Short-, Intermediate-, and Long-term Quality of Life Outcomes Following Radical Prostatectomy for Clinically Localized Prostate Cancer
Vinay Prabhu, MD, MS, Ted Lee, BS, Tyler R. McClintock, BS, Herbert Lepor, MD
Department of Urology, New York University School of Medicine, New York, NY
Many clinically localized prostate cancers that are diagnosed today are low risk, and prevention of disease-specific mortality may only be realized decades after treatment. Radical prostatectomy (RP) may adversely impact health-related quality of life (HRQOL) by causing both transient or permanent urinary incontinence and erectile dysfunction. In contrast, RP may also improve HRQOL via relief of lower urinary tract symptoms in men suffering from these symptoms prior to surgery. Because the average man treated for prostate cancer has a life expectancy of approximately 14 years, it is imperative to consider the long-term impact of RP on both survival and HRQOL in treatment decision making. This comprehensive literature review examines short-, intermediate-, and long-term HRQOL following RP. In addition, the long-term results of RP are compared with other treatment modalities for treating clinically localized prostate cancer.
[ Rev Urol. 2013;15(4):161-177 doi: 10.3909/riu0604]
© 2014 MedReviews®, LLC
Key words
Radical prostatectomy • Prostate cancer • Health-related quality of life • Lower urinary tract symptoms
In a study of over 1000 men evaluated for ≥ 6 months following RP, the majority reported similar or improved overall HRQOL scores compared with baseline, and 78% of responders indicated they would elect the same treatment again, despite any negative effects on urinary and sexual function.
LUTS adversely impact overall urinary HRQOL and increase resource utilization, work productivity loss, activity impairment, pain, anxiety, and depression. Medical and surgical treatments for BPH are estimated to cost the US economy $4 billion per year, in addition to causing potentially serious side effects.
Main Points
• Widespread adoption of prostate-specific antigen testing and prostate biopsy has increased the detection of clinically localized prostate cancer, resulting in increased use of radical prostatectomy (RP).
• The mean age of men undergoing RP is approximately 60 years; those undergoing curative intervention of prostate cancer are expected to live at least 14 years. Because of this, it is important to consider both the long-term impact of RP on survival and health-related quality of life (HRQOL) in treatment decision making.
• Men undergoing RP may experience significant short-term urinary incontinence, lower urinary tract symptoms (LUTS), and sexual dysfunction that may impair both their general and disease-specific HRQOL. In contrast, RP may also improve HRQOL via relief of LUTS in men suffering from these symptoms prior to surgery.
Based on the available literature, it appears that at short- and intermediate-term follow-up, RT has better SF outcomes than RP. SF was less likely to return following RP (P , .001) 4 years after RP in a study at UCLA, 5 years after RP in the PCOS, and 5 to 10 years after diagnosis in the PLCO.
Patients with solitary kidney, bilateral disease, poor renal function, small tumor burden, or low-grade disease force the clinician to consider other treatment options, such as segmental ureterectomy and ureteroscopic or percutaneous resection.
Unfortunately, there are no randomized studies comparing HRQOL outcomes following open versus robotic RP. The majority of studies comparing HRQOL outcomes between the two techniques are flawed in design.
In the Western world, prostate cancer is the most common cancer in men and the second most common cause of cancer death in men.1 Widespread adoption of prostate-specific antigen (PSA) testing and prostate biopsy has increased the detection of clinically localized prostate cancer,2 resulting in increased use of radical prostatectomy (RP).3 Of the over 240,000 cases of prostate cancer diagnosed annually in the United States, approximately one-third to one-half of men are managed initially with RP.4-6
Many clinically localized prostate cancers that are diagnosed today are low risk, and, therefore, prevention of disease-specific mortality may only be realized decades after treatment. RP may also adversely impact health-related quality of life (HRQOL) by causing both transient or permanent urinary incontinence and erectile dysfunction (ED).7,8 In contrast, RP may also improve HRQOL via relief of lower urinary tract symptoms (LUTS) in men who have these symptoms prior to surgery.9 Because the average man treated for prostate cancer has a life expectancy of approximately 14 years,10 it is imperative to consider both the long-term impact of RP on survival and HRQOL in treatment decision making.
This comprehensive literature review examines short-, intermediate-, and long-term HRQOL following RP. In addition, the long-term results of RP are compared with other treatment modalities for treating clinically localized prostate cancer.
Short-, Intermediate-, and Long-term Assessment of HRQOL Following RP
Most men experience some degree of urinary incontinence and ED immediately following RP. Because it was, and often still is, generally assumed that recovery of continence and potency plateaus at 2 years, there is abundant literature discussing this time period.11,12 Therefore, we define short-term outcomes as the first 2 years following RP. There is extensive literature examining short-term HRQOL following RP. There are fewer studies examining HRQOL between 2 and 10 years following RP, which we arbitrarily define as intermediate-term outcomes. There are two studies examining HRQOL at 10 years and beyond, which we define as long-term outcomes.
General HRQOL
General HRQOL represents a general assessment of well-being. General HRQOL is most commonly assessed by the Medical Outcome Study Short Form (SF-36),13 also called the RAND-36 when scored by a modified scale. The SF-36/RAND-36 assesses eight health concepts: physical, social, role limitation, emotional problems, bodily pain, mental health, vitality, and health perception.14 Other scales measuring general HRQOL have been less frequently employed for prostate cancer.8,15,16
In a study of over 1000 men evaluated for ≥ 6 months following RP, the majority reported similar or improved overall HRQOL scores compared with baseline, and 78% of responders indicated they would elect the same treatment again, despite any negative effects on urinary and sexual function.17 Favorable outcomes in physical and mental component summaries of the RAND-36 have been shown to persist for up to 4 years following RP.18
No observable differences in general HRQOL domains have been observed among men treated for prostate cancer and men electing active surveillance (AS) or age-matched control subjects without prostate cancer.8 Likewise, there is no observed difference in psychological symptoms, well-being, or subjective HRQOL among men treated with RP and AS.19
No demonstrable differences in HRQOL were observed among men undergoing RP and radiation therapy (RT). Men undergoing both RP and RT show similar short-term declines in vitality, energy, and role function scores shortly after treatment. These scores recover to baseline levels by 1 year following treatment.20,21 No differences were observed in studies comparing overall general HRQOL among RP, RT (external beam radiation therapy [EBRT] or brachytherapy [BT]), and control subjects.22 In one comparative study, RP was associated with better function for role physical, role emotional, vitality, and general health scales.23
Several baseline characteristics have been associated with better outcomes on general HRQOL surveys in men undergoing RP, including: age < 65 years,24 better general health,24 better preoperative functional status,15 fewer medical or psychiatric comorbidities,15 higher education level,25 and less pain after treatment.26 General HRQOL outcomes also depend on whether the assessment was made by the patient or surgeon.27
The clinical relevance of changes in general HRQOL domains varies amongst individuals. More recently, investigators have introduced individualized measurements that allow respondents to nominate significant HRQOL domains not captured on standard questionnaires and determine the relative weight of each domain.28,29
Satisfaction
Patient satisfaction, a component of general HRQOL, is an independent measure of quality of care and health status30,31 and is positively associated with global and disease-specific HRQOL.23,32,33 The National Cancer Institute recognizes satisfaction as a priority for outcomes assessment in cancer.34 Despite its recognized importance, assessment of satisfaction following all treatments for prostate cancer has been underreported. Satisfaction following RP has been most commonly ascertained via satisfaction with outcome or satisfaction with treatment or using institution-developed surveys, such as the Service Satisfaction Scale for Cancer Care7 or the Client Satisfaction Questionnaire.35
Outcome satisfaction after RP has only been studied by two groups. Sanda and colleagues observed that both patient and partner satisfaction were significantly associated with changes in sexual, hormonal, urinary, and bowel function, independent of treatment modality.7 Abraham and colleagues reported outcome satisfaction following RP in 1542 consecutive men undergoing RP by a single surgeon at various time intervals between 3 and 24 months.36 Satisfaction ranged between 92% and 94% at the various assessments.36 Perioperative factors, including duration of indwelling Foley catheterization, were associated with satisfaction at 3 months, whereas sexual function, urinary function, and biochemical failure were associated with satisfaction at the 2-year assessment.36
Several groups have reported on comparative treatment satisfaction. Of the 2365 men in the Prostate Cancer Outcomes Study (PCOS) who were surveyed at 2 years following RP, RT, androgen deprivation therapy (ADT), and no intervention, 83.1%, 90.9%, 90.9%, and 86.3% were at least mostly satisfied with their decision, respectively.32 The perception of being cancer free was the factor most significantly associated with treatment choice satisfaction; other significant factors included urinary, sexual, and bowel function; general health; and preservation of social relationships.32 In a prospective cohort study comparing self-assessment of satisfaction with care, RP treatment was associated with higher satisfaction than EBRT (odds ratio [OR] 7.9; P = .043).23
Disease-specific HRQOL
Disease-specific HRQOL following treatment for clinically localized prostate cancer includes side effects impacting urinary, sexual, and bowel function. The primary limitation of both RP and RT is related to their impact on these disease-specific HRQOL domains.8
Urinary Dysfunction: Urinary Incontinence and LUTS
The etiology for urinary dysfunction following treatment for clinically localized prostate cancer involves both bladder and sphincteric dysfunction, which are manifested by LUTS and urinary incontinence, respectively.
Incontinence
Incontinence following RP negatively impacts HRQOL and satisfaction.36-38 Continence following RP has been defined using a summary score incorporating a number of continence domains or as a binary variable (continent vs incontinent), using specific definitions for continence. The UCLA-Prostate Cancer Index Urinary Function Index (UCLA-PCI-UFI) is a commonly used continence summary score that captures five domains of urinary incontinence: leaking frequency, urinary control, diaper and pad use, dripping problems, and climacturia. These domains are individually scored out of 100 and the composite score is the mean of the components.39 The Expanded Prostate Cancer Index Composite (EPIC) Incontinence subscale captures the same domains as the UCLA-PCI except for climacturia, and is similarly scored out of 100.40 There is no consensus on what constitutes clinically significant changes in these urinary function scores.
Methodologies for assessing these continence variables can vary greatly and include: face-to-face interviews with or without a physician present, telephone interviews, self-administration of validated questionnaires, 24-hour pad weighing, and/or spousal report.11,41,42 The optimal continence assessment is utilization of a self-administered, validated questionnaire.
There is no universally agreed-upon definition of urinary continence. Definitions in the literature include: daily use of 0 pads,12,43-46 daily use of up to 1 pad,9,46-48 total control or occasional dribbling,9,49-51 no problem dripping or leaking urine,47 leaking once daily,47 or no leak, total control, and 0 pads.52 Lepor and Kaci have previously shown that 13.4% of men experienced occasional or frequent dribbling before RP.9 Therefore, if the definition of urinary continence is strictly total control, then 13.4% of men would be considered incontinent prior to undergoing RP. To better identify how men self-define their own continence, Lepor and associates correlated responses to the UCLA-PCI-UFI with patients’ self-assessments of whether they considered themselves continent or incontinent.53 At 3 and 24 months following RP, 82.5% and 100% of men using one pad per day considered themselves continent, respectively. Similarly, at 3 and 24 months, 92% and 100% of men reporting occasional dribbling of urine considered themselves continent, respectively. The fact that a greater percentage of men using one pad per day consider themselves continent suggests that the single pad is less saturated and often worn as a “safety” pad for the rare and minimal degree of urinary leakage. Therefore, we consider men continent at 24 months who require one pad in 24 hours and those who have occasional dribbling of urine, because the patient considers himself continent. Therefore, some “continent” men may experience further improvement in continence over time. Glickman and colleagues reported that almost one-quarter of continent men reported subjective improvements in continence between 2 and 4 years after RP.49
Short-term Outcomes
Due to different definitions of continence, methodology for ascertaining continence, and variation in patient selection,11,27,47,48 reported continence rates at 3, 6, 12, and 24 months range between 51% and 78%,9,12,53-57 70% and 89%,9,12,50,54-57 80% and 98%,9,12,50,54-57 and 86% and 98.5%,7,9,12,37,43,45,49 respectively. Despite these wide ranges, continence is consistently worst immediately after surgery and continues to improve through 2 years post-RP.11 Continence composite scores, such as the UCLA-PCI, show similar time-dependent increases until 2 years after RP.18,25,58
Several factors have been associated with better short-term continence following RP: lower age,7,18,38,46-48,50,59-63 fewer preoperative comorbidities,46,59,64-67 nerve sparing,47,55,68,69 higher socioeconomic status,64 nonblack race,7 higher education level,25 lower PSA,7 higher baseline urinary function,68 lack of preoperative ED,70 nonuse of ADT,68 lower body mass index (BMI),46 lower prostate volume,46 no preoperative LUTS,46 use of various anastomotic techniques,69,71,72 and absence of anastomotic stricture.69 The impact of prostate size on postoperative continence does not appear to have an impact on continence rates in adjusted analyses.41,59,69,73 In our prospective Institutional Review Board–approved longitudinal outcomes study, age, Gleason score, nerve sparing, estimated blood loss, baseline LUTS, and presence of benign prostatic tissue in the apical soft tissue margin were not associated with early continence recovery at 3 months.9
Intermediate and Long-term Outcomes
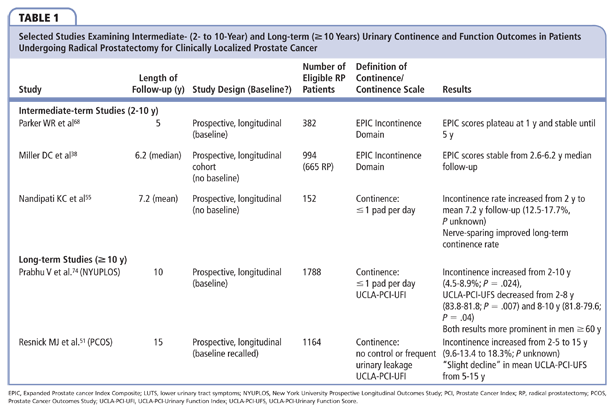
There is no agreement on time-dependent changes in urinary continence after 2 years (Table 1). In a study of 156 men at the Cleveland Clinic (Cleveland, OH) following RP by multiple surgeons, the proportion of patients using up to one pad per day increased from 12.5% to 17.7% between 2 years and mean 7.8-year follow-up (statistical significance not reported).55 In the 15-year follow-up study of the PCOS, the percentage of men experiencing no control or frequent urinary leakage increased progressively from 9.6% to 13.4% to 18.3% at 2, 5, and 15 years post-RP, respectively (statistical significance not reported).51 The increase in urinary incontinence was consistent with increasing bother due to incontinence. Between October 2000 and September 2012, 1788 of 1836 men who underwent RP by a single surgeon gave consent to participate in the New York University Prospective Longitudinal Outcomes Study (NYUPLOS). The UCLA-PCI-UFI was self-administered at baseline and at 6 time points over 10 years of follow-up.74 A change in the proportion of men using up to one pad daily was statistically significant between 2 and 10 years (4.5%– 8.9%; P = .024). The increase in the incontinence rate was driven by the subgroup of men aged ≥ 60 years.
The PCOS similarly demonstrated a “slight” decline in long-term mean UCLA-PCI-UFI score from 5 to 15 years.51 Parker and colleagues also prospectively administered the EPIC to 378 (77%) of 490 men undergoing RP at baseline and 246 (65.1%) of these men at 5 years after RP, showing that EPIC incontinence subscale scores plateaued at 12 months and subsequently stabilized until 5 years.68 In contrast, Miller and colleagues reported that incontinence subscale scores were stable for 665 men who underwent RP and completed the EPIC at a median of 2.6-year and 6.2-year follow-up.38 In the NYUPLOS, there was a modest, yet statistically significant, decrease in UCLA-PCI-UFI score between 2 and 8 years (83.8 vs 81.8; P = .007) and 8 and 10 years (81.8 vs 79.6; P = .04). The slight decrease in UCLA-PCI-UFI score was attributable to the subgroup of men aged ≥ 60 years. Overall, these data suggest that between 2 and 15 years following RP, there is a slight linear decline in both urinary continence rates and composite continence score. The deterioration of urinary continence appears to occur after 5 years and in men who underwent RP beyond age 60 years.
Continence rates, as well as continence summary scores, in men not undergoing RP are generally accepted to worsen with age.75,76 Therefore, the proportion of the observed progressive decline in urinary continence attributable to the long-term sequelae of RP versus the natural history of urinary dysfunction is unclear. In addition, it is unknown what the continence status of men undergoing RP would have been had they chosen AS, recognizing continence may have deteriorated due to aging or in surgical intervention for progressive, local disease causing bladder outlet obstruction.
Three studies comparing men undergoing RP to age-matched control subjects or those choosing AS provide insights into the natural history of continence in aging men with and without prostate cancer. Investigators from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial conducted telephone interviews with 529 men with screen-detected prostate cancer and 514 noncancer control subjects between 5 and 10 years following prostate cancer diagnosis in their randomized, controlled trial assessing the risks and benefits of screening for prostate cancer.42 Men undergoing RP (n = 201) exhibited worse EPIC Short Form Urinary Function scores (P < .001), which assesses both continence and two LUTS symptoms (weak stream, urinary frequency), as well as pain, burning, and bleeding with urination.40 In another study, 293 men with localized prostate cancer selected randomly from the New Mexico Tumor Registry were compared with age and ethnicity-matched noncancer control subjects. At 5 years, men undergoing RP demonstrated worse urinary function (P < .001).77 Baseline urinary function was recalled at 6 months posttreatment, introducing potential recall bias. In addition, the survey was not validated and captured both incontinence severity and one LUTS (frequency). The 41 men who chose conservative management (21 ADT, 20 watchful waiting [WW]) also exhibited significant declines in urinary function from baseline to 5 years (P < .03), suggesting that local disease progression may play a role in urinary function decline. The Scandinavian Prostate Cancer Group Study Number 4 (SPCG-4) compared degree of incontinence in 208 and 192 men who were randomly assigned to either RP or WW with a median 12.2-year follow-up.78 Men in the RP group demonstrated markedly higher rates of daily urinary leakage (41% vs 11%; relative risk [RR] 3.79; 95% confidence interval [CI], 2.36-6.06). Because baseline data were not collected, it is unknown how many men experienced daily urinary leakage prior to randomization.
Few studies compare intermediate- or long-term incontinence rates or continence scores between RP and RT. Two studies reported that incontinence rates following RP were significantly higher than RT at 4 to 5 years.51,58 The PLCO telephone interview of 201 RP and 110 RT patients between 5 and 10 years postdiagnosis demonstrated that men who underwent RP had poorer EPIC Short-Form Urinary Function scores compared to RT (71.48 vs 84.04; P < .001).42 Miller and associates prospectively compared outcomes in 665 and 231 men undergoing RP and RT, respectively, at a median of 2.6 and median 6.2 years. EPIC incontinence subscale scores in the RT group declined over the 3.6 years compared with stable scores in the RP group.38 This significant intermediate-term decline in urinary continence after RT explains why PCOS-reported continence rates were similar in the RP and RT groups at 15 years, despite better short-term outcomes after RT.51
LUTS
LUTS include storage and voiding urinary symptoms, most commonly caused in men by benign prostatic hyperplasia (BPH).79 LUTS adversely impact overall urinary HRQOL80,81 and increase resource utilization, work productivity loss, activity impairment, pain, anxiety, and depression.80-85 Medical and surgical treatments for BPH are estimated to cost the US economy $4 billion per year,86 in addition to causing potentially serious side effects.87,88
The American Urological Association Symptom Index (AUASI) was designed to capture the severity of baseline LUTS and the response of LUTS to treatment of BPH. The International Prostate Symptom Score (IPSS) is simply the AUASI combined with a single question querying urinary HRQOL.89 The AUASI independently scores four voiding (bladder emptying, intermittent urinary stream, caliber of urinary stream, and straining to urinate) and three storage (frequency, urgency, and nocturia) symptoms on a scale of 0 to 5, with a composite score (AUASS) calculated between 0 and 35. AUASS of 0 to 7, 8 to 19, and 20 to 35 define mild, moderate, and severe LUTS, respectively; therefore, a composite score > 7 is defined in clinical practice guidelines as clinically significant LUTS.79,90,91 Improvements in the AUASS of 8.8, 5.1, and 3.0 points correlate with marked, moderate, and slight changes in LUTS, respectively.92 The EPIC irritative/obstructive subscale is a less frequently employed instrument that captures three LUTS (weak stream, nocturia, and frequency) along with hematuria, pain with urination, and burning with urination symptoms not typically associated with LUTS.40
Short-term Outcomes
There are 2 competing factors that influence LUTS following RP. First, in the early phase, many men will time voiding to minimize incontinence. This voiding pattern leads to frequent voiding and a decreased stream due to decreased voided urine volume, which contributes to higher AUASS. In addition, up to 40% of men have coexisting clinically significant LUTS secondary to BPH, which has been shown to improve following removal of the prostate.
Several short-term studies have characterized time-dependent changes in LUTS during the first 2 years following RP (Table 2).7,9,93,94 The severity of LUTS generally increases immediately after RP and plateaus at 3 months. After 3 months, LUTS generally decreases, reaching a nadir by 1 or 2 years. Lepor and Kaci were the first to examine the short-term effect of RP on LUTS following RP and also stratified outcomes according to baseline LUTS severity.9 By 1 year following RP, the mean decrease in AUASS for men with baseline clinically insignificant (AUASS ≤ 7) and clinically significant (AUASS > 7) LUTS was 0.7 and 5.4, respectively. This 5.4-point decrease in mean LUTS corresponds to a moderate improvement in LUTS, whereas the decrease of 0.7 corresponds to a clinically insignificant change. Others have subsequently confirmed these findings.7,93,94 Several other factors aside from baseline clinically significant LUTS have been associated with improved short-term LUTS outcomes in men undergoing RP: a larger prostate,7 younger age,93 lower BMI,94 and urinary continence at 3 months.94
Intermediate-term Outcomes
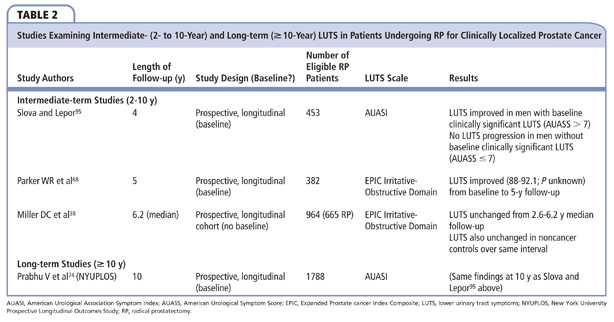
There is a paucity of intermediate-term studies examining LUTS following RP (Table 2). Slova and Lepor prospectively administered the AUASI to 453 men undergoing RP at baseline and several time points up to 4 years following RP. In men with baseline clinically significant LUTS, the early improvements persisted for 4 years.95 Interestingly, after an initial rise in AUASS postoperatively, the mean AUASS was unchanged between 1 and 4 years for the subgroup with baseline clinically insignificant LUTS, suggesting that RP may alter the natural history of progressive LUTS due to BPH.95 Parker and colleagues reported on 378 patients who completed the EPIC irritative/obstructive subscale at baseline and at multiple follow-up intervals over 5 years following RP. LUTS severity increased between baseline and 1 month and then progressively improved, plateauing at 24 months and remaining stable in the 246 evaluable men at 5 years following RP.68 The mean EPIC irritative/obstructive score at 5 years was higher (indicating an improvement of LUTS) than at baseline (92.1 vs 88.0), although statistical significance was not reported. Miller and associates similarly administered the EPIC survey to 665 men at a mean follow-up of 2.6 and 6.2 years: over the mean 3.6 years of follow-up, EPIC irritative/obstructive scores did not significantly change.38 However, no change was observed in control subjects without cancer over this same period.
Long-term Outcomes
The NYUPLOS is the only long-term study of LUTS following RP (Table 2).96 The AUASS was self-administered at baseline and nine scheduled assessment points over 10-year follow-up. Overall, the mean AUASS at baseline (6.87) and 10 years (5.90) was not significantly different.
The time-dependent changes in mean AUASS were also examined according to baseline severity of LUTS. The percentage of men with clinically significant LUTS (AUASS > 7) at baseline (35.5%) versus 10 years (26.9%) was significantly lower (P = .02). As expected, the benefit of RP on LUTS was greatest in those men presenting with clinically significant LUTS. In this subgroup, the mean AUASS at 10 years (8.81) was significantly lower than baseline (13.5; P < .001). The baseline (3.23) and 10-year (4.58) mean AUASS in the subgroup of men with baseline clinically insignificant LUTS were statistically, but not clinically, significantly different. After an initial rise of 1.7 points between baseline and 1 year, mean AUASS in these men did not increase by > 0.4 points between assessment intervals thereafter through 10 years.
The prevalence of clinically significant LUTS in community-dwelling men progressively increases beginning in the fifth decade of life and exceeds 40% by the eighth decade of life.83,97-106 In the NYUPLOS, the prevalence of clinically significant LUTS at baseline for men in their fifth, sixth, seventh, and eighth decades of life was 27%, 31%, 42%, and 46%, respectively, which parallels age-matched men in the general population.96 Based on the natural history of LUTS in age-matched control subjects without cancer, we would have anticipated LUTS to progress in men in the NYUPLOS. The fact that LUTS remained constant between 2 and 10 years following RP in both men with and without baseline clinically insignificant LUTS in the NYUPLOS suggests that RP interferes with the natural history of BPH by removing the prostate, the primary contributor to LUTS progression in men.
The SPCG-4 provides further insight into the natural history of LUTS in men with untreated, clinically localized prostate cancer. A total of 376 men were randomized to RP or WW and LUTS outcomes were compared at 4 years. One symptom (weak urinary stream) was worse in the WW arm at 4 years (RR 0.6; 95% CI, 0.5-0.9).19 In addition, the fraction of men with clinically significant LUTS at 4 years was greater in the WW arm (35% RP vs 49% WW; RR 0.7; 95% CI, 0.5-0.9). The lower proportion of men with clinically significant LUTS following RP compared with WW is due to the therapeutic benefit of RP and the natural history of untreated LUTS in the WW group attributable both to the development of progressive LUTS due to aging and local progression of the cancer.
Gore and associates administered the AUASI at baseline and multiple follow-up intervals to 307 and 90 men who underwent RP and BT, respectively.58 Voiding and storage symptoms were both more prevalent after BT than after RP (P < .001). Pardo and colleagues reported EPIC irritative/obstructive scores at baseline and at multiple time points up to 3 years in a prospective cohort of 123, 127, and 185 men undergoing RP, EBRT, and BT, respectively. Baseline EPIC irritative/obstructive scores were similar. BT and EBRT were associated with greater progression of LUTS at 3 years compared with RP (P = .002).107
The favorable impact of RP on short-, intermediate-, and long-term LUTS is highly clinically relevant. Millions of American men are on medical treatment for LUTS/BPH and many millions more undergo surgical resection and ablation of the prostate to alleviate bladder outlet obstruction, collectively amounting to annual expenditures of $4 billion.86 The HRQOL and economic benefits attributed to obviating the need for medical or surgical management of BPH are significant.
Combined Urinary Function
Because the EPIC questionnaire captures both incontinence and LUTS, it conveniently enables simultaneous assessment of these two outcomes using a single survey. The EPIC questionnaire is designed to independently report incontinence and irritative/obstructive subscores, or alternatively report a composite Urinary Summary, Urinary Function, or Urinary Bother score, which combine elements of both incontinence and irritative/obstructive symptoms.40 Most longitudinal studies following RP report incontinence and irritation/obstruction subscores separately,38,68,107 along with Urinary Function and Urinary Bother scores.38,42,68,107,108
Due to the pathophysiology of LUTS and continence, reporting a composite urinary function score is of limited utility for clinical management or research. Composite scores conflate physiologically unique aspects of urinary function and imply trends in urinary function and bother that are confusing and potentially misleading.
Sexual Function and Potency
ED, or impotence, is a major concern of men undergoing RP. As many as 45% to 64% of RP candidates suffer from ED preoperatively.19,78,109,110 The treatment-related incidence of ED is far more common than incontinence at all follow-up intervals. Therefore, a large proportion of men will be impacted temporarily and permanently by ED.
Global sexual function (SF) can be assessed using several validated instruments. The most common instrument is the International Index of Erectile Function (IIEF), a validated, self-administered 15-item questionnaire that queries five domains: erectile function (EF) (six items), orgasm function (two items), sexual desire (two items), intercourse satisfaction (three items), and overall satisfaction (two items).111,112 The UCLA-PCI Sexual Function Index (UCLA-PCI-SFI), or its slightly modified version, the EPIC Sexual Domain, is composed of eight questions addressing sexual desire, orgasm ability, intercourse frequency, erection upon awakening, erection ability, erection quality, erection frequency, and overall SF.39,40
It is generally believed that sexual dysfunction following RP is manifested primarily by ED. Thus, the nerve-sparing RP was designed to restore EF and not other problems associated with impaired SF, including climacturia, loss of emission, and diminished orgasmic pleasure. Elucidating the impact of RP and other treatments of prostate cancer requires assessing the many other factors contributing to SF captured by the UCLA-PCI-SFI or the EPIC Sexual Domain.
The Sexual Health Inventory for Men (SHIM) is a shortened, five-question version of the IIEF that specifically examines EF and demonstrates similar efficacy to the IIEF.113 EF can also be defined more specifically as erection adequate for intercourse with or without phosphodiesterase-5 inhibitors (PDE5-I),113,114 SHIM score > 15 to 21,115 sexual intercourse in the past month with or without PDE5-I,116 UCLA-PCI-SFI score 80 to 100,117 erection whenever wanted,117 good or very good SF,115,117 or ability to have erection spontaneously or elicited.78
The development of the nerve-sparing prostatectomy in the 1980s resulted in markedly improved SF and EF outcomes.12,118-122 The large variations in definitions of EF, patient selection, surgeon experience, and methodology for reporting outcomes account for the very wide range of reported potency rates.114,117,123,124 Ideally, potency outcomes should be ascertained using a validated, self-administered questionnaire administered at baseline and multiple predetermined time points following RP. It is also critical to determine interventions that are required to restore EF. The assessment of SF should go beyond EF. The operating surgeon must be uninvolved with data acquisition, collection, retrieval, and statistical analysis in order to minimize biases.
Short-term Outcomes
Initial studies examining EF and SF following nerve-sparing RP reported results up to 24 months, because it was assumed that potency rates plateaued by this time.12,122 Recovery of EF at 3, 6, 12, 18, and 24 to 36 months ranges between 19% and 68%,12,114 24% and 86%,12,114 39% and 90%,12,114 44% and 86%,12,50 and 35% to 94%,114,116,125 respectively. Because of these broad ranges, most men undergoing RP do not have realistic expectations regarding preservation of EF.
A number of factors have been shown to hold strong associations with postoperative return of EF or SF within 2 years, which include time after RP,18,126 nerve-sparing,45,116,125,127-129 lower age,18,116,122,125-131 better preoperative SF score,125,131 early treatment with PDE5-I,132 frequency of pre-RP intercourse,127 absence of diabetes mellitus,116 lower pathologic or clinical stage,122,128,129lower PSA,125 absence of seminal vesicle or lymph node involvement,127 greater education,25 absence of post-RP incontinence or strictures,127 lower cancer volume,127,130 and cautery-free technique.114
Intermediate-term Outcomes
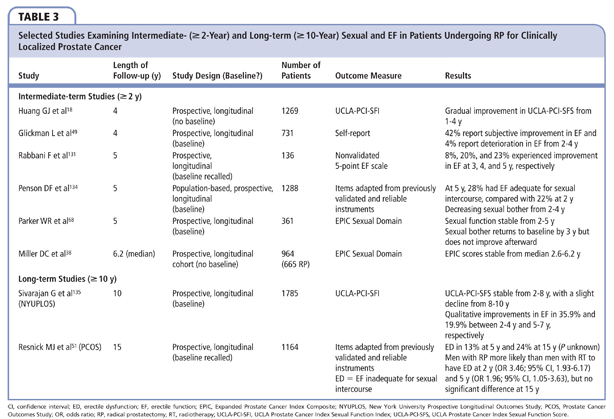
Studies examining intermediate-term SF outcomes provide compelling and consistent evidence that recovery of EF occurs beyond 2 years after RP (Table 3). Glickman and colleagues have shown that as many as 42% and 4% of men report subjective improvement or deterioration in EF from 2 to 4 years post-RP, respectively.49 Similarly, in a study of 136 preoperatively potent men who had not recovered potency by 2 years, Rabbani and associates reported that 8%, 20%, and 23% of men subsequently gained EF at 3, 4, and 5 years post-RP.133 The Cancer of the Prostate Strategic Urologic Research Endeavor cohort also demonstrated gradual improvement in UCLA-PCI-SFI scores from 1 to 4 years post-RP.18 Of the 1288 men who completed the UCLA-PCI-SFI in the PCOS at 2 and 5 years after RP, the proportion of men with erections adequate for sexual intercourse increased significantly from 22% to 28% (P = .003), with decreasing sexual bother.134 Independently derived erectile recovery models also demonstrate continued potency recovery through at least 4 years after RP.126,131 Other studies have shown stabilization of SF after 2 years: Miller and colleagues reported stable EPIC Sexual Summary Scores in 665 men longitudinally evaluated at median follow-up of 2.6 years and then 6.2 years,38 whereas Parker and associates demonstrated stable EPIC Sexual Function Scores from 2 to 5 years post-RP in their cohort of 361 men followed longitudinally.68 The apparent discordance between EF and SF outcomes may be explained by variable time-dependent effects of EF compared with the other domains captured by a composite SF assessment.
Long-term Outcomes
One of the limitations of the PCOS for assessing time-dependent changes in EF is that potency was preserved in only 24% of men at 5 years.51 Nevertheless, potency rates in the PCOS decreased from 24% to 13% between 5 and 15 years after RP, respectively.51 Interestingly, only half of men with ED indicated that this was a cause of bother and this percentage decreased over time,51 likely due to declining sexual interest or adjustment to ED over time.
The NYUPLOS reported that the mean UCLA-PCI-SFI scores were stable from 2 to 8 years post-RP, with a slight decline from 8 to 10 years.135 Mean EF scores, on the other hand, improved in a time-dependent manner until 8 years post-RP. The divergence between intermediate-term EF and SF scores has also been observed by other investigators, and suggests that EF is not the only parameter that drives SF scores. Overall, we observed that 35.9% and 19.9% of men reported subjective improvements in their EF between 2 and 4 years and 5 and 7 years post-RP, respectively. A total of 42% and 25.3% of men aged < 60 years reported improvements in their erections between 2 to 4 and 5 to 7 years, respectively, whereas only 14.8% and 6.6% reported a deterioration of their erections over these time intervals. Based on these responses, one would predict that mean EF scores would increase between 2 and 8 years for men aged < 60 years, which is consistent with their findings. Of men aged > 60 years, 28.4% and 13.1% reported subjective improvements of their erections between 2 to 4 and 5 to 7 years, respectively, whereas 12.4% and 14.3% reported deterioration of their erections over these time intervals. Based on these responses, one would predict that between 2 to 8 years, mean EF scores would essentially be unchanged for men aged > 60 years, which is also consistent with their findings. Mean SF scores were stable for men aged < 60 years between 2 to 8 years, whereas they significantly decreased for men aged > 60 years. The mean SF score captured frequency of intercourse and other parameters that appeared to be age dependent and independent of quality of erection.
It is well recognized that rates of ED steadily increase in community-dwelling men as they age.115,136 It is therefore unexpected that age-dependent factors influencing deterioration of EF were not operational in men following RP. The prevalence of ED in men treated for prostate cancer is consistently higher than that of age-matched control subjects, at any age.42,77,115 In a study of men chosen randomly from the New Mexico Tumor Registry, men with prostate cancer treated with RP had twice the rate of ED at 5 years than age- and ethnicity-matched control subjects (72% vs 36%), despite comparable prevalence of ED at baseline (27% vs 30%; between-group change over time, P < .001).77 Telephone interviews of men from the PLCO between 5 and 10 years postdiagnosis also showed that men undergoing RP had poorer SF scores than noncancer controls (P < .001), as measured by the EPIC Short-Form Sexual Function Score.42 It is conceivable with longer follow-up that the gap between men treated with RP and age-matched, untreated control subjects will converge, owing to stability of EF following RP observed in men during the eighth decade of life.
The SPCG-4 trial comparing RP versus WW provides insights into EF in those electing no treatment for localized prostate cancer. After 9 years, prerandomization ED rates increased from 24% to 75% in the WW arm but only from 66% to 81% in the RP arm. Unlike control subjects without cancer in the general population, men with clinically localized prostate cancer electing WW demonstrated much faster deterioration of EF and SF.78 The high rate of ED (75%) in the WW reflects both the natural history of ED in aging males and also a significantly greater likelihood of receiving ADT for disease progression, which affects all aspects of SF.
Based on the available literature, it appears that at short- and intermediate-term follow-up, RT has better SF outcomes than RP. SF was less likely to return following RP (P < .001) 4 years after RP in a study at UCLA,58 5 years after RP in the PCOS,77 and 5 to 10 years after diagnosis in the PLCO.42 It is important to consider that many men with intermediate- and high-risk prostate cancer may deliberately undergo partial or complete excision of the neurovascular bundle in order to maximize oncologic control at the expense of EF. In contrast, in the modern era, most men with intermediate- and high-risk prostate cancer undergoing RT receive neoadjuvant and adjuvant ADT.137 If these men are censored from comparisons of RP and RT due to the ADT, then the outcomes assessment is heavily biased in favor of RT. ADT not only causes ED, but also loss of libido, genital atrophy, loss of penile length, hot flashes, fatigue, and body feminization.138,139 The short-term advantage of RT on EF and SF for the subgroup not receiving neoadjuvant/adjuvant ADT appears to dissipate over time.56,113 In a comparative study of 147 and 665 men undergoing RT and RP, deterioration of EPIC Sexual Summary Scores was observed in the RT group compared with stability in the RP group between a median of 2.6- and 6.2-year follow-up.38 In the PCOS, the ability to achieve erections adequate for intercourse was similar among treatment groups at 15 years, with ED affecting a larger percentage of those in the RT group versus the RP group (93.9% vs 87%).51
Bowel Function
Bowel dysfunction is not considered an adverse side effect of RP, although it can develop and negatively impact HRQOL.8 Bowel function is most commonly assessed via the UCLA-PCI Bowel Function Score, which captures rectal urgency, loose stools, distress, and crampy pain,39 or the adapted EPIC Bowel Domain questionnaire which incorporates additional bowel symptoms: bloody stools, frequency of bowel movements, painful bowel movements, and uncontrolled stool leakage.40
Longitudinal data demonstrate an immediate decline in bowel function after RP, but there is improvement to baseline by 4 months, and subsequent stability up to a median follow-up of 6.2 years.38,68 The early changes in bowel function may be related to altered diet, self-imposed dehydration to decrease incontinence, and narcotic pain medications.
Bergman and Litwin showed that 5-year bowel function outcomes are similar in men undergoing RP and those choosing AS,140 indicating that RP does not negatively impact bowel function at intermediate-term follow-up. On the contrary, the PCOS reported increasing bowel urgency rates at 2, 5, and 15 years of 13.6%, 16.3%, and 21.9%, respectively.51 In the absence of a randomized control trial, most would assume the time-dependent increase in bowel urgency is attributable to aging. Despite possible increases in bowel dysfunction < 6% of men in the PCOS were bothered by decline in bowel function.51
In comparison, there is compelling evidence that RT causes short-, intermediate-, and long-term bowel dysfunction. Outcomes assessments at 1, 2, 3, 4, 5, and 10 years consistently demonstrate treatment-dependent bowel dysfunction following all forms of radiation delivery to the prostate.7,42,51,58,107,141 The data from the PCOS demonstrates that bowel dysfunction in men undergoing RP and RT converges at 15 years.51
Open RP Versus Robotic-assisted Laparoscopic RP
The ability to perform robotic-assisted laparoscopic RP (RALRP) was first described in 2000.142 Over the past decade, RALRP has gained widespread acceptance and now is the dominant approach to RP in the United States.143 Many attribute the adoption of the RALRP to marketing as opposed to any objective outcomes data showing superiority over the open approach.144
All of the intermediate- and long-term HRQOL outcomes reported in the literature are limited to open RP. The question is, are these findings relevant to RALRP?
Unfortunately, there are no randomized studies comparing HRQOL outcomes following open versus robotic RP. The majority of studies comparing HRQOL outcomes between the two techniques are flawed in design.145 First, the level of experience of surgeons performing the two techniques and their patient volume are often not comparable. In addition, validated instruments for capturing outcomes are usually not employed. Surgeons are sometimes involved in the data acquisition, entry, and interpretation, which can introduce bias. There are several studies that have examined large administrative databases, which often mitigate bias, but the quality of outcome measures is subject to criticism. A few studies stand out as objective and worthy of comment.
Hu and colleagues compared 1938 men who underwent minimally invasive RP with and without robotic assistance with 6899 men who underwent open RP using Surveillance, Epidemiology, and End Results (SEER) Medicare-linked data and found that minimally invasive RP was associated with higher rates of genitourinary complications (4.7% vs 2.1%; P = .001), diagnoses of incontinence (15.9 vs 12.2 per 100 person-years; P = .02), and ED (26.8 vs 19.2 per 100 person-years; P = .009).146
Schroeck and associates compared satisfaction between open and RALRP performed by experienced surgeons at Duke University Medical Center (Durham, NC).147 Men who underwent open RP were significantly more likely to be satisfied and over four times less likely to be regretful compared with those who underwent RALRP (OR 4.45; 95% CI, 1.90-10.4).147 The authors, who included experienced open and robotic surgeons and experts in health services research, speculated that men undergoing robotic surgery had unrealistic expectations about the “minimally invasive” new treatment, leading to greater dissatisfaction and more regret.147 A subsequent study by the same investigators prospectively ascertained expectations of outcome and reported that men undergoing RALRP expected better functional outcomes compared with those undergoing open RP.148
Barocas and colleagues compared men undergoing open and RALRP and failed to show any advantages of either approach for preventing biochemical recurrence.149
The most definitive comparison of continence and potency outcomes was recently reported by Barry and colleagues.150 In this study, a targeted disease-specific HRQOL survey was mailed to 797 men (86% response rate) in the SEER-Medicare database who had undergone open or RALRP from August 2008 through December 2008. Of the 406 and 220 responders undergoing RALRP and open RP, respectively, they reported a nonsignificant trend toward greater problems with continence following RALRP (OR 1.41; 95% CI, 0.97-2.05) and no differences in potency. These results, well beyond the so-called learning curve of RALRP, provide compelling evidence that HRQOL outcomes do not appreciably differ between the two techniques.
The majority of intermediate- and long-term outcomes data is derived from open RP series. Based on comparable short-term HRQOL outcomes between open RP versus RALRP, it is reasonable to speculate that the intermediate- and long-term HRQOL outcomes following RALRP will at best be comparable with open RP. Therefore, we believe the conclusions of this review can be extrapolated to RALRP.
Conclusions
Screening, diagnosis, and treatment for prostate cancer have evolved dramatically over the past two decades. Lower-risk prostate cancers are being diagnosed at a younger age. In the majority of contemporary RP series, the mean age of men undergoing the procedure is approximately age 60 years.7,51,68,96,151 Therefore, the average man undergoing curative intervention of his disease is expected to live at least 14 years.10 Many men undergoing RP will experience significant short-term urinary incontinence, LUTS, and sexual dysfunction that may impair both their general and disease-specific HRQOL. As men in the general population age, there are natural tendencies for LUTS, sexual dysfunction, and, to a lesser degree, urinary incontinence, to become clinically significant issues. In addition, as men age into and beyond their seventies, issues such as sexual dysfunction may be perceived as less bothersome relative to other issues such as LUTS. Knowledge of long-term urinary function and SF is mandatory in order to properly counsel men regarding short-, intermediate-, and long-term HRQOL issues following intervention. Equipped with realistic expectations, men will make informed decisions that balance disease control and HRQOL. ![]()
Supported in part by Grant UL1 TR000038 from the National Center for the Advancement of Translational Science (NCATS), National Institutes of Health. Dr. Lepor is co-owner of MedReviews, LLC; is on the Speakers’ Bureau for Actavis; is a Consultant for and investor in Serenity; is a Consultant for TheraCoat; and is a Lecturer for Amgen.
References
- Brawley OW. Prostate cancer epidemiology in the United States. 2012;30:195-200.
- Stephenson RA. Prostate cancer trends in the era of prostate-specific antigen. An update of incidence, mortality, and clinical factors from the SEER database. Urol Clin North Am. 2002;29:173-181.
- Pirtskhalaishvili G, Hrebinko RL, Nelson JB. The treatment of prostate cancer: an overview of current options. Cancer Pract. 2001;9:295-306.
- Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220-241.
- Underwood W 3rd, Jackson J, Wei JT, et al. Racial treatment trends in localized/regional prostate carcinoma: 1992-1999. Cancer. 2005;103:538-545.
- Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117-1123.
- Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358:1250-1261.
- Litwin MS, Hays RD, Fink A, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA. 1995;273:129-135.
- Lepor H, Kaci L. The impact of open radical retropubic prostatectomy on continence and lower urinary tract symptoms: a prospective assessment using validated self-administered outcome instruments. J Urol. 2004;171:1216-1219.
- Walz J, Gallina A, Saad F, et al. A nomogram predicting 10-year life expectancy in candidates for radical prostatectomy or radiotherapy for prostate cancer. J Clin Oncol. 2007;25:3576-3581.
- Loughlin KR, Prasad MM. Post-prostatectomy urinary incontinence: a confluence of 3 factors. J Urol. 2010;183:871-877.
- Walsh PC, Marschke P, Ricker D, Burnett AL. Patient-reported urinary continence and sexual function after anatomic radical prostatectomy. Urology. 2000;55:58-61.
- Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ. 1993;2:217-227.
- Eton DT, Lepore SJ. Prostate cancer and health-related quality of life: a review of the literature. Psychooncology. 2002;11:307-326.
- Schag CA, Ganz PA, Wing DS, et al. Quality of life in adult survivors of lung, colon and prostate cancer. Qual Life Res. 1994;3:127-141.
- Lilleby W, Fosså SD, Waehre HR, Olsen DR. Long-term morbidity and quality of life in patients with localized prostate cancer undergoing definitive radiotherapy or radical prostatectomy. Int J Radiat Oncol Biol Phys. 1999;43:735-743.
- Kao TC, Cruess DF, Garner D, et al. Multicenter patient self-reporting questionnaire on impotence, incontinence and stricture after radical prostatectomy. J Urol. 2000;163:858-864.
- Huang GJ, Sadetsky N, Penson DF. Health related quality of life for men treated for localized prostate cancer with long-term followup. J Urol. 2010;183:2206-2212.
- Steineck G, Helgesen F, Adolfsson J, et al. Quality of life after radical prostatectomy or watchful waiting. N Engl J Med. 2002;347:790-796.
- Lubeck DP, Litwin MS, Henning JM, et al. Changes in health-related quality of life in the first year after treatment for prostate cancer: results from CaPSURE. Urology. 1999;53:180-186.
- Beard CJ, Propert KJ, Rieker PP, et al. Complications after treatment with external-beam irradiation in early-stage prostate cancer patients: a prospective multiinstitutional outcomes study. J Clin Oncol. 1997;15:223-229.
- Wei JT, Dunn RL, Sandler HM, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20:557-566.
- Jayadevappa R, Schwartz JS, Chhatre S, et al. Satisfaction with care: a measure of quality of care in prostate cancer patients. Med Decis Making. 2010;30:234-245.
- Hu JC, Elkin EP, Pasta DJ, et al. Predicting quality of life after radical prostatectomy: results from CaPSURE. J Urol. 2004;171(2 Pt 1):703-707; discussion 707-708.
- Knight SJ, Latini DM, Hart SL, et al. Education predicts quality of life among men with prostate cancer cared for in the Department of Veterans Affairs: a longitudinal quality of life analysis from CaPSURE. Cancer. 2007;109:1769-1776.
- Heim HM, Oei TP. Comparison of prostate cancer patients with and without pain. Pain. 1993;53:159-162.
- Litwin MS, Lubeck DP, Henning JM, Carroll PR. Differences in urologist and patient assessments of health related quality of life in men with prostate cancer: results of the CaPSURE database. J Urol. 1998;159:1988-1992.
- Willener R, Hantikainen V. Individual quality of life following radical prostatectomy in men with prostate cancer. Urol Nurs. 2005;25:88-90, 95-100.
- Stone PC, Murphy RF, Matar HE, Almerie MQ. Measuring the individual quality of life of patients with prostate cancer. Prostate Cancer Prostatic Dis. 2008;11:390-396.
- Donabedian A. The quality of care. How can it be assessed? JAMA. 1988;260:1743-1748.
- Cleary PD, McNeil BJ. Patient satisfaction as an indicator of quality care. Inquiry. 1988;25:25-36.
- Hoffman RM, Hunt WC, Gilliland FD, et al. Patient satisfaction with treatment decisions for clinically localized prostate carcinoma. Results from the Prostate Cancer Outcomes Study. Cancer. 2003;97:1653-1662.
- Brédart A, Razavi D, Robertson C, et al. Assessment of quality of care in an oncology institute using information on patients’ satisfaction. Oncology. 2001;61:120-128.
- Gotay CC, Lipscomb J, Snyder CF. Reflections on findings of the Cancer Outcomes Measurement Working Group: moving to the next phase. J Natl Cancer Inst. 2005;97:1568-1574.
- Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann 1979;2:197-207.
- Abraham NE, Makarov DV, Laze J, et al. Patient centered outcomes in prostate cancer treatment: predictors of satisfaction up to 2 years after open radical retropubic prostatectomy. J Urol. 2010;184:1977-1981.
- Penson DF, McLerran D, Feng Z, et al. 5-year urinary and sexual outcomes after radical prostatectomy: results from the Prostate Cancer Outcomes Study. J Urol. 2008;179(suppl 5):S40-S44.
- Miller DC, Sanda MG, Dunn RL, et al. Long-term outcomes among localized prostate cancer survivors: health-related quality-of-life changes after radical prostatectomy, external radiation, and brachytherapy. J Clin Oncol. 2005;23:2772-2780.
- Litwin MS, Hays RD, Fink A, et al. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36:1002-1012.
- Wei JT, Dunn RL, Litwin MS, et al Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899-905.
- Steiner MS, Morton RA, Walsh PC. Impact of anatomical radical prostatectomy on urinary continence. J Urol. 1991;145:512-514; discussion 514-515.
- Taylor KL, Luta G, Miller AB, et al. Long-term disease-specific functioning among prostate cancer survivors and noncancer controls in the prostate, lung, colorectal, and ovarian cancer screening trial. J Clin Oncol. 2012;30:2768-2775.
- Goluboff ET, Saidi JA, Mazer S, et al. Urinary continence after radical prostatectomy: the Columbia experience. J Urol. 1998;159:1276-1280.
- Fowler FJ Jr, Barry MJ, Lu-Yao G, et al. Effect of radical prostatectomy for prostate cancer on patient quality of life: results from a Medicare survey. Urology. 1995;45:1007-1013; discussion 1013-1015.
- Kundu SD, Roehl KA, Eggener SE, et al. Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. J Urol. 2004;172(6 Pt 1):2227-2231.
- Ficarra V, Novara G, Rosen RC, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. 2012;62:405-417.
- Wei JT, Dunn RL, Marcovich R, et al. Prospective assessment of patient reported urinary continence after radical prostatectomy. J Urol. 2000;164(3 Pt 1):744-748.
- Sacco E, Prayer-Galetti T, Pinto F, et al. Urinary incontinence after radical prostatectomy: incidence by definition, risk factors and temporal trend in a large series with a long-term follow-up. BJU Int. 2006;97:1234-1241.
- Glickman L, Godoy G, Lepor H. Changes in continence and erectile function between 2 and 4 years after radical prostatectomy. J Urol. 2009;181:731-735.
- Stanford JL, Feng Z, Hamilton AS, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283:354-360.
- Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368:436-445.
- Hurtes X, Roupret M, Vaessen C, et al. Anterior suspension combined with posterior reconstruction during robot-assisted laparoscopic prostatectomy improves early return of urinary continence: a prospective randomized multicentre trial. BJU Int. 2012;110:875-883.
- Lepor H, Kaci L, Xue X. Continence following radical retropubic prostatectomy using self-reporting instruments. J Urol. 2004;171:1212-1215.
- Hammerer P, Huland H. Urodynamic evaluation of changes in urinary control after radical retropubic prostatectomy. J Urol. 1997;157:233-236.
- Nandipati KC, Raina R, Agarwal A, Zippe CD. Nerve-sparing surgery significantly affects long-term continence after radical prostatectomy. Urology. 2007;70:1127-1130.
- Donnellan SM, Duncan HJ, MacGregor RJ, Russell JM. Prospective assessment of incontinence after radical retropubic prostatectomy: objective and subjective analysis. Urology. 1997;49:225-230.
- Patel VR, Tully AS, Holmes R, Lindsay J. Robotic radical prostatectomy in the community setting—the learning curve and beyond: initial 200 cases. J Urol. 2005;174:269-272.
- Gore JL, Kwan L, Lee SP, et al. Survivorship beyond convalescence: 48-month quality-of-life outcomes after treatment for localized prostate cancer. J Natl Cancer Inst. 2009;101:888-892.
- Anderson CB, Kaufman MR, Dietrich MS, et al. Recovery of urinary function after radical prostatectomy: identification of trajectory cluster groups. J Urol. 2012;187:1346-1351.
- Konety BR, Sadetsky N, Carroll PR, for the CaPSURE Investigators. Recovery of urinary continence following radical prostatectomy: the impact of prostate volume—analysis of data from the CaPSURE Database. J Urol. 2007;177:1423-1425; discussion 1425-1426.
- Loeb S, Roehl KA, Helfand BT, Catalona WJ. Complications of open radical retropubic prostatectomy in potential candidates for active monitoring. Urology. 2008;72:887-891.
- Pick DL, Osann K, Skarecky D, et al. The impact of cavernosal nerve preservation on continence after robotic radical prostatectomy. BJU Int. 2011;108:1492-1496.
- Abdollah F, Sun M, Suardi N, et al. A novel tool to assess the risk of urinary incontinence after nerve-sparing radical prostatectomy. BJU Int. 2013;111:905-913.
- Karakiewicz PI, Bhojani N, Neugut A, et al. The effect of comorbidity and socioeconomic status on sexual and urinary function and on general health-related quality of life in men treated with radical prostatectomy for localized prostate cancer. J Sex Med. 2008;5:919-927.
- Novara G, Ficarra V, D’Elia C, et al. Evaluating urinary continence and preoperative predictors of urinary continence after robot assisted laparoscopic radical prostatectomy. J Urol. 2010;184:1028-1033.
- Teber D, Sofikerim M, Ates M, et al. Is type 2 diabetes mellitus a predictive factor for incontinence after laparoscopic radical prostatectomy? A matched pair and multivariate analysis. J Urol. 2010;183:1087-1091.
- Mohamed NE, Bovbjerg DH, Montgomery GH, et al. Pretreatment depressive symptoms and treatment modality predict post-treatment disease-specific quality of life among patients with localized prostate cancer. Urol Oncol. 2012;30:804-812.
- Parker WR, Wang R, He C, Wood DP Jr. Five year expanded prostate cancer index composite-based quality of life outcomes after prostatectomy for localized prostate cancer. BJU Int. 2011;107:585-590.
- Eastham JA, Kattan MW, Rogers E, et al. Risk factors for urinary incontinence after radical prostatectomy. J Urol. 1996;156:1707-1713.
- Wille S, Heidenreich A, Hofmann R, Engelmann U. Preoperative erectile function is one predictor for post prostatectomy incontinence. Neurourol Urodyn. 2007;26:140-143; discussion 144.
- Schlomm T, Heinzer H, Steuber T, et al. Full functional-length urethral sphincter preservation during radical prostatectomy. Eur Urol. 2011;60:320-329.
- Walsh PC. Anatomic radical prostatectomy: evolution of the surgical technique. J Urol. 1998;160(6 Pt 2):2418-2424.
- Foley CL, Bott SR, Thomas K, et al. A large prostate at radical retropubic prostatectomy does not adversely affect cancer control, continence or potency rates. BJU Int. 2003;92:370-374.
- Prabhu V, Sivarajan G, Taksler GB, et al. Long-term continence outcomes in men undergoing radical prostatectomy for clinically localized prostate cancer. Eur Urol. 2014;65:52-57.
- Litwin MS. Health related quality of life in older men without prostate cancer. J Urol. 1999;161:1180-1184.
- Thom D. Variation in estimates of urinary incontinence prevalence in the community: effects of differences in definition, population characteristics, and study type. J Am Geriatr Soc. 1998;46:473-480.
- Hoffman RM, Gilliland FD, Penson DF, et al. Cross-sectional and longitudinal comparisons of health-related quality of life between patients with prostate carcinoma and matched controls. Cancer. 2004;101:2011-2019.
- Johansson E, Steineck G, Holmberg L, et al. Long-term quality-of-life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Prostate Cancer Group-4 randomised trial. Lancet Oncol. 2011;12:891-899.
- Sarma AV, Wei JT. Clinical practice. Benign prostatic hyperplasia and lower urinary tract symptoms. N Engl J Med. 2012;367:248-257.
- Girman CJ, Jacobsen SJ, Tsukamoto T, et al. Health-related quality of life associated with lower urinary tract symptoms in four countries. Urology. 1998;51:428-436.
- Coyne KS, Wein AJ, Tubaro A, et al. The burden of lower urinary tract symptoms: evaluating the effect of LUTS on health-related quality of life, anxiety and depression: EpiLUTS. BJU Int. 2009;103(suppl 3):4-11.
- Kannan H, Radican L, Turpin RS, Bolge SC. Burden of illness associated with lower urinary tract symptoms including overactive bladder/urinary incontinence. Urology. 2009;74:34-38.
- Wu MP, Hsu YW, Weng SF, et al. Healthcare-seeking prevalence of lower urinary tract symptoms among national health insurance enrollees in Taiwan, 2000-2009. Urology. 2013;81:61-65.
- Collins MM, Barry MJ, Bin L, et al. Diagnosis and treatment of benign prostatic hyperplasia. Practice patterns of primary care physicians. J Gen Intern Med. 1997;12:224-229.
- Jacobsen SJ, Girman CJ, Guess HA, et al. Do prostate size and urinary flow rates predict health care-seeking behavior for urinary symptoms in men? Urology. 1995;45:64-69.
- Taub DA, Wei JT. The economics of benign prostatic hyperplasia and lower urinary tract symptoms in the United States. Curr Urol Rep. 2006;7:272-281.
- Carbone DJ Jr, Hodges S. Medical therapy for benign prostatic hyperplasia: sexual dysfunction and impact on quality of life. Int J Impot Res. 2003;15:299-306.
- O’Leary MP. Treatment and pharmacologic management of BPH in the context of common comorbidities. Am J Manag Care. 2006;12(suppl 5):S129-S140.
- Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148:1549-1557; discussion 1564.
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol. 2011;185:1793-1803.
- Madersbacher S, Alivizatos G, Nordling J, et al. EAU 2004 guidelines on assessment, therapy and follow-up of men with lower urinary tract symptoms suggestive of benign prostatic obstruction (BPH guidelines). Eur Urol. 2004;46:547-554.
- Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol. 1995;154:1770-1774.
- Namiki S, Ishidoya S, Saito S, et al. Natural history of voiding function after radical retropubic prostatectomy. Urology. 2006;68:142-147.
- Kim JH, Ha YS, Jeong SJ, et al. Impact of robot-assisted radical prostatectomy on lower urinary tract symptoms and predictive factors for symptom changes: a longitudinal study. Urology. 2013;81:787-793.
- Slova D, Lepor H. The short-term and long-term effects of radical prostatectomy on lower urinary tract symptoms. J Urol. 2007;178:2397-2400; discussion 2400-2401.
- Prabhu V, Taksler GB, Sivarajan G, et al. Radical prostatectomy improves and prevents age-dependent progression of lower urinary tract symptoms [published online ahead of print August 13, 2013]. J Urol. doi: 1016/j.juro.2013.08.010.
- Berges RR, Pientka L, Höfner K, et al. Male lower urinary tract symptoms and related health care seeking in Germany. Eur Urol. 2001;39:682-687.
- Kok ET, Bohnen AM, Groeneveld FP, et al. Changes in disease specific and generic quality of life related to changes in lower urinary tract symptoms: the Krimpen study. J Urol. 2005;174:1055-1058.
- Berges R, Oelke M. Age-stratified normal values for prostate volume, PSA, maximum urinary flow rate, IPSS, and other LUTS/BPH indicators in the German male community-dwelling population aged 50 years or older. World J Urol. 2011;29:171-178.
- Chute CG, Panser LA, Girman CJ, et al. The prevalence of prostatism: a population-based survey of urinary symptoms. J Urol. 1993;150:85-89.
- Sarma AV, Jacobsen SJ, Girman CJ, et al. Concomitant longitudinal changes in frequency of and bother from lower urinary tract symptoms in community dwelling men. J Urol. 2002;168(4 Pt 1):1446-1452.
- Temml C, Brossner C, Schatzl G, et al. The natural history of lower urinary tract symptoms over five years. Eur Urol. 2003;43:374-380.
- Madersbacher S, Pycha A, Schatzl G, et al. The aging lower urinary tract: a comparative urodynamic study of men and women. Urology. 1998;51:206-212.
- Terai A, Matsui Y, Ichioka K, et al. Comparative analysis of lower urinary tract symptoms and bother in both sexes. Urology. 2004;63:487-491.
- Boyle P, Robertson C, Mazzetta C, et al. The prevalence of lower urinary tract symptoms in men and women in four centres. The UrEpik study. BJU Int. 2003;92:409-414.
- Diokno AC, Brown MB, Goldstein N, Herzog AR. Epidemiology of bladder emptying symptoms in elderly men. J Urol. 1992;148:1817-1821.
- Pardo Y, Guedea F, Aguiló F, et al. Quality-of-life impact of primary treatments for localized prostate cancer in patients without hormonal treatment. J Clin Oncol. 2010;28:4687-4696.
- Rice K, Hudak J, Peay K, et al. Comprehensive quality-of-life outcomes in the setting of a multidisciplinary, equal access prostate cancer clinic. Urology 2010;76:1231-1238.
- Long JA, Lebret T, Saporta F, et al. Evaluation of sexuality and erectile function of candidates for radical prostatectomy. [Article in French] Prog Urol. 2006;16:450-456.
- Salonia A, Zanni G, Gallina A, et al. Baseline potency in candidates for bilateral nerve-sparing radical retropubic prostatectomy. Eur Urol. 2006;50:360-365.
- Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49:822-830.
- Rosen RC, Cappelleri JC, Gendrano N 3rd. The International Index of Erectile Function (IIEF): a state-of-the-science review. Int J Impot Res. 2002;14:226-244.
- Cappelleri JC, Rosen RC. The Sexual Health Inventory for Men (SHIM): a 5-year review of research and clinical experience. Int J Impot Res. 2005;17:307-319.
- Ficarra V, Novara G, Ahlering TE, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol. 2012;62:418-430.
- Bacon CG, Mittleman MA, Kawachi I, et al. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Ann Intern Med. 2003;139:161-168.
- Marien T, Sankin A, Lepor H. Factors predicting preservation of erectile function in men undergoing open radical retropubic prostatectomy. J Urol. 2009;181:1817-1822.
- Krupski TL, Saigal CS, Litwin MS. Variation in continence and potency by definition. J Urol. 2003;170(4 Pt 1):1291-1294.
- Lepor H, Gregerman M, Crosby R, et al. Precise localization of the autonomic nerves from the pelvic plexus to the corpora cavernosa: a detailed anatomical study of the adult male pelvis. J Urol. 1985;133:207-212.
- Walsh PC, Lepor H, Eggleston JC. Radical prostatectomy with preservation of sexual function: anatomical and pathological considerations. Prostate. 1983;4:473-485.
- Walsh PC. Radical prostatectomy, preservation of sexual function, cancer control. The controversy. Urol Clin North Am. 1987;14:663-673.
- Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982;128:492-497.
- Van der Aa F, Joniau S, De Ridder D, Van Poppel H. Potency after unilateral nerve sparing surgery: a report on functional and oncological results of unilateral nerve sparing surgery. Prostate Cancer Prostatic Dis. 2003;6:61-65.
- Boorjian SA, Eastham JA, Graefen M, et al. A critical analysis of the long-term impact of radical prostatectomy on cancer control and function outcomes. Eur Urol. 2012;61:664-675.
- Ayyathurai R, Manoharan M, Nieder AM, et al. Factors affecting erectile function after radical retropubic prostatectomy: results from 1620 consecutive patients. BJU Int. 2008;101:833-836.
- Alemozaffar M, Regan MM, Cooperberg MR, et al. Prediction of erectile function following treatment for prostate cancer. JAMA. 2011;306:1205-1214.
- Kilminster S, Muller S, Menon M, et al. Predicting erectile function outcome in men after radical prostatectomy for prostate cancer. BJU Int. 2012;110:422-426.
- Geary ES, Dendinger TE, Freiha FS, Stamey TA. Nerve sparing radical prostatectomy: a different view. J Urol. 1995;154:145-149.
- Quinlan DM, Epstein JI, Carter BS, Walsh PC. Sexual function following radical prostatectomy: influence of preservation of neurovascular bundles. J Urol. 1991;145:998-1002.
- Catalona WJ, Basler JW. Return of erections and urinary continence following nerve sparing radical retropubic prostatectomy. J Urol. 1993;150:905-907.
- Drago JR, Badalament RA, York JP, et al. Radical prostatectomy: OSU and affiliated hospitals’ experience 1985-1989. Urology. 1992;39:44-47.
- Rabbani F, Stapleton AM, Kattan MW, et al. Factors predicting recovery of erections after radical prostatectomy. J Urol. 2000;164:1929-1934.
- Padma-Nathan H, McCullough AR, Levine LA, et al. Randomized, double-blind, placebo-controlled study of postoperative nightly sildenafil citrate for the prevention of erectile dysfunction after bilateral nerve-sparing radical prostatectomy. Int J Impot Res. 2008;20:479-486.
- Rabbani F, Schiff J, Piecuch M, et al. Time course of recovery of erectile function after radical retropubic prostatectomy: does anyone recover after 2 years? J Sex Med. 2010;7:3984-3990.
- Penson DF, McLerran D, Feng Z, et al. 5-year urinary and sexual outcomes after radical prostatectomy: results from the prostate cancer outcomes study. J Urol. 2005;173:1701-1705.
- Sivarajan G, Prabhu V, Taksler GB, et al. Ten-year outcomes of sexual function after radical prostatectomy: results of a prospective longitudinal study. Eur Urol. 2014;65:58-65.
- Marumo K, Nakashima J, Murai M. Age-related prevalence of erectile dysfunction in Japan: assessment by the International Index of Erectile Function. Int J Urol. 2001;8:53-59.
- Park S, Meng MV, Elkin EP, et al. Androgen deprivation use with external beam radiation for prostate cancer: results from CaPSURE. J Urol. 2005;174:1802-1807.
- Higano CS. Side effects of androgen deprivation therapy: monitoring and minimizing toxicity. Urology. 2003;61(2 suppl 1):32-38.
- Walker LM, Robinson JW. Sexual adjustment to androgen deprivation therapy: struggles and strategies. Qual Health Res. 2012;22:452-465.
- Bergman J, Litwin MS. Quality of life in men undergoing active surveillance for localized prostate cancer. J Natl Cancer Inst Monogr. 2012;2012:242-249.
- Litwin MS, Gore JL, Kwan L, et al. Quality of life after surgery, external beam irradiation, or brachytherapy for early-stage prostate cancer. Cancer. 2007;109:2239-2247.
- Binder J, Kramer W. Robotically-assisted laparoscopic radical prostatectomy. BJU Int. 2001;87:408-410.
- Trinh QD, Sammon J, Sun M, et al. Perioperative outcomes of robot-assisted radical prostatectomy compared with open radical prostatectomy: results from the nationwide inpatient sample. Eur Urol. 2012;61:679-685.
- Mirkin JN, Lowrance WT, Feifer AH, et al. Direct-to-consumer Internet promotion of robotic prostatectomy exhibits varying quality of information. Health Aff (Millwood). 2012;31:760-769.
- Lowrance WT, Tarin TV, Shariat SF. Evidence-based comparison of robotic and open radical prostatectomy. ScientificWorld Journal. 2010;10:2228-2237.
- Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA. 2009;302:1557-1564.
- Schroeck FR, Krupski TL, Sun L, et al. Satisfaction and regret after open retropubic or robot-assisted laparoscopic radical prostatectomy. Eur Urol. 2008;54:785-793.
- Schroeck FR, Krupski TL, Stewart SB, et al. Pretreatment expectations of patients undergoing robotic assisted laparoscopic or open retropubic radical prostatectomy. J Urol. 2012;187:894-898.
- Barocas DA, Salem S, Kordan Y, et al. Robotic assisted laparoscopic prostatectomy versus radical retropubic prostatectomy for clinically localized prostate cancer: comparison of short-term biochemical recurrence-free survival. JUrol. 2010;183:990-996.
- Barry MJ, Gallagher PM, Skinner JS, Fowler FJ Jr. Adverse effects of robotic-assisted laparoscopic versus open retropubic radical prostatectomy among a nationwide random sample of medicare-age men. J Clin Oncol. 2012;30:513-518.
- Isbarn H, Jeldres C, Budaus L, et al. Effect of body mass index on histopathologic parameters: results of large European contemporary consecutive open radical prostatectomy series. Urology. 2009;73:615-619.