Acute and Chronic Cardiovascular Effects of Hyperkalemia: New Insights Into Prevention and Clinical Management
Sandeep K. Krishnan, MD, Norman E. Lepor, MD, FACC, FAHA, FSCAI
Cedars-Sinai Heart Institute, Los Angeles, CA
Hyperkalemia is a common electrolyte disorder associated with life-threatening cardiac arrhythmias and increased mortality. Patients at greatest risk for hyperkalemia include those with diabetes and those with impaired renal function in whom a defect in the excretion of renal potassium may already exist. Hyperkalemia is likely to become more common clinically because angiotensin receptor blockers and angiotensin-converting enzyme inhibitors are increasingly being used in higher doses and are thought to confer cardiovascular and renal protection. Until recently, options for treating hyperkalemia were limited to the use of thiazide and loop diuretics and sodium polystyrene sulfonate. Newer options such as sodium zirconium cyclosilicate will allow for the safe and effective treatment of hyperkalemia while maintaining patients on prescribed renin-angiotensin-aldosterone system inhibitors.
[Rev Cardiovasc Med. 2016;17(suppl 1):S9-S21 doi: 10.3909/ricm17S1S0002]
© 2016 MedReviews®, LLC
Acute and Chronic Cardiovascular Effects of Hyperkalemia: New Insights Into Prevention and Clinical Management
Sandeep K. Krishnan, MD, Norman E. Lepor, MD, FACC, FAHA, FSCAI
Cedars-Sinai Heart Institute, Los Angeles, CA
Hyperkalemia is a common electrolyte disorder associated with life-threatening cardiac arrhythmias and increased mortality. Patients at greatest risk for hyperkalemia include those with diabetes and those with impaired renal function in whom a defect in the excretion of renal potassium may already exist. Hyperkalemia is likely to become more common clinically because angiotensin receptor blockers and angiotensin-converting enzyme inhibitors are increasingly being used in higher doses and are thought to confer cardiovascular and renal protection. Until recently, options for treating hyperkalemia were limited to the use of thiazide and loop diuretics and sodium polystyrene sulfonate. Newer options such as sodium zirconium cyclosilicate will allow for the safe and effective treatment of hyperkalemia while maintaining patients on prescribed renin-angiotensin-aldosterone system inhibitors.
[Rev Cardiovasc Med. 2016;17(suppl 1):S9-S21 doi: 10.3909/ricm17S1S0002]
© 2016 MedReviews®, LLC
Acute and Chronic Cardiovascular Effects of Hyperkalemia: New Insights Into Prevention and Clinical Management
Sandeep K. Krishnan, MD, Norman E. Lepor, MD, FACC, FAHA, FSCAI
Cedars-Sinai Heart Institute, Los Angeles, CA
Hyperkalemia is a common electrolyte disorder associated with life-threatening cardiac arrhythmias and increased mortality. Patients at greatest risk for hyperkalemia include those with diabetes and those with impaired renal function in whom a defect in the excretion of renal potassium may already exist. Hyperkalemia is likely to become more common clinically because angiotensin receptor blockers and angiotensin-converting enzyme inhibitors are increasingly being used in higher doses and are thought to confer cardiovascular and renal protection. Until recently, options for treating hyperkalemia were limited to the use of thiazide and loop diuretics and sodium polystyrene sulfonate. Newer options such as sodium zirconium cyclosilicate will allow for the safe and effective treatment of hyperkalemia while maintaining patients on prescribed renin-angiotensin-aldosterone system inhibitors.
[Rev Cardiovasc Med. 2016;17(suppl 1):S9-S21 doi: 10.3909/ricm17S1S0002]
© 2016 MedReviews®, LLC
KEY WORDS
Hyperkalemia • Potassium • Chronic kidney disease • Congestive heart failure • Patiromer • Sodium zirconium cyclosilicate
KEY WORDS
Hyperkalemia • Potassium • Chronic kidney disease • Congestive heart failure • Patiromer • Sodium zirconium cyclosilicate
Hyperkalemia is likely to become more common clinically because ARBs and ACE inhibitors are increasingly being used in higher doses, and are known to confer cardiovascular and renal protection.
… pseudohyperkalemia should be recognized as a spurious increase of potassium level and should not be treated, as it usually does not have the life-threatening consequences of true hyperkalemia.
The largest reservoir of potassium is in the large intestine, where most of the sodium-potassium exchange takes place, leading to potassium excretion in the stool.
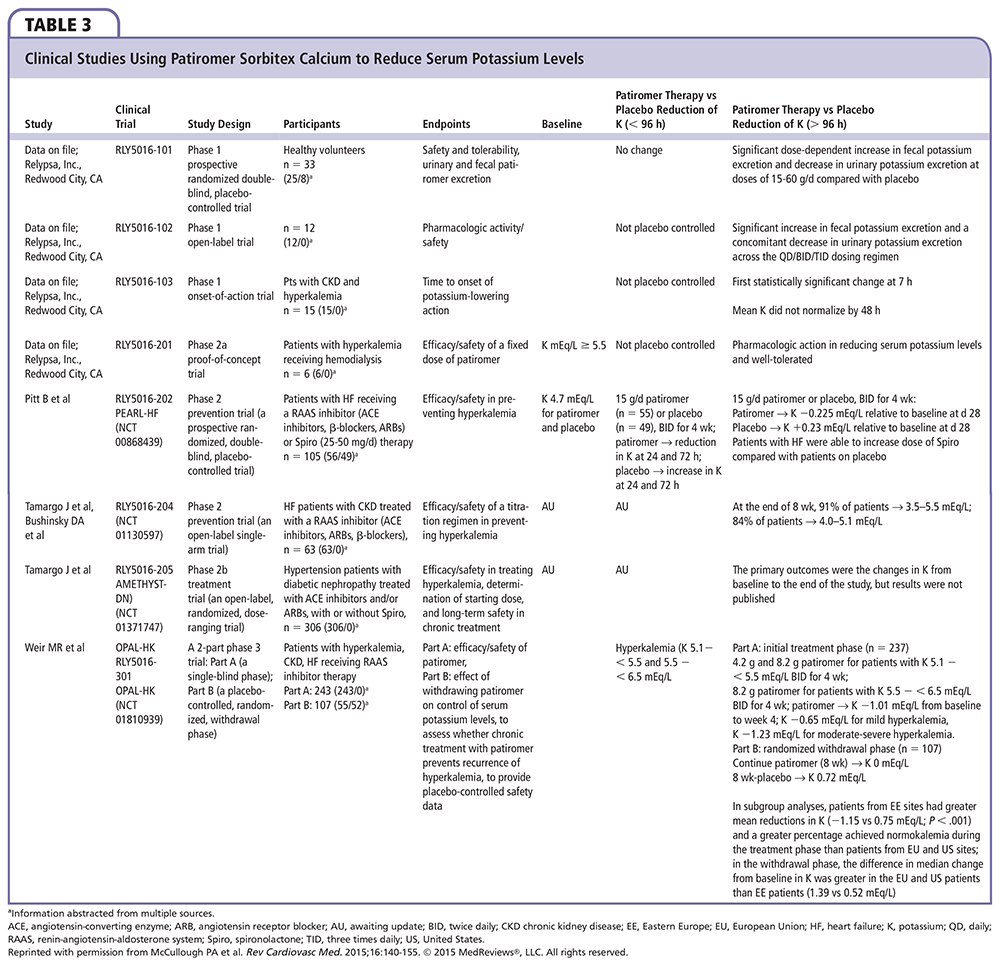
Figure 1. Study design for the ZS003 trial. Patients whose serum potassium level decreased to 3.5 to 4.9 mEq/L at 48 hours during the initial phase of the study were randomly assigned to receive either their original sodium zirconium cyclosilicate dose or placebo once daily before breakfast on days 3 to 15 (maintenance phase). Patients assigned to the placebo group in the initial phase were randomly assigned to receive either 1.25 g or 2.5 g of sodium zirconium cyclosilicate in the maintenance phase. ZS-9, sodium zirconium cyclosilicate (ZS Pharma, Coppell, TX). Reprinted with permission from McCullough PA et al. Rev Cardiovasc Med. 2015;16:140-155. © 2015 MedReviews®, LLC. All rights reserved.
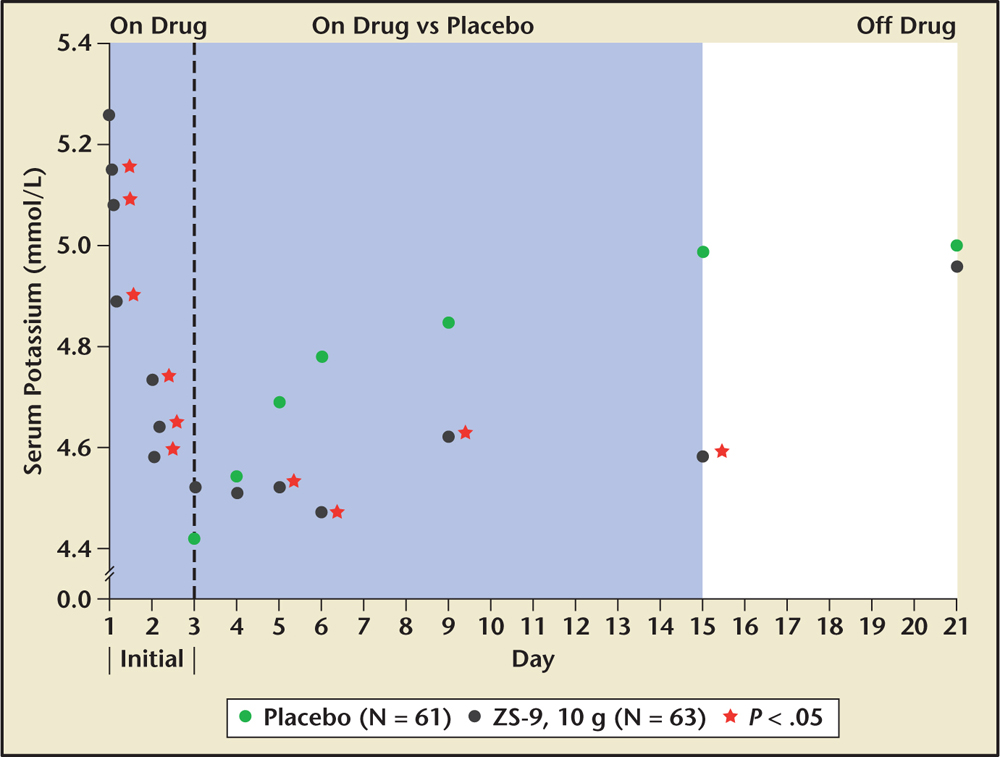
Figure 2. Extended-use sodium zirconium cyclosilicate (ZS-9), 10 g three times daily by mouth versus placebo in those who initially achieved normokalemia and were followed for 21 days. ZS-9, sodium zirconium cyclosilicate (ZS Pharma, Coppell, TX). Reprinted with permission from McCullough PA et al. Rev Cardiovasc Med. 2015;16:140-155. © 2015 MedReviews®, LLC. All rights reserved.
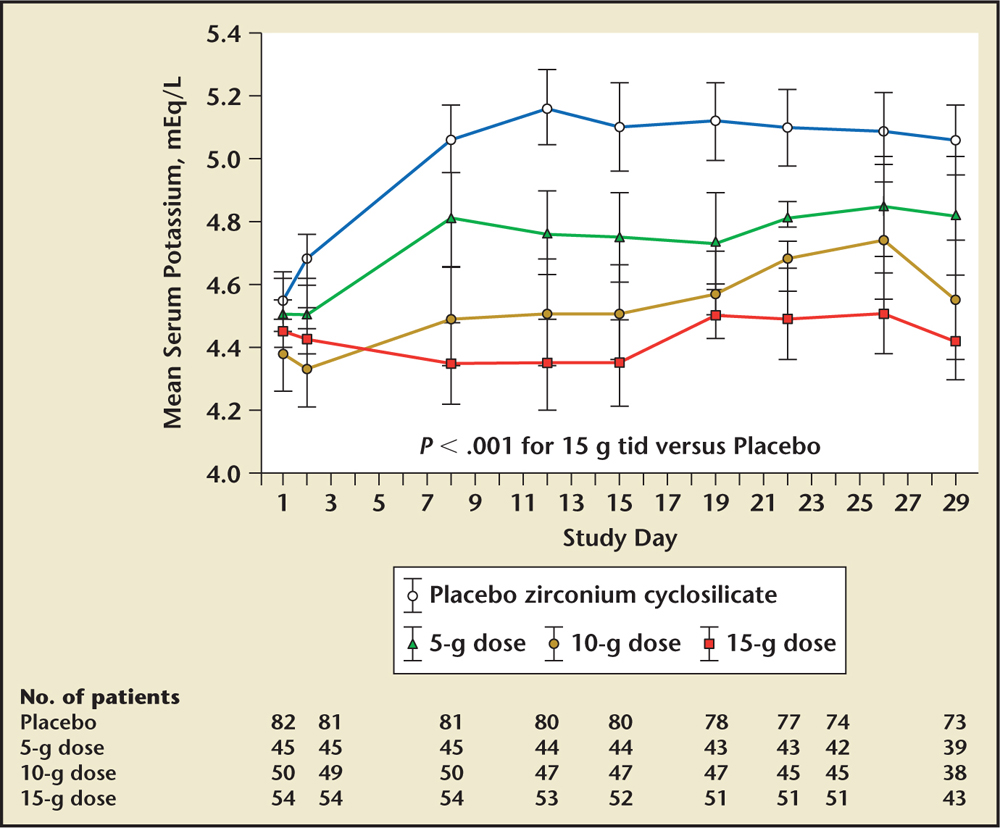
Figure 3. Results from the HARMONIZE trial with serial potassium concentrations over time. HARMONIZE, Hyperkalemia Randomized Intervention Multidose ZS-9 Maintenance; tid, three times daily. ZS-9, sodium zirconium cyclosilicate (ZS Pharma, Coppell, TX). Reprinted with permission from McCullough PA et al. Rev Cardiovasc Med. 2015;16:140-155. © 2015 MedReviews®, LLC. All rights reserved.
Main Points
• Hyperkalemia (defined as a serum potassium level > 5 mmol/L) is a common electrolyte disorder associated with life-threatening cardiac arrhythmias and increased mortality. It is likely to become more common clinically because angiotensin receptor blockers and angiotensin-converting enzyme inhibitors are increasingly being used in higher doses.
• Patients at greatest risk for hyperkalemia include those with diabetes and those with impaired renal function in whom a defect in the excretion of renal potassium may already exist.
• Sodium polystyrene sulfonate use is associated with a variety of shortcomings, including the high dose of sorbitol leading to gastrointestinal intolerance, sodium loading, and the resultant volume overload, making it a poor candidate for the chronic treatment of hyperkalemia.
• Patiromer, a recent entrant into the marketplace, is indicated for the treatment of hyperkalemia. Its safety and effectiveness were demonstrated in a series of modest-sized clinical trials.
• Sodium zirconium cyclosilicate is a highly selective cation exchanger that entraps potassium in the intestinal tract in exchange for sodium and hydrogen; US Food and Drug Administration approval is pending.
Main Points
• Hyperkalemia (defined as a serum potassium level > 5 mmol/L) is a common electrolyte disorder associated with life-threatening cardiac arrhythmias and increased mortality. It is likely to become more common clinically because angiotensin receptor blockers and angiotensin-converting enzyme inhibitors are increasingly being used in higher doses.
• Patients at greatest risk for hyperkalemia include those with diabetes and those with impaired renal function in whom a defect in the excretion of renal potassium may already exist.
• Sodium polystyrene sulfonate use is associated with a variety of shortcomings, including the high dose of sorbitol leading to gastrointestinal intolerance, sodium loading, and the resultant volume overload, making it a poor candidate for the chronic treatment of hyperkalemia.
• Patiromer, a recent entrant into the marketplace, is indicated for the treatment of hyperkalemia. Its safety and effectiveness were demonstrated in a series of modest-sized clinical trials.
• Sodium zirconium cyclosilicate is a highly selective cation exchanger that entraps potassium in the intestinal tract in exchange for sodium and hydrogen; US Food and Drug Administration approval is pending.
Potassium is one of the most abundant ions in the body (50-75 mmol/kg body weight) and approximately 98% of potassium is located intracellularly (~ 140 mmol/L).1 Potassium hemostasis is a very important aspect of electrolyte regulation; hyperkalemia (defined as a serum potassium level > 5 mmol/L) is a common electrolyte disorder associated with life-threatening cardiac arrhythmias and increased mortality.2-5 This is, in large part, due to the increasing use of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) in clinical practice as antihypertensive agents, heart failure treatments, and to decrease cardiovascular events in a large subset of high-risk patients.6
The prevalence of hyperkalemia in the general population is unknown and difficult to quantify.1 However, it is present in up to 10% of hospitalized patients, depending on how hyperkalemia is defined.7 Patients at greatest risk for hyperkalemia include those with diabetes and those with impaired renal function in whom a defect in the excretion of renal potassium may already exist. The incidence of hyperkalemia with renin-angiotensin-aldosterone system (RAAS) inhibitor monotherapy is low (≤ 2%) in patients without predisposing factors, but increases with dual RAAS inhibitor use (5%) and in patients with risk factors such as chronic kidney disease (CKD), congestive heart failure (CHF), and/or diabetes (5%-10%).8 And because one third to one half of patients with CHF have CKD, in actual practice a large proportion of patients being treated with these drugs are at increased risk for hyperkalemia.9
Hyperkalemia is likely to become more common clinically because ARBs and ACE inhibitors are increasingly being used in higher doses, and are known to confer cardiovascular and renal protection.10-13 They are often prescribed in combination with aldosterone receptor blockers secondary to the evidence that these medications provide incremental and added hard outcomes benefits in patients with CHF.14,15 These medications have also been shown in combination to slow the progression of CKD.16
Predisposing factors for hyperkalemia are numerous. Hyperkalemia may result from impaired potassium distribution between intracellular and extracellular spaces, increased potassium intake, and/or conditions that reduce potassium excretion, including CKD, hypertension, diabetes, and chronic CHF.4 Additionally, various drugs, including those taken for CKD and CHF, can produce hyperkalemia in up to 88% of hospitalized patients by interfering with normal potassium regulation.17
Drug mechanisms leading to hyperkalemia include those that decrease aldosterone synthesis/action (ACE inhibitors, ARBs, heparins, mineralocorticoid receptor antagonists); those that suppress renin release (nonsteroidal anti-inflammatory drugs [NSAIDs], cyclosporine, tacrolimus); those that inhibit sodium-potassium adenosine triphosphatase, including β-blockers and digoxin (as well as digitalis-like remedies); drugs that decrease adrenal steroid synthesis (azole antifungals); antibiotics such as penicillin G, which increase potassium intake into cells (several herbal supplements fall into this category as well, including alfalfa, dandelion, etc); those that impair renal potassium secretion (amiloride, pentamidine, triamterene, trimethoprim); and drugs that shift potassium into the extracellular space (amino acids, aminocaproic acid, succinylcholine).1,2,8,18,19 The risk of hyperkalemia with the use of the aforementioned drugs increases substantially when the glomerular filtration rate is < 30 mL/min.6
In normal physiology, potassium is freely filtered by the glomerulus. Most of this filtered potassium is reabsorbed in the proximal tubule and loop of Henle, with only 10% of the filtered load reaching the distal nephron. In addition to this small amount of potassium, which is filtered, potassium is also secreted into urine in the collecting duct. Potassium secretion in this segment is regulated and varies according to physiologic needs. The two most important physiologic determinants of potassium excretion are the serum aldosterone concentration and the delivery of sodium to the distal nephron.6
Aldosterone secretion is influenced by potassium concentration in the plasma and the renin-angiotensin system. The juxtaglomerular cells in the afferent arteriole secrete renin when renal perfusion pressure is low (hypovolemia, CHF, cirrhosis). Renin then acts on angiotensinogen to form angiotensin I, which is then converted to angiotensin II by ACE. Angiotensin II stimulates the release of aldosterone from the zona glomerulosa in the adrenal gland. Plasma potassium also has a direct stimulatory effect on aldosterone secretion.20 The stimulatory effects of angiotensin II and potassium on the release of aldosterone appear to be synergistic because the presence of one factor increases the response to the other.21 This interaction between potassium and angiotensin II involves the activation of a local intra-adrenal renin-angiotensin system.22
The most common method of drug-induced hyperkalemia results from ACE inhibition and ARB use, which impair urinary potassium excretion by interfering with the stimulatory effect of angiotensin II on aldosterone secretion in the adrenal gland. ACE inhibition blocks the formation of angiotensin II, whereas ARBs prevent angiotensin II from binding to its adrenal receptor. Additionally, these drugs may interfere with the angiotensin II that is generated locally within the adrenal zona glomerulosa.22
Clinicians are occasionally confronted with the finding of an elevated serum or plasma potassium level in an otherwise healthy person. Such an abnormality may herald the presence of occult mineralocorticoid deficiency or a defect in renal tubular transport.23 Alternatively, it may represent pseudohyperkalemia—a condition caused by the release of potassium from formed elements in the blood in patients with severe leukocytosis or thrombocytosis.24,25 There are not extensive systemic data on pseudohyperkalemia but there are multiple case reports in the literature describing pseudohyperkalemia in various clinical scenarios including repeated fist clenching during venipuncture,26,27 thrombocytosis,28 and extreme leukocytosis,29 to name a few. Pseudohyperkalemia has also been linked to traumatic transport of blood samples in hospital pneumatic tube transport systems.30-32 Whatever the cause, pseudohyperkalemia should be recognized as a spurious increase of potassium level and should not be treated, as it usually does not have the life-threatening consequences of true hyperkalemia.
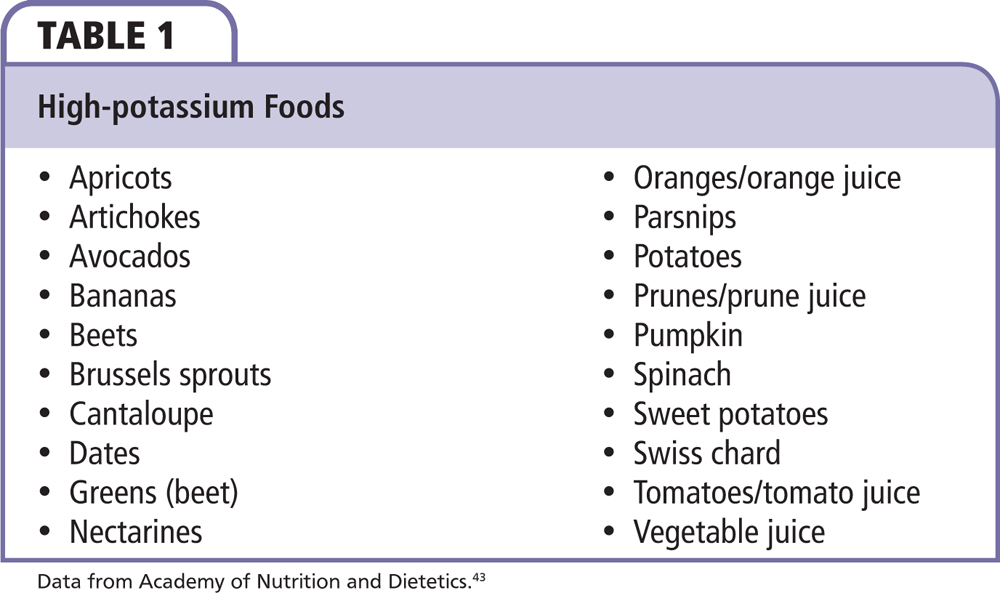
The goal of managing nonemergent hyperkalemia is to prevent the progression to the more life-threatening emergent state and treat the underlying causes of potassium imbalance. Eliminating modifiable causes, including a high potassium intake in foods or with supplements or nonessential medications likely to predispose to hyperkalemia, is a first step (Table 1). A normal amount of potassium in a typical diet of a healthy American is approximately 3500 to 4500 mg/d. A potassium-restricted diet typically includes approximately 2000 mg/d.
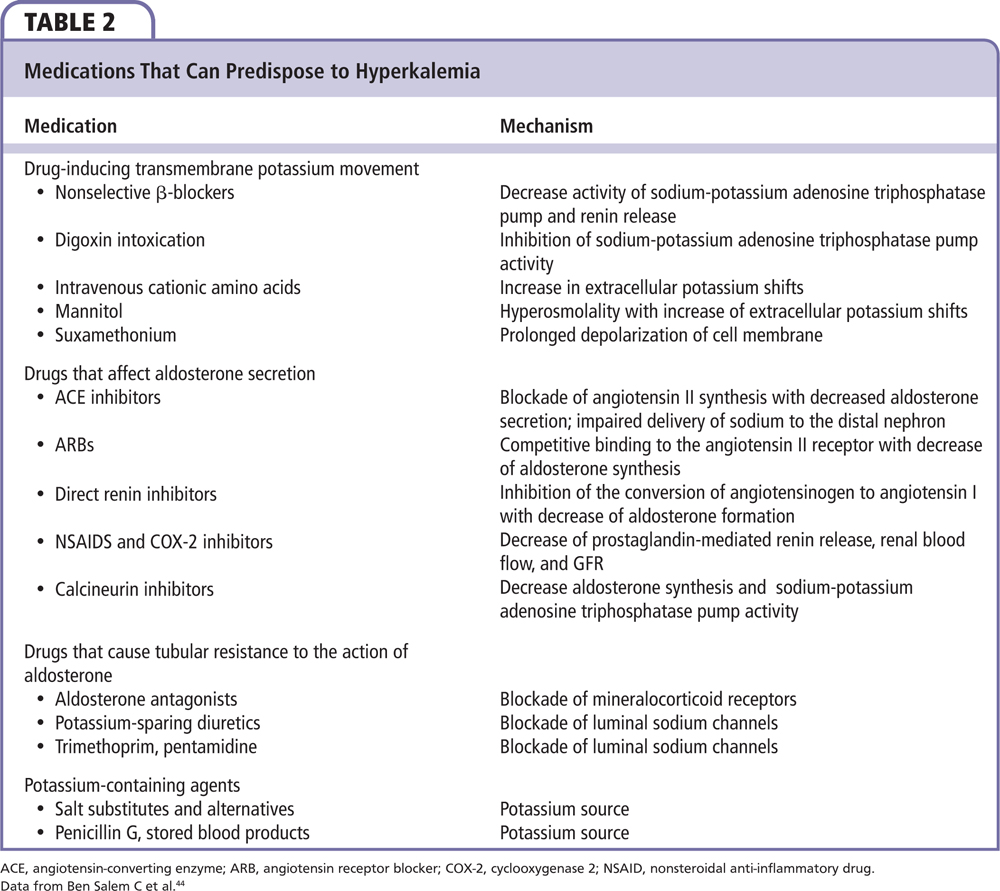
Carefully reviewing a patient’s updated and comprehensive list of all medications and supplements will help to avoid inadvertent use of potassium supplements and removing those medications that can adversely affect potassium balance (Table 2). According to the US Department of Agriculture, herbs including alfalfa, noni, and dandelion, and herbs including chervil, coriander, parsley, tarragon, turmeric, basil, and dill weed are high in potassium.
Until recently, little had changed regarding the treatment of nonemergent hyperkalemia. Approaches to preventing and treating mild hyperkalemia include initiating a low-potassium diet (< 2000 mg/d), and avoiding the use of potassium supplements, NSAIDs and cyclooxygenase-2 inhibitors. Use of RAAS inhibitors, including direct renin inhibitors, ACE inhibitors, ARBs, and mineralocorticoid receptor antagonists, are all associated with an increased risk of hyperkalemia, especially in those most in need of these treatments (those with CKD, heart failure, and diabetes). It is not infrequent that the development of hyperkalemia can interfere with their being utilized for their cardiac and renal protective effects in patients being treated for hypertension and CHF. For treatment of hypertension, ACE inhibitors and ARBs are accorded top-tier recommendations for use in patients with known cardiac and vascular disease and diabetes.33 In those with heart failure with reduced ejection fraction, blockade of the RAAS has lifesaving and quality-of-life (QoL)-enhancing effects and are critical components of national societal guideline recommendations. In an ambulatory practice, ACE inhibitor/ARB therapy contributed to hyperkalemia in up to 10% of patients and has been implicated in hyperkalemia observed in heart failure clinical trials.34-36
In the recently reported Prospective Comparison of ARNI with ACE-I to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) study that compared the combination of the neprilysin inhibitor sacubitril and the ARB valsartan with enalapril in patients with Class II-IV heart failure with left ventricular dysfunction, hyperkalemia was reported as an adverse event in 12% of patients treated with the combination agent and 14% of patients treated with enalapril during the double-blind period.37 Preventing and treating hyperkalemia will allow clinicians to maintain important heart failure therapies and therefore have important life-saving and QoL-enhancing implications.
In the past, options for treating hyperkalemia that does not correct with conservative measures were limited to the use of diuretics (thiazide and loop diuretics) and the sodium-potassium exchange resin, sodium polystyrene sulfonate. Unfortunately, diuretics can predispose patients to prerenal azotemia and other complications. Sodium polystyrene sulfonate was introduced over 50 years ago as a treatment for hyperkalemia based on very limited data. As it passes along the intestine, sodium ions are released and exchanged for potassium ions. The largest reservoir of potassium is in the large intestine, where most of the sodium-potassium exchange takes place, leading to potassium excretion in the stool. Because of the time needed to transit to the colon, it may take hours to days for a potassium-reducing effect to occur with sodium polystyrene sulfonate (Kayexalate®; Covis Pharmaceuticals, Inc., Cary, NC). By replacing potassium with sodium, sodium polystyrene sulfonate causes a sodium load; therefore, caution is needed when using in patients with severe CHF, severe hypertension, and edema. Because the cation exchange is not specific to potassium and can lead to inadvertent losses of magnesium and calcium, monitoring electrolyte levels is recommended. The only clinical data supporting the use of sodium polystyrene sulfonate are from one retrospective, uncontrolled analysis.38 Sodium polystyrene sulfonate is contraindicated in patients with obstructive bowel disease. Cases of colonic necrosis have been reported and are probably related to the large dose of sorbitol associated with its use, leading to a black box warning. Sodium polystyrene sulfonate use is associated with a variety of shortcomings, including the high dose of sorbitol leading to gastrointestinal (GI) intolerance, sodium loading, and the resultant volume overload, making it a poor candidate for the chronic treatment of hyperkalemia
Patiromer
Patiromer is indicated for the treatment of hyperkalemia and is a recent entrant into the marketplace. It is not to be used as an emergency treatment for life-threatening hyperkalemia because of its delayed onset of action. It is a nonabsorbed, cation exchange polymer that contains a calcium-sorbitol counter-ion. Patiromer increases fecal potassium excretion by binding potassium in the GI tract, leading to greater excretion of potassium, thereby lowering serum levels. Its safety and effectiveness were demonstrated in a series of modest-sized clinical trials (Table 3). The efficacy and safety of patiromer were studied in two key studies, the Two-Part, Single-Blind, Phase 3 Study Evaluating the Efficacy and Safety of Patiromer for the Treatment of Hyperkalemia (OPAL-HK) and Patiromer in the Treatment of Hyperkalemia in Patients With Hypertension and Diabetic Nephropathy (AMETHYST-DN).39,40
THE OPAL-HK study was a single-blind randomized trial of 243 hyperkalemic patients with CKD on stable doses of at least one RAAS inhibitor.39 Subjects with potassium levels of 5.1 to 5.5 mEq/L received a starting daily dose of 8.4 g of patiromer and those with levels of 5.5 to 6.5 mEq/L received 16.8 g of patiromer per day. Dose titrations were designed to maintain potassium levels between 3.8 to 5.1 mEq/L. The primary endpoint was the mean change in serum potassium levels from baseline to week 4, which was 20.65 mEq/L in subjects treated with 8.4 g patiromer per day (total daily dose), 21.23 mEq/L in patients treated with 16.8 g patiromer per day (total daily dose), and 21.01 mEq/L in the overall population.
The AMETHYST-DN trial was a 52-week open-label trial of 304 hyperkalemic patients with type 2 diabetes and CKD on a RAAS inhibitor.40 Patients with a baseline serum potassium of > 5.0 to 5.5 mEq/L or baseline serum potassium of > 5.5 to 6.0 mEq/L were randomized to receive one of three starting doses.
Similar to sodium polystyrene sulfonate, patiromer contains sorbitol, but at a much lower exposure. Because patiromer can bind to other orally administered agents, leading to a potential reduction of their bioavailability, a black box warning mandates that other medicines be administered either 6 hours before or after taking patiromer. This 6-hour timeframe may affect compliance in patients taking medications at different times of the day. Use of patiromer should be avoided in patients with severe constipation, bowel obstruction or impaction, including abnormal postoperative bowel motility disorder, as it may be ineffective and may worsen GI conditions.
Because patiromer can also bind to magnesium, leading to increased excretion, and cause hypomagnesemia, magnesium levels should be monitored regularly. The most common adverse reactions of patiromer include constipation, hypomagnesemia, diarrhea, nausea, abdominal discomfort, and flatulence.
Sodium Zirconium Cyclosilicate (ZS-9)
Sodium zirconium cyclosilicate is a highly selective cation exchanger that entraps potassium in the intestinal tract in exchange for sodium and hydrogen that is pending US Food and Drug Administration approval. Cyclosilicate is not a polymer, as is the case with sodium polystyrene sulfate and patiromer, nor is it delivered with sorbitol; it is a crystal that is highly selective, capturing only potassium and ammonium ions. It was engineered to have a high-capacity, highly selective crystalline lattice that entraps potassium cations over other divalent cations such as calcium or magnesium. A result of sodium zirconium cyclosilicate binding the ammonium ion is a net loss of acid, blood urea nitrogen, and elevation of serum bicarbonate levels, which may be favorable in patients with CKD who often have a relative metabolic acidosis. A robust clinical trial program has evaluated the safety and efficacy of sodium zirconium cyclosilicate in treating hyperkalemia in a number of clinical scenarios (Table 4).
The clinical trial program for sodium zirconium cyclosilicate includes ZS003, the multicenter, two-phase, multidose, prospective, randomized, double-blind placebo-controlled study of 753 patients with mild to moderate hyperkalemia (potassium levels of 5.0-6.5 mEq/L), including patients with CKD, heart failure, and diabetes who are on ACE inhibitors, ARBs, or mineralocorticoid antagonists. Treatment included four different doses of sodium zirconium cyclosilicate (1.25 g, 2.5 g, 5 g, and 10 g) or placebo given three times daily for the initial 48-hour acute phase (Figure 1).41 Patients who became normokalemic at 48 hours were then randomly assigned on day 3 to receive either sodium zirconium cyclosilicate or placebo once daily for the next 12 days followed by a 7-day follow-up phase.
The primary endpoint was the rate of change of potassium from baseline throughout the 48-hour acute phase. At 48 hours the mean reductions for the four doses tested was 0.46 mEq/L in the 2.5-g group, 0.54 mEq/L in the 5.0-g group, and 0.73 mEq/L in the 10-g group (Figure 2). At 1 hour following the first 10-g dose, a potassium level reduction of 0.11 mEq/L was observed. The more rapid reduction of potassium levels observed with sodium zirconium cyclosilicate than with other agents indicates that the potassium-binding effect starts higher in the GI tract, perhaps in the stomach or small bowel.
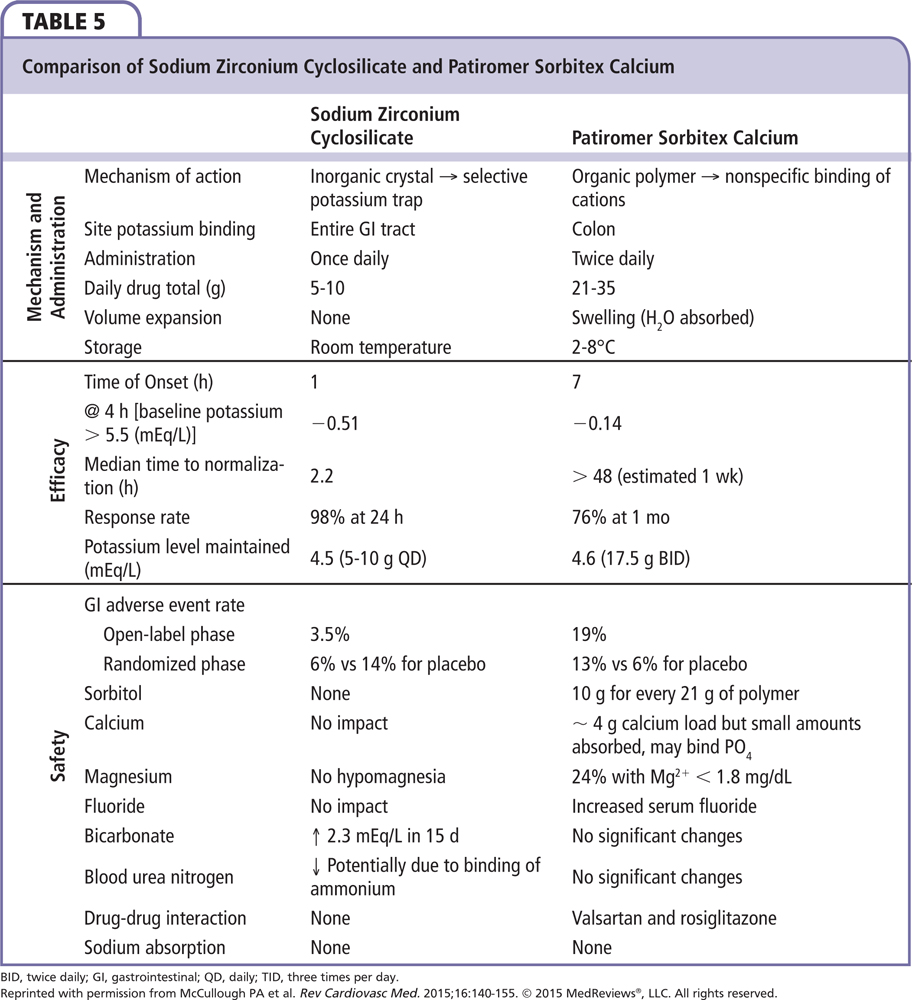
In the phase 3, multicenter, double-blind, placebo-controlled Hyperkalemia Randomized Intervention Multidose ZS-9 Maintenance (HARMONIZE) trial, 258 ambulatory outpatients with a potassium concentration > 5.1 mEq/L at baseline received 10 g of sodium zirconium cyclosilicate three times daily during an initial 48-hour open-label phase.42 Patients achieving normokalemia (3.5-5.0 mEq/L) were then randomized to one of three daily doses of sodium zirconium cyclosilicate (5 g, 10 g, or 15 g) or placebo for 28 days. Potassium was significantly reduced by 0.2 mEq/L at 1 hour following the first 10-g dose from baseline, with reductions at 2 and 4 hours after the first dose of 0.4 mEQ/L and 0.5 mEq/L, respectively. At 24 and 48 hours postdose, reductions of potassium were 0.7 mEQ/L and 1.1 mEq/L, respectively. Median time to normokalemia was 2.2 hours. The primary endpoint was a comparison of mean serum potassium levels among the different doses and placebo. Patients receiving 5 g, 10 g, or 15 g of sodium zirconium cyclosilicate were maintained at 4.8, 4.5, and 4.4 mEq/L versus 5.1 mEq/L for placebo patients (Figure 3). Of importance was that the potassium-lowering effect and maintenance of normokalemia occurred in patients with CKD, heart failure, and diabetes without the need to remove them from their RAAS inhibitor treatment. There was no difference from placebo in GI adverse events, with no observed increase in body weight, blood pressure, or urinary excretion. There was an increase in the incidence of edema at the 10- and 15-g doses. A comparison of the mechanism of action, efficacy, and safety of sodium zirconium cyclosilicate and patiromer are provided in Table 5.
Conclusions
Hyperkalemia is a common problem observed in both the acute care and chronic ambulatory care settings by primary care practitioners, cardiologists, nephrologists, and endocrinologists. It is especially seen among patients with diabetes, heart failure, and CKD who are treated with the renal and cardioprotective RAAS inhibitors. Until recently, the treatment of hyperkalemia was limited to discontinuation of these important and potentially life-saving RAAS inhibitors and, in worse cases, the use of sodium polystyrene sulfonate with its associated high incidence of adverse reactions. Newer options will allow for effective treatment of hyperkalemia while maintaining patients on prescribed RAAS inhibitors. The introduction of patiromer to the marketplace provides one new option to treat hyperkalemia that appears to be better tolerated then sodium polystyrene sulfonate, but it has a black box warning not to be given within 6 hours of other medications. The anticipated introduction of ZS-9 to the marketplace will provide another new option for treating hyperkalemia. It appears to be well tolerated, with a more rapid onset of action; with its high specificity for potassium the timing of its use is not limited by concomitant medication. The introduction of these new options for treating hyperkalemia is expected to lead to treatment at the time of diagnosis by cardiologists. ![]()
Dr. Lepor is a consultant for ZS Pharma (San Mateo, CA) and serves as a speaker for Relypsa (Redwood City, CA).
References
- Tamargo J, Caballero R, Delpón E. New drugs for the treatment of hyperkalemia in patients treated with renin-angiotensin-aldosterone system inhibitors—hype or hope? Discov Med. 2014;18:249-254.
- Khanagavi J, Gupta T, Aronow WS, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10:251-257.
- An JN, Lee JP, Jeon HJ, et al. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care. 2012;16:R225.
- Jain N, Kotla S, Little BB, et al. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2012;109:1510-1513.
- Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156-1162.
- Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004;351:585-592.
- McMahon GM, Mendu ML, Gibbons FK, Christopher KB. Association between hyperkalemia at critical care initiation and mortality. Intensive Care Med. 2012;38:1834-1842.
- Weir MR, Rolfe M. Potassium homeostasis and renin-angiotensin-aldosterone system inhibitors. Clin J Am Soc Nephrol. 2010;5:531-548.
- Shlipak MG. Pharmacotherapy for heart failure in patients with renal insufficiency. Ann Intern Med. 2003;138:917-924.
- Nakao N, Yoshimura A, Morita H, et al. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet. 2003;361:117-124.
- Cohn JN, Tognoni G; Valsartan Heart Failure Trial I. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667-1675.
- McMurray JJ, Ostergren J, Swedberg K, et al; CHARM Investigators and Committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767-771.
- Pfeffer MA, McMurray JJ, Velazquez EJ, et al; Valsartan in Acute Myocardial Infarction Trial Investigators. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893-1906.
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309-1321.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709-717.
- Hostetter TH, Ibrahim HN. Aldosterone in chronic kidney and cardiac disease. J Am Soc Nephrol. 2003;14:2395-2401.
- Hollander-Rodriguez JC, Calvert JF Jr. Hyperkalemia. Am Fam Physician. 2006;73:283-290.
- Gennari FJ. Disorders of potassium homeostasis. Hypokalemia and hyperkalemia. Crit Care Clin. 2002;18:273-288, vi.
- Sorensen MV, Matos JE, Praetorius HA, Leipziger J. Colonic potassium handling. Pflugers Arch. 2010;459:645-656.
- Young DB, Smith MJ Jr, Jackson TE, Scott RE. Multiplicative interaction between angiotensin II and K concentration in stimulation of aldosterone. Am J Physiol. 1984;247:E328-E335.
- Pratt JH, Rothrock JK, Dominguez JH. Evidence that angiotensin-II and potassium collaborate to increase cytosolic calcium and stimulate the secretion of aldosterone. Endocrinology. 1989;125:2463-2469.
- Shier DN, Kusano E, Stoner GD, et al. Production of renin, angiotensin II, and aldosterone by adrenal explant cultures: response to potassium and converting enzyme inhibition. Endocrinology. 1989;125:486-491.
- Sebastian A, Schambelan M. Renal hyperkalemia. Semin Nephrol. 1987;7:223-238.
- Hartmann RC, Auditore JV, Jackson DP. Studies on thrombocytosis. I. Hyperkalemia due to release of potassium from platelets during coagulation. J Clin Invest. 1958;37:699-707.
- Bronson WR, DeVita VT, Carbone PP, Cotlove E. Pseudohyperkalemia due to release of potassium from white blood cells during clotting. N Engl J Med. 1966;274:369-375.
- Don BR, Sebastian A, Cheitlin M, et al. Pseudohyperkalemia caused by fist clenching during phlebotomy. N Engl J Med. 1990;322:1290-1292.
- Seimiya M, Yoshida T, Sawabe Y, et al. Reducing the incidence of pseudohyperkalemia by avoiding making a fist during phlebotomy: a quality improvement report. Am J Kidney Dis. 2010;56:686-692.
- Ingram RH Jr, Seki M. Pseudohyperkalemia with thrombocytosis. N Engl J Med. 1962;267:895-900.
- Colussi G, Cipriani D. Pseudohyperkalemia in extreme leukocytosis. Am J Nephrol. 1995;15:450-452.
- Dickinson H, Webb NJ, Chaloner C, et al. Pseudohyperkalaemia associated with leukaemic cell lysis during pneumatic tube transport of blood samples. Pediatr Nephrol. 2012;27:1029-1031.
- Garwicz D, Karlman M. Early recognition of reverse pseudohyperkalemia in heparin plasma samples during leukemic hyperleukocytosis can prevent iatrogenic hypokalemia. Clin Biochem. 2012;45:1700-1702.
- Kellerman PS, Thornbery JM. Pseudohyperkalemia due to pneumatic tube transport in a leukemic patient. Am J Kidney Dis. 2005;46:746-748.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
- Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004;351:585-592.
- Massie BM, Armstrong PW, Cleland JG, et al. Toleration of high doses of angiotensin-converting enzyme inhibitors in patients with chronic heart failure: results from the ATLAS trial. Arch Intern Med. 2001;161: 165-171.
- de Denus S, Tardif JC, White M, et al. Quantification of the risk and predictors of hyperkalemia in patients with left ventricular dysfunction: a retrospective analysis of the Studies of Left Ventricular Dysfunction (SOLVD) trials. Am Heart J. 2006;152: 705-712.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004.
- Chernin G, Gal-Oz A, Ben-Assa E, et al. Secondary prevention of hyperkalemia with sodium polystyrene sulfonate in cardiac and kidney patients on renin-angiotensin-aldosterone system inhibition therapy. Clin Cardiol. 2012;35:32-36.
- Weir MR, Bakris GL, Bushinsky DA, et al; OPAL-HK Investigators. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211-221.
- Bakris GL, Pitt B, Weir MR, et al; AMETHYST-DN Investigators. Effect of patiromer on serum potassium in patients with hyperkalemia and diabetic kidney disease: the AMETHYST-DN randomized clinical trial. JAMA. 2015;314:151-161.
- Packham DK, Rasmussen HS, Lavin PT, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372:222-231.
- Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312:2223-2233.
- Kidney disease: high- and moderate-potassium foods. Academy of Nutrition and Dietetics website. http://www.eatright.org/resource/health/diseases-and-conditions/kidney-disease/kidney-disease-high-and-low-potassium-foods. Accessed May 30, 2016.
- Ben Salem C, Badreddine A, Fathallah N, et al. Drug-induced hyperkalemia. Drug Saf. 2014;37:677-692.
Dr. Lepor is a consultant for ZS Pharma (San Mateo, CA) and serves as a speaker for Relypsa (Redwood City, CA).