failed prior SWL and one with a mechanical heart valve had formed an abscess despite antibiotic therapy and needed definitive management. Following unroofing, stone extraction, and fulguration, the authors filled the cavities with surgical mesh impregnated with gelatin resorcinol formaldehyde glue. Average operative time was 80 minutes, blood loss was minimal in all cases, and no complications were reported. At 6-month follow-up, all patients were stone free and asymptomatic, with no recurrences noted.94
Miller and colleagues described five patients who underwent retroperitoneoscopic management that included freehand suturing of the diverticular neck in two cases, with injection of indigo carmine to confirm watertight closure.96 Argon was used to obliterate the cavity lining. Mean operative time was 133.8 minutes, estimated blood loss was 70 mL, and length of stay was 1.5 days, including four patients who were discharged home in the first 24 hours. Diverticula were obliterated in all patients on postoperative imaging at 6 weeks.
Although it may be the most “invasive” of the minimally invasive approaches, perioperative outcomes of laparoscopic surgery for calyceal diverticula are encouraging, and its long-term results appear to be durable. Larger series, which may require a multi-institutional effort due to the relative rarity of the disease, are needed for further analysis of both the retroperitoneal and transperitoneal approaches (Table 5).
Conclusions
Calyceal diverticula are rare outpouchings of the upper collecting system that likely have a congenital origin. Stones are found in up to 50% of cases, although over the combined reported series, 96% of patients presented with stones. Diagnosis is best made by IVU or CTU. SWL is an option for first-line therapy in patients with stone-bearing diverticula that have radiologically patent necks in mid- to upper-pole diverticula and small stone burdens. Stone-free rates are the lowest with SWL, although patients report being asymptomatic following therapy in up to 75% of cases with extended follow-up. URS is best suited for management of mid- to upper-pole anteriorly located diverticular stones. Drawbacks to URS include difficulty of identifying the ostium and low rate of obliteration. Percutaneous management is best used in posteriorly located mid- to lower-pole stones, and offers the ability to directly ablate the diverticulum. PCNL remains effective in the management of upper-pole diverticula, but carries the risk of pulmonary complications unless subcostal access strategies such as triangulation or renal displacement are used. Finally, laparoscopic surgery provides definitive management, but should be reserved for cases with large stones in anteriorly located diverticula with thin overlying parenchyma, and cases that are refractory to other treatment. ![]()
References
- Timmons JW Jr, Malek RS, Hattery RR, Deweerd JH. Caliceal diverticulum. J Urol. 1975;114: 6-9.
- Rayer PP. Traitements des maladies des reins 3. Paris: Baillere; 1841:507.
- Migliardi L. Pelvic cysts with multiple calculosis. Urologia. 1954; 21:63.
- Gambaccini P. Study of cystic dysplasia with respect to new morphogenetic findings. [Article in undetermined language] Radiol Med. 1954;40:338-369.
- Thorsen G. Pyelorenal cysts. Acta Chir Scand. 1949;98:476-488.
- Loeb MJ. Solitary cyst of the kidney. Urol Cutan Rev. 1944;48:105.
- Holm H. On pyelogenic cysts. Acta Radiol. 1948;29:87.
- Moore T. Hydrocalicosis. Br J Urol. 1950;22:304-319.
- Steinert R. Renal tuberculosis. Acta Radiol. 1943;(suppl):50.
- Bennett JP. Serous cyst communicating with the pelvis. Illinois Med J. 1941;79:222.
- Wolfromm G, Chalochet P, Gilson M. Cystoid deformity of a calyx associated with a congenital dysplasia of the opposite kidney. [Article in undetermined language] J Urol Medicale Chir. 1951;57:422-425.
- Le Maitre G. Pseudocystic cavities appended to the calices. J Radiol Electrol. 1955;36:384.
- Jomaine J. Cavite kystique juxta-calicielle. J Urol. 1951;57:425.
- Ask-Upmark E. Malignant juvenile nephrosclerosis, relation to disturbance in renal development. Acta Path Microbiol Scand. 1929;6:383.
- Itikawa T, Tanio H. Prolapse of the left ureter and congenital diverticulum of a calyx. Z Urol. 1939;33:395.
- Oschner HC. Cysts of the kidney. Amer J Roentgenol. 1951;51:1855.
- Abeshouse BS, Abeshouse GA. Calycealdiverticulum: a report of sixteen cases and review of the literature. Urol Int. 1963;329-357.
- Prather GC. Calyceal diverticulum. J Urol. 1941;45:55.
- Wulfsohn MA. Pyelocaliceal diverticula. J Urol. 1980;123:1-8.
- Middleton AW Jr, Pfister RC. Stone-containing pyelocaliceal diverticulum: embryogenic, anatomic, radiologic and clinical characteristics. J Urol. 1974;111:2-6.
- Michel W, Funke PJ, Tunn UW, Senge T. Pyelocalyceal diverticula. Int Urol Nephrol. 1985;17:225-230.
- Mathieson AJ. Calyceal diverticulum: a case with a discussion and review of the condition. Br J Urol. 1953;25:147-154.
- Yow RM, Bunts RC. Calyceal diverticulum. J Urol. 1955;73:663-670.
- Devine CJ Jr, Guzman JA, Devine PC, Poutasse EF. Calyceal diverticulum. J Urol. 1969;101:8-11.
- Narath PA. Renal Pelvis and Ureter. New York: Grune and Stratton; 1951.
- Schwartz A, Pfau A, Weinberg H. Association of calyceal diverticulum and butterfly vertebra. J Uro. 1960;8:32-35.
- Fey B, Gouygou C, Teinturier. [Cystic diverticula of the calyces.] J Urol Medicale Chir. 1951;57:192-195.
- Braasch WF, Hendrick JA. Renal cysts, simple and otherwise. J Urol. 1944;51:1.
- Hyams JA, Kenyon HR. Localized obliterating pyelonephritis. J Urol. 1941;46:380.
- Narath PA. The hydromechanics of the calyx renalis. J Urol. 1940;43:145-176 .
- Watkins KH. Cysts of the kidney due to hydrocalycosis. Br J Urol. 1939;11:207-215.
- Winsbury-White HP. A case of hydrocalycosis. Br J Urol. 1939;11:245-247.
- Dretler SP. Anew useful endourologic classification of calyceal diverticula. J Endourol. 1992;6(suppl):81.
- Burns JR, Finlayson B, Gauthier J. Calcium oxalate retention in subjects with crystalluria. Urol Int. 1984;39:36-39.
- Hsu TH, Streem SB. Metabolic abnormalities in patients with caliceal diverticular calculi. J Urol. 1998;160:1640-1642.
- Auge BK, Maloney ME, Mathias BJ, et al. Metabolic abnormalities associated with calyceal diverticular stones. BJU Int. 2006;97:1053-1056.
- Matlaga BR, Miller NL, Terry C, et al. The pathogenesis of calyceal diverticular calculi. Urol Res. 2007;35:35-40.
- Liatsikos EN, Bernardo NO, Dinlenc CZ, et al. Caliceal diverticular calculi: is there a role for metabolic evaluation? J Urol. 2000;164:18-20.
- Siegel MJ, McAlister WH. Calyceal diverticula in children: unusual features and complications. Radiology. 1979;131:79-82.
- Davidson AJ, Hartman DS, Choyke PL, Wagner BJ. Davidson’s Radiology of the Kidney and Genitourinary Tract. 3rd ed. Philadelphia: W.B. Saunders Company; 1999:416-421.
- Patriquin H, Lafortune M, Filiatrault D. Urinary milk of calcium in children and adults: use of gravity-dependent sonography. AJR Am J Roentgenol. 1985;144:407-413.
- Jacobs RP, Kane RA. Sonographic appearance of calculi in renal calyceal diverticula. J Clin Ultrasound. 1984;12:289-291.
- Widder DJ, Newhouse JH. The sonographic appearance of milk of calcium in renal caliceal diverticuli. J Clin Ultrasound. 1982;10:448-450.
- Dunnick NR, Sandler CM, Newhouse JH, Amis Jr ES. Textbook of Uroradiology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2008.
- Gayer G, Apter S, Heyman Z, Morag B. Pyelocalyceal diverticula containing milk of calcium—CT diagnosis. Clin Radiol. 1998;53:369-371.
- Clayman RV, Hunter D, Surya V, et al. Percutaneous intrarenal electrosurgery. J Urol. 1984;131:864-867.
- Hulbert JC, Hernandez-Graulau.JM, Hunter DW, et al. Current concepts in the management of pyelocaliceal diverticula. J Endourol. 1988;2:11.
- Rapp DE, Gerber GS. Management of caliceal diverticula. J Endourol. 2004;18:805-810.
- Collins JW, Keeley FX Jr. Is there a role for prophylactic shock wave lithotripsy for asymptomatic calyceal stones? Curr Opin Urol. 2002;12:281-286.
- Garcia Reboll L, Pontones J, Boronat F, et al. Extracorporeal shockwave lithotripsy: an alternative treatment for lithiasis of caliceal diverticula. [Article in Spanish] Actas Urol Esp, 1992;16:467-470.
- Ritchie AW, Parr NJ, Moussa SA, Tolley DA. Lithotripsy for calculi in caliceal diverticula? Br J Urol. 1990;66:6-8.
- Psihramis KE, Dretler SP. Extracorporeal shock wave lithotripsy of caliceal diverticula calculi. J Urol. 1987;138:707-711.
- Streem SB, Yost A. Treatment of caliceal diverticular calculi with extracorporeal shock wave lithotripsy: patient selection and extended followup. J Urol. 1992;148(3 Pt 2):1043-1046.
- Bilgasem S, Pace KT, Dyer S, Honey RJ. Erect and supine radiographs to assess effectiveness of SWL for stones in a caliceal diverticulum or dilated calix. J Endourol. 2003;17:7-9.
- Puppo P, Bottino P, Germinale F, et al. Management of caliceal stones resistant to extracorporeal shock wave lithotripsy. J Endourol. 1989;3:367-373.
- Nakada SY, Streem S, Preminger GM, et al. Controversial cases in endourology. Caliceal diverticular calculi. J Endourol. 1999;13:61-64.
- Cohen TD, Preminger GM. Management of calyceal calculi. Urol Clin North Am. 1997;24:81-96.
- Matlaga BR, Kim SC, Watkins SL, et al. Pre-percutaneous nephrolithotomy opacification for caliceal diverticular calculi. J Endourol. 2006;20:175-178.
- Karlin GS, Smith AD. Approaches to the superior calix: renal displacement technique and review of options. J Urol. 1989;142:774-777.
- Bellman GC, Silverstein JI, Blickensderfer S, Smith AD. Technique and follow-up of percutaneous management of caliceal diverticula. Urology. 1993;42:21-25.
- Auge BK, Munver R, Kourambas J, et al. Neoinfundibulotomy for the management of symptomatic caliceal diverticula. J Urol. 2002;167:1616-1620.
- Al-Basam S, Bennett JD, Layton ZA, et al. Treatment of caliceal diverticular stones: transdiverticular percutaneous nephrolithotomy with creation of a neoinfundibulum. J Vasc Interv Radiol. 2000;11:885-889.
- Lang EK. Percutaneous infundibuloplasty: management of calyceal diverticula and infundibular stenosis. Radiology. 1991;181:871-877.
- Matsumoto ED, Pearle MS. Treatment of caliceal diverticula. In: Nakada SY, Pearle MS, eds. Advanced Endourology: The Complete Clinical Guide.Totowa, NJ: Humana Press; 2006.
- Schwartz BF, Stoller ML. Percutaneous management of caliceal diverticula. Urol Clin North Am. 2000;27:635-645.
- Eshghi M, Tuong W, Fernandez R, Addonizio JC. Percutaneous (Endo) infundibulotomy. J Endourol. 1987;1:107-114.
- Hedelin H, Geterud K, Grenabo L, et al. Percutaneous surgery for stones in pyelocaliceal diverticula. Br J Urol. 1988;62:206-208.
- Ellis JH, Patterson SK, Sonda LP, et al. Stones and infection in renal caliceal diverticula: treatment with percutaneous procedures. AJR Am J Roentgenol. 1991;156: 995-1000.
- Shalhav AL, Soble JJ, Nakada SY, et al. Long-term outcome of caliceal diverticula following percutaneous endosurgical management. J Urol. 1998;160:1635-1639.
- Monga M, Smith R, Ferral H, Thomas R.. Percutaneous ablation of caliceal diverticulum: long-term -followup. J Urol. 2000;163:28-32.
- Donnellan SM, Harewood LM, Webb DR. Percutaneous management of caliceal diverticular calculi: technique and outcome. J Endourol. 1999;13:83-88.
- Kim SC, Kuo RL, Tinmouth WW, et al. Percutaneousnephrolithotomy for caliceal diverticular calculi: a novel single stage approach. J Urol. 2005;173:
1194-1198. - Krambeck AE, Lingeman JE. Percutaneous management of caliceal diverticuli. J Endourol. 2009;23:1723-1729.
- Hendrikx AJ, Bierkens AF, Bos R, et al. Treatment of stones in caliceal diverticula: extracorporeal shock wave lithotripsy versus percutaneous nephrolitholapaxy. Br J Urol. 1992;70:478-482.
- Jones JA, Lingeman JE, Steidle CP. The roles of extracorporeal shock wave lithotripsy and percutaneous nephrostolithotomy in the management of pyelocaliceal diverticula. J Urol. 1991;146:724-727.
- Turna B, Raza A, Moussa S, et al. Management of calyceal diverticular stones with extracorporeal shock wave lithotripsy and percutaneous nephrolithotomy: long-term outcome. BJU Int. 2007;100:151-156.
- Staios D, Andrews HO, Shaik T, Buchholz NN. Quality of life after percutaneous nephrolithotomy for caliceal diverticulum and secluded lower-pole renal stones. J Endourol. 2007;21:515-519.
- Chong TW, Bui MH, Fuchs GJ. Calyceal diverticula. Ureteroscopic management. Urol Clin North Am. 2000;27:647-654.
- Fuchs GJ, David RD. Flexible ureterorenoscopy, dilatation of narrow caliceal neck, and ESWL: a new, minimally invasive approach to stones in caliceal diverticula. J Endourol. 1989;3:255-263.
- Batter SJ, Dretler SP. Ureterorenoscopic approach to the symptomatic caliceal diverticulum. J Urol. 1997;158(3 Pt 1):709-713.
- Grasso M, Lang G, Loisides P, et al. Endoscopic management of the symptomatic caliceal diverticular calculus. J Urol.1995;153:1878-1881.
- Auge BK, Munver R, Kourambas J, et al. Endoscopic management of symptomatic caliceal diverticula: a retrospective comparison of percutaneous nephrolithotripsy and ureteroscopy. J Endourol. 2002;16:557-563.
- Canales B, Monga M. Surgical management of the calyceal diverticulum. Curr Opin Urol. 2003;13:255-260.
- Wolf JS Jr. Caliceal diverticulum and hydrocalyx. Laparoscopic management. Urol Clin North Am. 2000;27:655-660.
- Ramakumar S, Segura JW. Laparoscopic surgery for renal urolithiasis: pyelolithotomy, caliceal diverticulectomy, and treatment of stones in a pelvic kidney. J Endourol. 2000;14:829-832.
- Gluckman GR, Stoller M, Irby P. Laparoscopic pyelocaliceal diverticula ablation. J Endourol. 1993;7:315-317.
- Winfield HN, Donovan JF, Godet AS, Clayman RV. Laparoscopic partial nephrectomy: initial case report for benign disease. J Endourol. 1993;7:521-526.
- Wong C, Zimmerman RA. Laparoscopy-assisted transperitoneal percutaneous nephrolithotomy for renal caliceal diverticular calculi. J Endourol. 2005;19:608-613; discussion 613.
- Brunet P, Meria P, Mahe P, Danjou P. Laparoscopically-assisted percutaneous nephrolithotomy for the treatment of anterior calyceal diverticula. BJU Int. 2000;86:1088-1089.
- Wyler SF, Bachmann A, Jayet C. Retroperitoneoscopic management of caliceal diverticular calculi. Urology. 2005;65:380-383.
- Curran MJ, Little AF, Bouyounes B, et al. Retroperitoneoscopic technique for treating symptomatic caliceal diverticula. J Endourol. 1999;13:723-725.
- Terai A, Habuchi T, Terachi T, et al. Retroperitoneoscopic treatment of caliceal diverticular calculi: report of two cases and review of the literature. J Endourol. 2004;18:672-674.
- Harewood LM, Agarwal D, Lindsay S, et al. Extraperitoneal laparoscopic caliceal diverticulectomy. J Endourol. 1996;10:425-430.
- Hoznek A, Herard A, Ogiez N, et al. Symptomatic caliceal diverticula treated with extraperitoneal laparoscopic marsupialization fulguration and gelatin resorcinol formaldehyde glue obliteration. J Urol. 1998;160:352-355.
- Miller SD, Ng CS, Streem SB, Gill IS. Laparoscopic management of caliceal diverticular calculi. J Urol. 2002;167:1248-1252.
- Landry JL, Colombel M, Rouviere O, et al. Long term results of percutaneous treatment of caliceal diverticular calculi. Eur Urol. 2002;41:474-477.