The Role of Medical Therapy in Moderate to Severe Degenerative Mitral Regurgitation
Leandro Slipczuk, MD, PhD,1 Asim M. Rafique, MD,1 Carlos D. Davila, MD,2 Roy Beigel, MD,1 Gregg S. Pressman, MD,2 Robert J. Siegel, MD1
1Cedars-Sinai Heart Institute, Los Angeles, CA; 2Einstein Medical Center, Philadelphia, PA
Mitral regurgitation (MR) is a common valvular disorder that has important health and economic consequences. Standardized guidelines exist regarding when and in whom to perform mitral valve surgery, but little information is available regarding medical treatment of MR. Many patients with moderate or severe MR do not meet criteria for surgery or are deemed to be at high risk for surgical therapy. We reviewed the available published data on medical therapy in the treatment of patients with primary MR. β-blockers and renin-angiotensin-aldosterone system inhibitors had the strongest supporting evidence for providing beneficial effects. β-blockers appear to lessen MR, prevent deterioration of left ventricular function, and improve survival in asymptomatic patients with moderate to severe primary MR. Angiotensin-converting enzyme inhibitor and angiotensin receptor blocker therapy reduces MR, especially in asymptomatic patients. However, in the setting of hypertrophic cardiomyopathy or mitral valve prolapse, vasodilators can increase the severity of MR. To define the precise role of medical therapy, a larger randomized controlled trial is needed to confirm benefit and assess in which subsets of patients medical therapy is most useful. Medical therapy in some patients improves symptoms, lessens MR, and may delay the need for surgical intervention.
[Rev Cardiovasc Med. 2016;17(1/2):28-39 doi: 10.3909/ricm0835]
© 2016 MedReviews®, LLC
The Role of Medical Therapy in Moderate to Severe Degenerative Mitral Regurgitation
Leandro Slipczuk, MD, PhD,1 Asim M. Rafique, MD,1 Carlos D. Davila, MD,2 Roy Beigel, MD,1 Gregg S. Pressman, MD,2 Robert J. Siegel, MD1
1Cedars-Sinai Heart Institute, Los Angeles, CA; 2Einstein Medical Center, Philadelphia, PA
Mitral regurgitation (MR) is a common valvular disorder that has important health and economic consequences. Standardized guidelines exist regarding when and in whom to perform mitral valve surgery, but little information is available regarding medical treatment of MR. Many patients with moderate or severe MR do not meet criteria for surgery or are deemed to be at high risk for surgical therapy. We reviewed the available published data on medical therapy in the treatment of patients with primary MR. β-blockers and renin-angiotensin-aldosterone system inhibitors had the strongest supporting evidence for providing beneficial effects. β-blockers appear to lessen MR, prevent deterioration of left ventricular function, and improve survival in asymptomatic patients with moderate to severe primary MR. Angiotensin-converting enzyme inhibitor and angiotensin receptor blocker therapy reduces MR, especially in asymptomatic patients. However, in the setting of hypertrophic cardiomyopathy or mitral valve prolapse, vasodilators can increase the severity of MR. To define the precise role of medical therapy, a larger randomized controlled trial is needed to confirm benefit and assess in which subsets of patients medical therapy is most useful. Medical therapy in some patients improves symptoms, lessens MR, and may delay the need for surgical intervention.
[Rev Cardiovasc Med. 2016;17(1/2):28-39 doi: 10.3909/ricm0835]
© 2016 MedReviews®, LLC
The Role of Medical Therapy in Moderate to Severe Degenerative Mitral Regurgitation
Leandro Slipczuk, MD, PhD,1 Asim M. Rafique, MD,1 Carlos D. Davila, MD,2 Roy Beigel, MD,1 Gregg S. Pressman, MD,2 Robert J. Siegel, MD1
1Cedars-Sinai Heart Institute, Los Angeles, CA; 2Einstein Medical Center, Philadelphia, PA
Mitral regurgitation (MR) is a common valvular disorder that has important health and economic consequences. Standardized guidelines exist regarding when and in whom to perform mitral valve surgery, but little information is available regarding medical treatment of MR. Many patients with moderate or severe MR do not meet criteria for surgery or are deemed to be at high risk for surgical therapy. We reviewed the available published data on medical therapy in the treatment of patients with primary MR. β-blockers and renin-angiotensin-aldosterone system inhibitors had the strongest supporting evidence for providing beneficial effects. β-blockers appear to lessen MR, prevent deterioration of left ventricular function, and improve survival in asymptomatic patients with moderate to severe primary MR. Angiotensin-converting enzyme inhibitor and angiotensin receptor blocker therapy reduces MR, especially in asymptomatic patients. However, in the setting of hypertrophic cardiomyopathy or mitral valve prolapse, vasodilators can increase the severity of MR. To define the precise role of medical therapy, a larger randomized controlled trial is needed to confirm benefit and assess in which subsets of patients medical therapy is most useful. Medical therapy in some patients improves symptoms, lessens MR, and may delay the need for surgical intervention.
[Rev Cardiovasc Med. 2016;17(1/2):28-39 doi: 10.3909/ricm0835]
© 2016 MedReviews®, LLC
KEY WORDS
Mitral regurgitation • Medical therapy • Mitral insufficiency • Heart failure
KEY WORDS
Mitral regurgitation • Medical therapy • Mitral insufficiency • Heart failure
… the increase in systemic arterial pressure during activity and/or exercise could also be a target for medical therapy of primary MR.
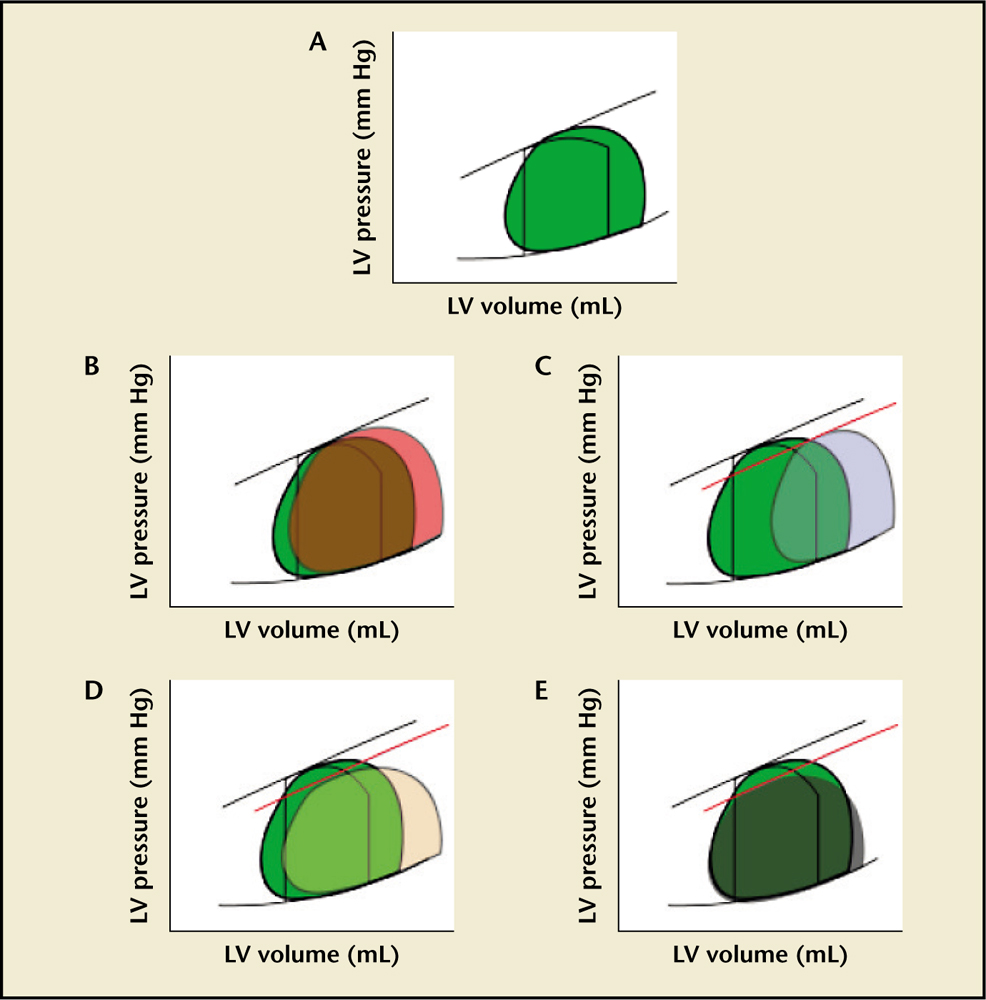
Figure 1. Pressure-volume loops of mitral regurgitation (MR) and its change with therapy. Graphs show pressure on the y-axis and volume on the x-axis. (A) Acute MR. The black lined loop represents normal look. The green filled loop represents changes after acute MR occurs. After acute MR there is no true isovolumetric contraction as blood flows back into the left atrium (LA) as soon as systole starts. This reduces afterload, increasing ejection fraction (EF). There is also no isovolumetric relaxation as blood continues to flow back to the LA even after aortic valve closure. This additional flow into the LA increases its pressure and thus increasing left ventricular end-diastolic pressure (LVEDP). The ventricle also uses its preload reserve to maintain EF. Even though total EF is increased, forward stroke volume can be decreased. (B) Chronic compensated MR. The orange loop represents changes with chronic compensated MR. The volume overload leads to dilation of the left ventricle. Preload returns to normal or nearly normal. With time, left ventricular end-diastolic volume (LVESV) increases and afterload starts to increase as well. An enlarged compliant LA permits maintenance of lower pulmonary venous pressures. (C) Chronic decompensated MR. The light blue loop represents changes with decompensated MR. In this stage there is progressive LV dilatation with increased afterload and a decrease in EF. At this point, there is an increase in pulmonary venous pressure and symptomatic heart failure. (D) Effects of afterload reduction on MR. The light orange loop represents changes after vasodilator therapy. Vasodilator effects on arteries potentially increase forward stroke volume (angiotensin-converting enzyme inhibitors, hydralazine, β-blockers) without significant changes in LVEDV. (E) Effects of preload reduction on MR. The grey loop represents changes with decreased preload. Venodilation decreases preload with a reduction in LVEDV and LVESV, increasing LVEF. Moreover, this provides better workload conditions.

Figure 1. Pressure-volume loops of mitral regurgitation (MR) and its change with therapy. Graphs show pressure on the y-axis and volume on the x-axis. (A) Acute MR. The black lined loop represents normal look. The green filled loop represents changes after acute MR occurs. After acute MR there is no true isovolumetric contraction as blood flows back into the left atrium (LA) as soon as systole starts. This reduces afterload, increasing ejection fraction (EF). There is also no isovolumetric relaxation as blood continues to flow back to the LA even after aortic valve closure. This additional flow into the LA increases its pressure and thus increasing left ventricular end-diastolic pressure (LVEDP). The ventricle also uses its preload reserve to maintain EF. Even though total EF is increased, forward stroke volume can be decreased. (B) Chronic compensated MR. The orange loop represents changes with chronic compensated MR. The volume overload leads to dilation of the left ventricle. Preload returns to normal or nearly normal. With time, left ventricular end-diastolic volume (LVESV) increases and afterload starts to increase as well. An enlarged compliant LA permits maintenance of lower pulmonary venous pressures. (C) Chronic decompensated MR. The light blue loop represents changes with decompensated MR. In this stage there is progressive LV dilatation with increased afterload and a decrease in EF. At this point, there is an increase in pulmonary venous pressure and symptomatic heart failure. (D) Effects of afterload reduction on MR. The light orange loop represents changes after vasodilator therapy. Vasodilator effects on arteries potentially increase forward stroke volume (angiotensin-converting enzyme inhibitors, hydralazine, β-blockers) without significant changes in LVEDV. (E) Effects of preload reduction on MR. The grey loop represents changes with decreased preload. Venodilation decreases preload with a reduction in LVEDV and LVESV, increasing LVEF. Moreover, this provides better workload conditions.
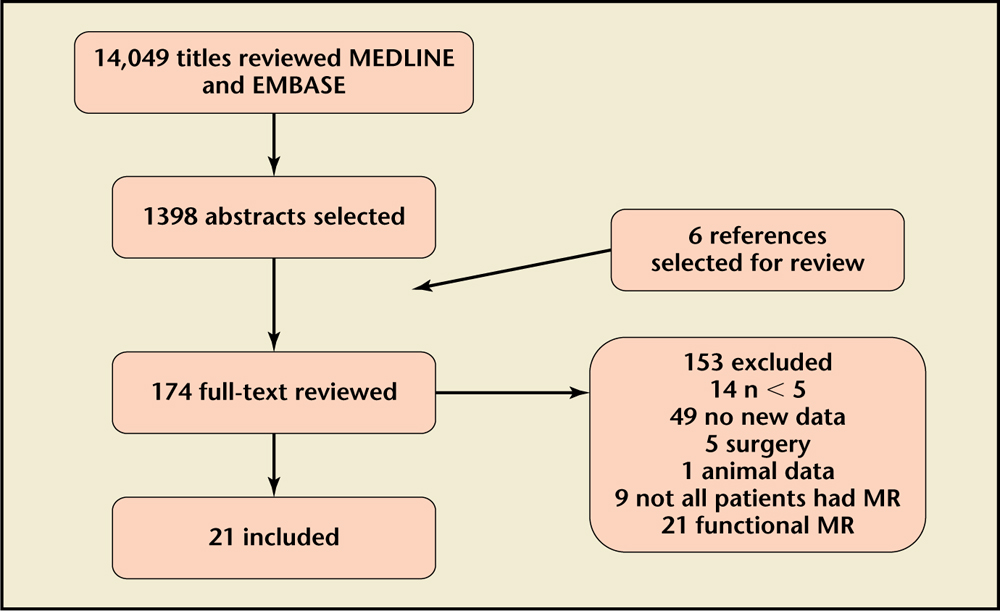
Figure 2. Selection process flow chart. MR, mitral regurgitation

Figure 2. Selection process flow chart. MR, mitral regurgitation

In primary MR, ACE inhibitor/ARB therapy has been associated with reduced RF, RV, LVEDV, and increased stroke volume, especially in patients without symptoms or LV dysfunction.


Attenuation of systolic blood pressure with medical therapy using vasodilators might improve exercise tolerance and reduce MR.
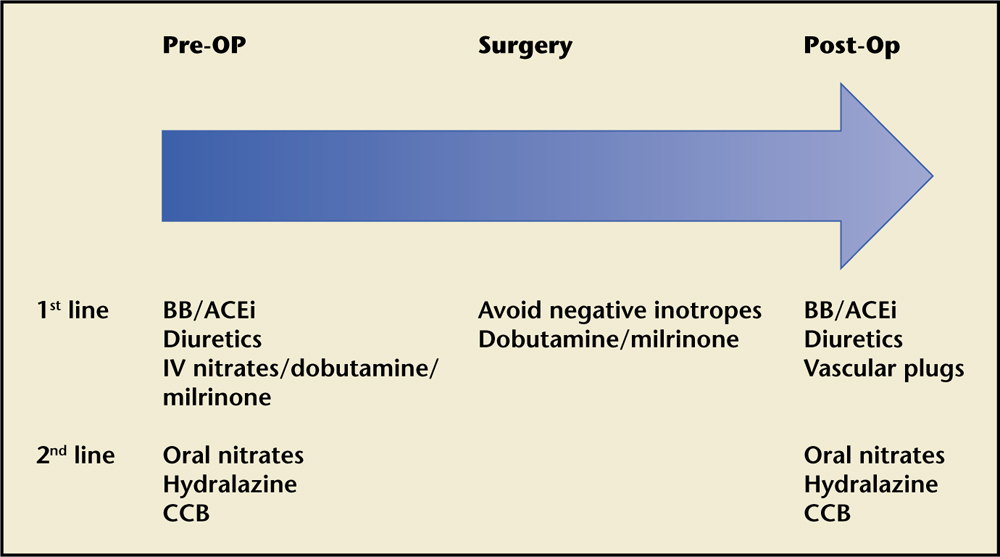
Figure 3. Suggested perioperative therapy. Graph shows suggested therapy in the preoperative, operative, and postoperative period. ACEi, angiotensin-converting enzyme inhibitor; BB, β-blocker; CCB, calcium channel blocker; IV, intravenous.

Figure 3. Suggested perioperative therapy. Graph shows suggested therapy in the preoperative, operative, and postoperative period. ACEi, angiotensin-converting enzyme inhibitor; BB, β-blocker; CCB, calcium channel blocker; IV, intravenous.
Main Points
• At present, there are no standardized guidelines to pharmacologically treat patients with moderate to severe primary mitral regurgitation (MR).
• Medical therapy, especially with β-blockers and angiotensin converting enzyme inhibitors/angiotensin receptor blockers, is potentially useful in primary MR by reducing afterload, improving MR, and preventing left ventricular (LV) remodeling or causing reverse re-modeling. These medications are therefore preferred as first-line treatment with other medications (nitrates, hydralazine, calcium channel blocker) held in reserve as add-on/second-line therapy.
• Reduction of pre- and afterload with anesthesia and vasodilator agents can worsen MR due to mitral valve prolapse or hypertrophic obstructive cardiomyopathy.
• Surgery is recommended in patients with primary severe MR who have symptoms, LV dysfunction, LV dilatation (LV end-systolic diameter ≥ 40 mm), or that are undergoing cardiac surgery for other indications. Moreover, when there is a high likelihood of successful and durable repair and resting pulmonary hypertension (pulmonary artery systolic pressure > 50 mm Hg) or new onset of atrial fibrillation, it is reasonable to proceed with surgery.
• During the in-hospital waiting period for surgery, hemodynamic monitoring with a pulmonary artery catheter and the combination of diuresis and intravenous nitrates ± dobutamine/milrinone have been shown to have beneficial effects in patients with severe congestive heart failure due to systolic dysfunction.
• Medical therapy could potentially delay the need for surgery, improve functional status, and delay trading one disease condition (MR) for another, namely, mitral valve placement or repair which have their own morbidity and complications. However, large-scale randomized clinical trials are needed to clarify the utility of medical therapy.
Main Points
• At present, there are no standardized guidelines to pharmacologically treat patients with moderate to severe primary mitral regurgitation (MR).
• Medical therapy, especially with β-blockers and angiotensin converting enzyme inhibitors/angiotensin receptor blockers, is potentially useful in primary MR by reducing afterload, improving MR, and preventing left ventricular (LV) remodeling or causing reverse re-modeling. These medications are therefore preferred as first-line treatment with other medications (nitrates, hydralazine, calcium channel blocker) held in reserve as add-on/second-line therapy.
• Reduction of pre- and afterload with anesthesia and vasodilator agents can worsen MR due to mitral valve prolapse or hypertrophic obstructive cardiomyopathy.
• Surgery is recommended in patients with primary severe MR who have symptoms, LV dysfunction, LV dilatation (LV end-systolic diameter ≥ 40 mm), or that are undergoing cardiac surgery for other indications. Moreover, when there is a high likelihood of successful and durable repair and resting pulmonary hypertension (pulmonary artery systolic pressure > 50 mm Hg) or new onset of atrial fibrillation, it is reasonable to proceed with surgery.
• During the in-hospital waiting period for surgery, hemodynamic monitoring with a pulmonary artery catheter and the combination of diuresis and intravenous nitrates ± dobutamine/milrinone have been shown to have beneficial effects in patients with severe congestive heart failure due to systolic dysfunction.
• Medical therapy could potentially delay the need for surgery, improve functional status, and delay trading one disease condition (MR) for another, namely, mitral valve placement or repair which have their own morbidity and complications. However, large-scale randomized clinical trials are needed to clarify the utility of medical therapy.
Mitral regurgitation (MR) is the most common valve disease in the United States,1 with a prevalence in the general population of approximately 19%.2,3 Its incidence increases with age, especially in countries with a low prevalence of rheumatic heart disease. It can be classified as primary (degenerative or rheumatic) or secondary (functional or ischemic). Degenerative MR is due to leaflet disease whereas functional MR is the consequence of left ventricular (LV) dilation and papillary muscle displacement. Degenerative MR was originally thought to be due to myxomatous changes or fibroelastic deficiency of the valve and chordae; more recent work (at least in posterior leaflet disease) suggests thickening is due to fibrous tissue accumulation resulting from abnormal leaflet contact, with chordal rupture being the initiating event.4 Current American College of Cardiology/American Heart Association guidelines give a Class I (Level of Evidence B) recommendation for surgical referral of patients with severe primary MR and symptoms. Improvement in MR after treatment of heart failure in patients with functional MR is well established.5 However, the role of traditional medical therapy for normotensive patients with degenerative MR and preserved LV function is more controversial,6 with significant variability in its clinical management.7 Notably, the use of standard medical therapy has not been compared with placebo, surgery, or interventional therapy.
Once mitral valve regurgitation becomes symptomatic or LV dysfunction occurs, prognosis is impaired and patients should be referred for surgery.2 In the Euro Heart Survey, 26% of the patients with severe MR did not have symptoms; 60% of patients with symptomatic degenerative severe MR responded to medical therapy and this was found to be the most frequent reason (45% of cases) for practitioners to delay surgery. However, in 37% of cases, other comorbidities were listed as reasons for not referring patients for mitral surgery.80 In patients with primary MR on no medical therapy, Rosenhek and colleagues8 found that approximately half of the patients with severe MR could safely be treated with the strategy of watchful waiting, namely, serial follow-up at 3, 6, or 12 months. With this strategy, half the patients developed an indication for surgical therapy at 8 years (eg, symptoms, LV dysfunction, pulmonary hypertension, and atrial fibrillation). However, at present, there is no consensus or guideline recommendation on the role of medical therapy in patients with primary MR.
Physiology
As early as 1922 the effect of vasodilation on MR was studied by Wiggers and Feely.9 They showed that experimental MR could be increased by aortic constriction and decreased by administration of nitrates. The Torricelli equation provides a tool to understand MR determinants. This equation states that the flow through an orifice varies according to the square root of the pressure gradient across the orifice10:
MRV = MROA × C × (√MPG × Ts)
where MRV is the mitral regurgitant volume; MROA is the mitral regurgitant orifice area; C is the constant; MPG is the mean pressure gradient between left atrial systolic mean pressure (LAsm) and left ventricular systolic mean pressure (LVsm); and Ts is the time of duration of systole.
As MROA is dynamic and can vary according to LV size, there is the potential to reduce MR with medical therapy that reduces either LV dimension, the LV systolic pressure, or both.
LVsm is a modifiable factor for determining MR severity in this equation (Figure 1). Unlike aortic regurgitation, MR by itself usually does not produce systemic hypertension11 and right ventricular dysfunction appears to occur earlier and to have greater prognostic impact than LV changes.12 Nevertheless, in the setting of increased LV size, MR patients also have an increase in LV afterload (wall tension = LV dimension × LV systolic pressure/2 × LV wall thickness). In addition to an increase in afterload, hemodynamically compensated MR is associated with an increase in catecholamines,13 increased preload with normal wall thickness, and supranormal diastolic function.14 Chronic severe MR causes LV volume overload,11 producing a specific pattern of remodeling.15 Once LV dysfunction ensues, the LV dilates and LV afterload rises.16 Furthermore, any increase in arterial pressure (as generally occurs with exercise or variable degrees of exertion) increases LVsm, and therefore increases mitral regurgitant volume,17 and—as indicated by the Torricelli equation—increases the MROA.18 Similarly, the increase in systemic arterial pressure during activity and/or exercise could also be a target for medical therapy of primary MR. Theoretically, preload and afterload reduction with medical therapy could reduce/prevent LV dysfunction and/or reduce symptoms with possible reverse remodeling of the left ventricle. As is seen in Figure 1, this, in turn, can favorably shift the pressure-volume loop and reduce myocardial stress. One exception to this is in cases of mitral valve prolapse (MVP) and hypertrophic obstructive cardiomyopathy (HOCM), in which the mitral valve apparatus is redundant with excessive tissue relative to the LV during mid to late systole. Therefore, anything that decreases LV volume (eg, standing, decreasing afterload, or peripheral vasodilation) can worsen the MR.19 In the case of hypertrophic cardiomyopathy, any increase in contractility can produce a similar effect.
For almost 20 years, several research groups have focused on understanding the remodeling of the LV in the early phases of MR. Animal studies have shown that early changes in the LV arise with a low pressure-volume overload. An increase in LV apex tension induces activation of metalloproteinases and chymase from mast cells with consequent myocardial hypertrophy. These changes appear to start in the endocardium (see Dillon and colleagues20 for an extensive analysis of this process).
In addition, animal experiments have provided important insights on medical therapy for MR. Tsutsui and colleagues21 and Nemoto and associates22 initially demonstrated that β-blockers ameliorated contractile dysfunction caused by experimental chronic MR in dogs. In another study,23 metoprolol improved LV function in dogs 4 months after the induction of severe MR. This effect appeared to be mediated in part by bradycardia as it can be altered by pacing,24 and mitigation of adrenergic damage to cardiomyocytes.25
Treatment with angiotensin-converting enzyme (ACE) inhibitors has had varied results in preclinical studies. One study failed to show LV mass, myocyte length, and collagen distribution improvement in early MR despite reduction in wedge pressure and LV angiotensin (Ang) II levels. The authors found a compensatory increase in Ang II type 1 (AT1) receptors that could explain the lack of effect.26 However, Nemoto and associates22 found that when atenolol was added to lisinopril therapy, forward stroke volume and contractility returned to normal. In addition, Tallaj and colleagues27 demonstrated that dogs with MR treated with long-acting metoprolol had an attenuation of their LV ACE expression, and Ang II-mediated norepinephrine and epinephrine release in the cardiac interstitial fluid and circulation. These findings support the concept that combining renin-angiotensin-aldosterone system (RAAS) inhibitors with β-blockers may be a beneficial treatment strategy. Aiming to further characterize the effects of medical therapy on moderate to severe and severe primary MR in humans, we conducted a systematic search of current evidence on the subject.
Data Sources and Searches
We searched English language papers in the Ovid/MEDLINE and EMBASE electronic databases from their inception through May 1, 2015. We used the terms mitral regurgitation OR mitral insufficiency as MeSH keywords. We supplemented the search with references from articles reviewed (Figure 2).
Study Selection and Data Collection
Prospective and nonprospective studies reporting the outcomes of medical therapy on primary moderate to severe MR were included in this systematic review. Two investigators (Drs. Slipczuk and Davila) independently assessed the studies for eligibility. Inclusion criteria were (1) isolated native moderate or severe MR in the absence of > mild stenosis, and in the absence of > mild regurgitation or stenosis of another valve; (2) N > 5; and (3) medical therapy outcomes reported. We excluded papers that reported treatments of functional MR, prosthetic valves or ones applied intraoperatively, and papers that reported outcomes of different medications as a group without specific data for a medication or combinations of them. We also excluded papers that only studied acute effects of a drug (< 24 h).
Medical Therapy
As shown in Figure 2, our search in MEDLINE and EMBASE resulted in 14,049 papers. We included 21 studies in our review. β-blockers were studied in 4 reports; RAAS inhibitors in 13; nitrates in 1; nitrates and calcium channel blockers (CCBs) in 1; hydralazine in 1; and a combination of medications in 1 report.
β-Blockers
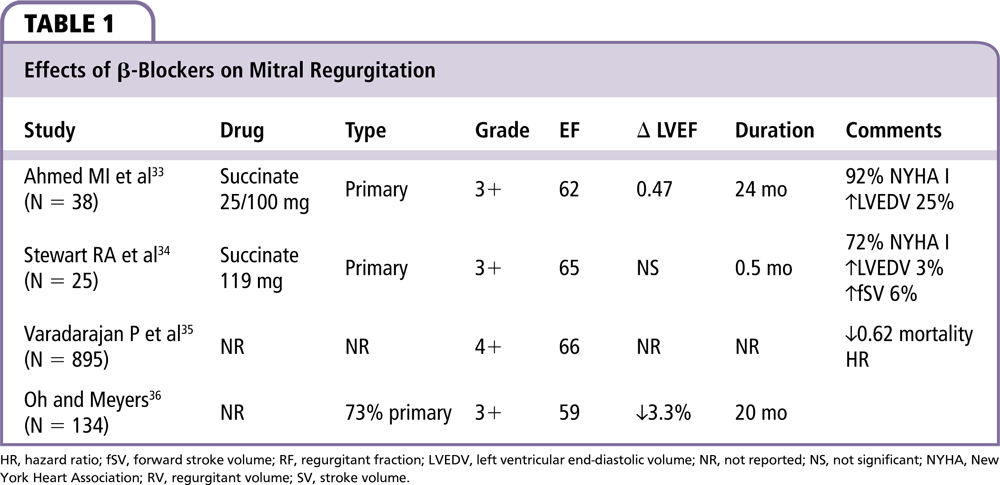
Evidence of β-blocker effects on MR comes mostly from studies of secondary MR in patients with dilated cardiomyopathy. However, most of these trials were designed to evaluate therapy for heart failure, with MR only as a secondary outcome. The sympathetic nervous system activity plays an important role in the adaptation of the LV to the volume overload that occurs with MR.13,28 Catecholamines are known to affect myocyte viability29 and can lead to adverse remodeling.30 Conversely, β-blockers decrease morbidity and mortality in LV systolic heart failure31,32 and might be useful in treating MR in certain settings. We included 4 studies on β-blockers with a total of 1092 patients; 2 analyzed long-acting metoprolol; 2 did not specify the type of β-blocker.
As can be seen in Table 1, trials in primary MR were mainly conducted in asymptomatic patients or those with limited symptoms. Effects of β-blocker therapy in patients with severe MR and congestive heart failure have been shown to be beneficial. However, this has been presumed to be due to the beneficial effects of β-blockers on congestive heart failure.
Ahmed and colleagues33 performed a small randomized, double-blind placebo-controlled trial on 38 patients with compensated, asymptomatic, moderate to severe degenerative MR with thickened leaflets and MVP. The authors excluded patients with systemic hypertension that required therapy, those with functional class III or IV heart failure, previous MI, or significant coronary artery disease. No patients had a flail leaflet. Patients who received long-acting metoprolol demonstrated preservation of LV systolic and diastolic function over a 2-year period as measured by cardiac magnetic resonance. Six patients out of 19 in the placebo group required surgery compared with 2 out of 19 in the medical therapy group (P = .23).
In a prospective, randomized double-blind crossover study of 25 patients with moderate to severe degenerative MR, 44% of patients had flail or partial flail mitral valve leaflets.34 Treatment with long-acting β-blockers for 2 weeks increased the forward stroke volume (fSV) mildly (+5 mL) without changing ejection fraction (EF) or the MR volume. β-blockers also decreased the cardiac workload by 20% and the cardiac output (CO) to a lesser degree (9%). Long-term outcome data were not reported.
Varadarajan and associates35 conducted the largest study of β-blockers in patients with MR. All patients had an LVEF > 55%. In their retrospective study of 895 patients with severe MR and normal EF, the use of β-blockers was associated with a reduction in mortality (hazard ratio 0.62; P = .002). However, 70% of patients receiving β-blockers were hypertensive; entire β-blocker group also received more aspirin (56% vs 21%; P < .0001), ACE inhibitors (52% vs 28%; P > .0001), and statins (8% vs 30%; P > .0001). The mortality benefit persisted after adjustment for differences between groups. Patients in the β-blocker group had less mitral valve surgery (19% vs 37%; P < .0001) over maximum 11 years of follow-up.
Oh and Meyers36 retrospectively studied 134 asymptomatic patients with moderate to severe MR and LVEF > 50%. At 20 months, the β-blocker group had a small reduction in EF of 3.3% as compared with improvement of 3.4% in the group with afterload reduction without β-blockers when treatment was newly started. However, this change became nonsignificant with continued treatment (P = .06). Semiquantitative MR severity grade decreased after initiation of afterload reduction (4.0 vs 3.5; P = .05) but this did not persist with longer exposure to β-blockers. β-blockers did not affect heart rate and therefore patients were probably not treated with adequate doses of β-blockers to have a significant pharmacologic effect.
In summary, β-blocker therapy in primary MR has been studied in 1092 patients. β-blocker therapy is associated with less deterioration in EF, reduced cardiac workload,34 reduced MR grade,36 and reduced mortality,35 while possibly increasing fSV34 and preserving LV systolic and diastolic function.33 These effects are also associated with a reduction in heart rate possibly leading to (1) reduction in oxygen consumption,37 (2) improved calcium handling,38 (3) improved myocardium metabolism restoring high-energy phosphate,39 and (4) preservation of the curvature of the apex preventing global rounding of the left ventricle.40
RAAS Inhibitors
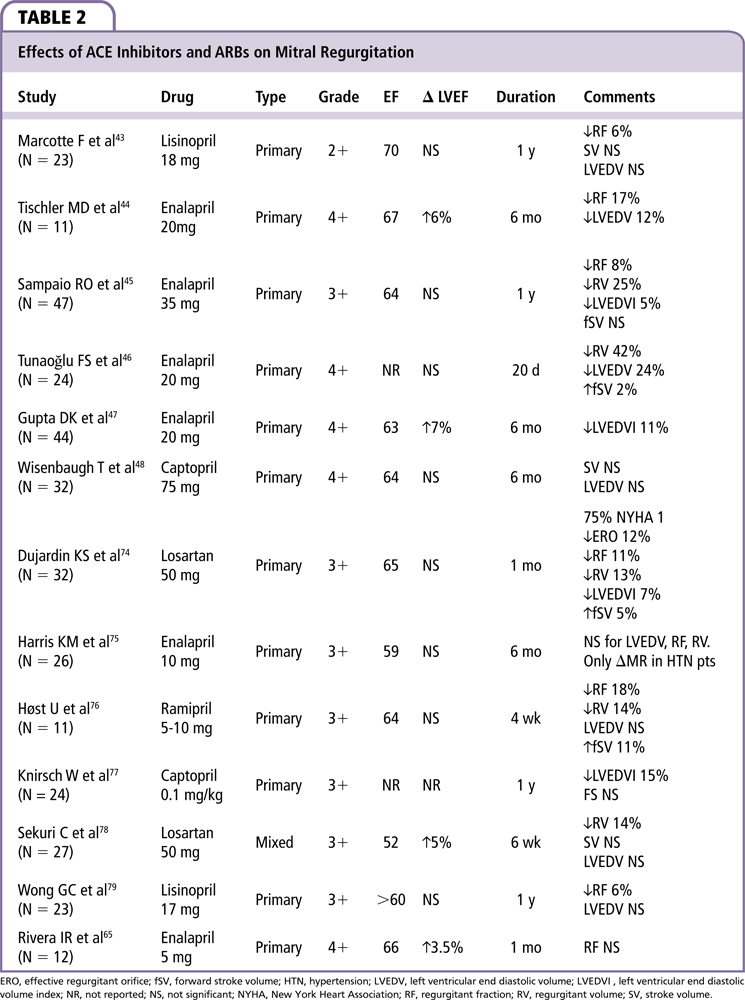
RAAS regulates blood volume and systemic vascular resistance. Ang II constricts resistance vessels, mainly through AT1 receptors. ACE inhibitors block the conversion of Ang I to Ang II. ACE, or kininase II, in the enzyme is responsible for cleaving the C-terminal dipeptide from Ang I, bradykinin, and a number of other small peptides that lack a penultimate proline residue. ACE also catalyzes the degradation of bradykinin further by possibly inhibiting desensitization of its receptor, increasing both bradykinin levels and signaling.41 Bradykinin causes smooth muscle contraction in certain organs and increases vascular permeability, stimulates peripheral and C fibers, and augments mucous secretion. Angiotensin receptor blockers (ARBs) inhibit the AT1 receptor directly. ARBs, as opposed to ACE inhibitors, do not increase bradykinin levels.42 We found 13 papers reporting effects on ACE inhibitors/ARBs on MR, with a total of 336 patients (Table 2).
Four small trials evaluated the efficacy of ACE inhibitors in asymptomatic patients with chronic primary MR; only the study by Marcotte and associates43 was randomized. In this study, asymptomatic patients with chronic, moderate MR of varying etiologies were randomized to lisinopril or placebo, and followed for 1 year. Those treated with lisinopril showed a decrease in regurgitant fraction (RF) of 6.4% compared with a 3.7% increase among control subjects (P < .05). Tischler and colleagues44 treated 11 asymptomatic patients with severe MR due to MVP with enalapril for 6 months. Treatment was associated with a reduction in LV end-systolic volume (LVESV) and LV end-diastolic volume (LVEDV), at rest and during exercise. There was a small but significant increase in EF of 6% (P < .01) with a reduction in LV mass index (P < .001). Enalapril reduced both regurgitant volume (RV) and RF at rest and during exercise without changes in exercise capacity; however, there was no control group in the study. Sampaio and associates45 reported a randomized study of 47 asymptomatic or minimally symptomatic patients with moderate to severe MR treated with enalapril for 1 year. They also found reductions in regurgitant orifice area, RV, and RF.
Tunaoğlu and coworkers46 followed 24 patients with severe MR from rheumatic heart disease (RHD) (mean age 14 y) for 20 days. These patients received lisinopril or placebo. Lisinopril decreased LVEDV (P < .05) and showed a trend for a reduction in regurgitant volume (P > .05) and minimally increased fSV (P < .05). In this study, patients were also taking digoxin. Gupta and colleagues47 found similar results in 44 patients on enalapril with severe MR secondary to RHD (mean age 18.8 y) who were treated for 6 months. Improvement to New York Heart Association (NYHA) class I was seen in 30% of the treated NYHA class II patients at 6 months. The authors also found that patients had a 7% increase in LVEF and a decrease of 11% in LVEDV.
In a randomized controlled trial, Wisenbaugh and associates48 compared captopril with placebo in 32 patients (NYHA class I to II) with chronic rheumatic MR. At 6 months, there was no difference in systolic arterial pressure, LV volume, or EF between groups. Malev and coworkers49 studied 233 asymptomatic patients with severe MR secondary to MVP in a retrospective, nonrandomized, single-center study. Groups did not differ in most demographic and clinical characteristics; 81.1% received an ACE inhibitor or ARB and 18.9% did not receive these medications. Transforming growth factor (TGF)-β1 and TGF-β2 levels were significantly higher in subjects without ARB therapy than in the ARB group (55.9 ± 70.7 vs 16.1 ± 32.9 ng/mL; P = .0003; and 7.6 ± 0.5 vs 2.2 ± 1.5 ng/ mL; P < .0001). There was also improvement in systolic function (EF 59.2 ± 9.6% vs 47.5 ± 10.6%; P > .0001). TGF-β signaling has shown to induce myocardial fibrosis and contribute to the development of a myxomatous mitral valve and therefore its attenuation by RAAS inhibitors could represent an interesting strategy to mitigate the progression of MVP and MR.50
In primary MR, ACE inhibitor/ARB therapy has been associated with reduced RF, RV, LVEDV, and increased stroke volume, especially in patients without symptoms or LV dysfunction.49 Possible beneficial effects of ACE inhibitor/ARB therapy have also been found in the pediatric population. This was proven in a meta-analysis by Strauss and associates51 of ACE inhibitor/ARB for MR that included 19 studies. These authors combined data and noted a mean decrease of 7.7% in RF, 7.9 mL in RV, and a decrease of 11.5 mL/m2 in the LVEDV index. However, data are sparse and somewhat conflicted. Even though ACE inhibitors and ARBs seem to have beneficial hemodynamic effects, there are no data on hospitalizations for heart failure or mortality. More research is needed before any definitive conclusions can be made.
Nitrates
The role of vasodilator therapy to reduce ventricular load has been evaluated in several small clinical trials. Most of the studies with nitrates used intravenous dosing assessing short-term response. We included two studies of nitrates in 50 patients with MR (Table 3).
Nitroglycerin (NTG) and other vasodilators may reduce LV end-systolic size, thereby allowing better MV leaflet coaptation. Alternatively, vasodilation by reducing afterload may enhance LV closing forces. As opposed to hydralazine, which mainly affects afterload, nitrates decrease LVEDV and LVESV. NTG has been reported to result in changes in the mitral annulus size if it is not rheumatic or calcified.52,53 This is potentially important when MR is due to dysfunction of the subvalvular apparatus.54,55 The effects of NTG appear to be additional to those of hydralazine.56
The decrease in LVEDV produced by nitrates has the potential to influence remodeling and reduce ventricular dilatation. A limitation of the available data is that they are primarily based on acute effects of intravenous medications. Chronic effects of long-acting oral nitrates may not be the same.57 Studies have not found any beneficial effects of oral nitrates on MR either in acute57,58 or 2-week administration of the drugs.57 Moreover, in patients with rheumatic valve disease, they may have a detrimental effect.58 Nitrates are expected to decrease the backward impedance by decreasing the impedance at the left atrial and pulmonary vein levels. However, because of the corresponding decrease in the LV filling volume, which causes a decrease in the effective size of the regurgitant orifice, the overall backward impedance may be unchanged. Data from Jeang and colleagues58 suggest that this is true for nonrheumatic MR; however, in the rheumatic MR group, because the commissures are frequently fused, and the leaflets and chordae thickened, the effective regurgitant area may not change significantly with LV volume.
Unloading of the LV with vasodilator therapy is associated with a variable reduction in MR severity and LV volumes.59 Many patients with severe MR who are apparently asymptomatic and have normal LVEF may be symptomatic during stress testing, with an associated afterload increase on exertion. Attenuation of systolic blood pressure with medical therapy using vasodilators might improve exercise tolerance and reduce MR.
Vasodilators may be harmful in certain situations such as HOCM with MR secondary to systolic anterior motion (SAM) of the mitral valve and in patients with MVP and preserved LV systolic function.60 In addition, SAM and LV outflow tract (LVOT) gradients (HOCM physiology) can be precipitated in hypertensive patients with LV hypertrophy and small LV cavities. Thus, vasodilators should be used with caution in patients with hyperdynamic LV function or small LV chamber size because of the potential for SAM to produce LVOT gradients and worsen MR.61
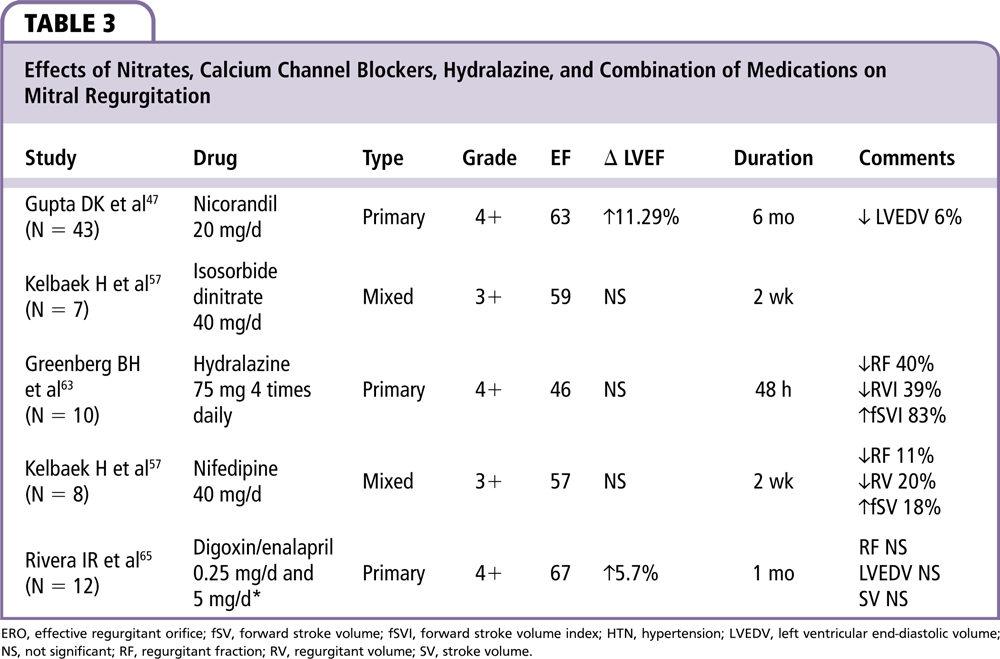
As shown in Table 3, Gupta and associates47 randomized 87 patients with severe MR secondary to RHD to nicorandil (a nitrate not available in the United States) or enalapril and followed them for 6 months. EF increased 7% and 4% in both the nicorandil and enalapril groups, respectively, with statistical significance. Unfortunately, there was no placebo control group and the study was not blinded.
Kelbaek and coworkers57 studied both the acute and 2-week effects of isosorbide dinitrate, 20 mg twice daily. We only included data from the 2-week results in our study. Patients were randomly allocated and the study was double-blinded. MR was primary in 16 patients, with MVP in 9, and secondary to cardiomyopathy or ischemic heart disease in the remaining patients. No significant hemodynamic changes were found in this study.
Hydralazine
Hydralazine dilates the resistance arterioles, reducing peripheral resistance and aortic impedance. Consequently, the gradient between the LV and LA falls, which can result in an increase in fSV and a decrease in MR. Hydralazine is a pure arterial vasodilator and does not produce venodilation; on the contrary, it generates baroreflex-mediated venoconstriction with increased blood return to the heart.62
As noted in Table 3, Greenberg and associates63 studied both intravenous and oral hydralazine in severe primary MR. The authors studied 10 patients with NYHA III or IV heart failure, a mean EF of 51%, and severe MR of various etiologies as determined by angiography.63 They found an acute decrease in RF, RV, systemic vascular resistance, and wedge pressure, with an increase in fSV but no changes in LVEF. Hydralazine did not change the LV end-diastolic pressure or LVEDV. The effects were sustained for at least 48 hours with oral hydralazine. Similar hemodynamic changes were noted during exercise in a group of 12 heart failure patients with severe MR and a mean EF of 55%. At 13-month follow-up, 44% of patients had sustained symptomatic improvement, whereas the other patients had no benefit or intolerable side effects.64 The hemodynamic improvement with hydralazine appears to be enhanced when combined with nitrates, as shown in patients studied at rest and during exercise.56
CCBs
The heterogeneous group of CCBs produces vasodilation by blocking long-acting (L-type) calcium channels in vascular smooth muscle and the myocardium. Different binding sites divide the calcium blockers into dihydropyridines (DHT) and non-dihydropyridines (non-DHT). The DHT group acts more on the vascular bed and the non-DHT group in the myocardium and conduction system. One study evaluating the use of CCB is shown in Table 3. Kelbaek and associates57 studied eight patients with moderate to severe chronic MR randomized to placebo or nifedipine. After 2 weeks of treatment, their RF decreased 11%, RV decreased 20%, and fSV increased 18% with no changes noted in the placebo group.
Combination Therapy
Rivera and colleagues65 carried out a double-blind placebo-controlled study in 12 patients with a mean age of 12.5 years, with mildly symptomatic MR from rheumatic etiology with hemodynamic repercussion (Table 3). Patients were randomized in four phases of 30 days each: digoxin, enalapril, digoxin + enalapril, and placebo. Both independent drug groups showed improved hemodynamics with greatest improvement seen in the combination group, with an improvement in LVEF from 65% to 69% (P < .01), change in LV end-systolic dimension from 38 to 36 mm (P < .01), and improvement in LV ventricular contractility measure (dP/dT) from 1042 to 1409 mm Hg/s (P < .01). No significant changes were seen in blood pressure or heart rate.
Miscellaneous
A small number of trials have shown beneficial effects with α-blockers such as prazosin,66 phosphodiesterase III inhibitors,67 and digoxin.65 Mehta and coworkers66 studied eight patients and found an increase of 33% in SV and 28% in cardiac index, with a 30% decrease in systemic vascular resistance, 8 hours after a single dose of prazosin without any long-term effects reported.
Perioperative Therapy
Though studies evaluating intraoperative or prosthetic valve regurgitation were excluded from the current review, it seems reasonable to apply the same principles of reducing preload and afterload to these clinical settings (Figure 3). Medications with the highest level of evidence (ACE inhibitors and β-blockers) are preferred as a first line of treatment, with others (nitrates, hydralazine, CCBs) held in reserve as add-on/second-line therapy. In patients with heart failure due to LV systolic dysfunction, the initiation of β-blockers should be avoided, especially in the setting of shock. ACE inhibitor use should be avoided if renal function is compromised. During the in-hospital waiting period for surgery, hemodynamic monitoring with a pulmonary artery catheter and the combination of diuresis and intravenous nitrates with or without dobutamine and/or milrinone have been shown to have beneficial effects in patients with severe congestive heart failure due to systolic dysfunction.5,68-70
In the operating room, anesthesia induction should be done carefully trying to avoid negative inotropes in patients with severe heart failure; the surgical team should be present in case of acute decompensation and inotropes such as milrinone or dobutamine used if needed.70 Reassessment of MR severity in this setting should be done with caution, as general anesthesia affects loading conditions and decreases regurgitant volume.71 It should be noted that the reduction of preload and afterload with anesthesia and vasodilator agents can worsen MR due to MVP or HOCM.
After surgery, if the EF is reduced, cardiomyopathy guideline-directed medical therapy should be initiated.72 If the EF is preserved but there is residual regurgitation not deemed feasible for reoperation, we believe that hemodynamic optimization with β-blockers and ACE inhibitors should be implemented. When there is significant paravalvular regurgitation, the use of minimally invasive options, such as placement of a vascular plug device, should be considered.73
Limitations
The data analyzed on our review have multiple limitations. The studies reviewed are mostly small studies with different MR etiologies, different patient populations, different durations of therapy, and different measurement points. However, there are no large, randomized prospective trial data. Some studies lacked control groups. In several of the studies, patients were taking other cardioactive medications and there is a lack of quantitative analysis of the MR. In many of the studies, patients had hypertension, heart failure, and reduced EF; in this setting it is difficult to differentiate whether the beneficial effects of treatment are due to improvement in LV function or a reduction in MR severity.
Conclusions
At present, there are no standardized guidelines to pharmacologically treat patients with moderate to severe primary MR. Primary MR is a structural lesion, which has traditionally been treated with surgical correction. MR is a highly prevalent progressive disorder that can lead to heart failure. Surgery is recommended in patients with primary severe MR who have symptoms, LV dysfunction, LV dilatation (LV end-systolic diameter ≥ 40 mm), or that are undergoing cardiac surgery for other indications. Moreover, when there is a high likelihood of successful and durable repair and resting pulmonary hypertension (pulmonary artery systolic pressure [PASP] > 50 mm Hg) or new onset atrial fibrillation, it is reasonable to proceed with surgery. The role of medical therapy in asymptomatic normotensive patients with preserved LV functions and dimensions has not been established. Further, there is a sizable group of patients with significant MR who do not meet surgical criteria or for whom surgery would entail high risk. This review shows that medical therapy, especially β-blockers and ACE inhibitors/ARBs, is potentially useful in primary MR by reducing afterload, improving MR, and preventing LV remodeling or causing reverse remodeling. Thus, there may be a role for medical therapy in patients with asymptomatic severe primary MR. However; it is necessary to have verification in a large-scale randomized clinical trial on the utility of medical therapy. Unfortunately, although there are promising data, it remains uncertain if medical therapy has any beneficial impact on the natural history of MR. Medical therapy could potentially delay the need for surgery, improve functional status, and delay trading one disease condition (MR) for another, namely, for mitral valve placement or repair, which have their own morbidity and complication rates. ![]()
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368:1005-1011.
- Bonow RO, Carabello BA, Chatterjee K, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e1-e142.
- Enriquez-Sarano M, Basmadjian AJ, Rossi A, et al. Progression of mitral regurgitation: a prospective Doppler echocardiographic study. J Am Coll Cardiol. 1999;34:1137-1144.
- Roberts WC, Vowels TJ, Ko JM, Hebeler RF Jr. Gross and histological features of excised portions of posterior mitral leaflet in patients having operative repair of mitral valve prolapse and comments on the concept of missing (= ruptured) chordae tendineae. J Am Coll Cardiol. 2014;63:1667-1674.
- Hamilton MA, Stevenson LW, Child JS, et al. Sustained reduction in valvular regurgitation and atrial volumes with tailored vasodilator therapy in advanced congestive heart failure secondary to dilated (ischemic or idiopathic) cardiomyopathy. Am J Cardiol. 1991;67: 259-263.
- Borer JS, Sharma A. Drug therapy for heart valve diseases. Circulation. 2015;132:1038-1045.
- Harris KM, Pastorius CA, Duval S, et al. Practice variation among cardiovascular physicians in management of patients with mitral regurgitation. Am J Cardiol. 2009;103:255-261.
- Rosenhek R, Rader F, Klaar U, et al. Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation. 2006;113:2238-2244.
- Wiggers CJ, Feely H. The cardiodynamics of mitral insufficiency. Heart Bull. 1922;149-183.
- Gorlin R, Gorlin SG. Hydraulic formula for calculation of the area of the stenotic mitral valve, other cardiac valves, and central circulatory shunts. I. Am Heart J. 1951;41:1-29.
- Wisenbaugh T, Spann JF, Carabello BA. Differences in myocardial performance and load between patients with similar amounts of chronic aortic versus chronic mitral regurgitation. J Am Coll Cardiol. 1984;3: 916-923.
- Borer JS, Hochreiter CA, Supino PG, et al. Importance of right ventricular performance measurement in selecting asymptomatic patients with mitral regurgitation for valve surgery. Adv Cardiol. 2002;39:144-152.
- Mehta RH, Supiano MA, Oral H, et al. Relation of systemic sympathetic nervous system activation to echocardiographic left ventricular size and performance and its implications in patients with mitral regurgitation. Am J Cardiol. 2000;86:1193-1197.
- Zile MR, Tomita M, Nakano K, et al. Effects of left ventricular volume overload produced by mitral regurgitation on diastolic function. Am J Physiol. 1991;261(5 Pt 2):H1471-H1480.
- Carabello BA. The relationship of left ventricular geometry and hypertrophy to left ventricular function in valvular heart disease. J Heart Valve Dis. 1995;4(suppl 2):S132-S138.
- Corin WJ, Monrad ES, Murakami T, et al. The relationship of afterload to ejection performance in chronic mitral regurgitation. Circulation. 1987;76: 59-67.
- Braunwald E, Welch GH Jr, Sarnoff SJ. Hemodynamic effects of quantitatively varied experimental mitral regurgitation. Circ Res. 1957;5:539-545.
- Jose AD, Bernstein L, Taylor RR. The influence of arterial pressure on mitral incompetence in man. J Clin Invest. 1964;43:2094-2103.
- Kowalski SE. Mitral valve prolapse. Can Anaesth Soc J. 1985;32:138-141.
- Dillon AR, Dell’Italia LJ, Tillson M, et al. Left ventricular remodeling in preclinical experimental mitral regurgitation of dogs. J Vet Cardiol. 2012;14:73-92.
- Tsutsui H, Spinale FG, Nagatsu M, et al. Effects of chronic beta-adrenergic blockade on the left ventricular and cardiocyte abnormalities of chronic canine mitral regurgitation. J Clin Invest. 1994;93:2639-2648.
- Nemoto S, Hamawaki M, De Freitas G, Carabello BA. Differential effects of the angiotensin-converting enzyme inhibitor lisinopril versus the beta-adrenergic receptor blocker atenolol on hemodynamics and left ventricular contractile function in experimental mitral regurgitation. J Am Coll Cardiol. 2002;40:149-154.
- Pat B, Killingsworth C, Denney T, et al. Dissociation between cardiomyocyte function and remodeling with beta-adrenergic receptor blockade in isolated canine mitral regurgitation. Am J Physiol Heart Circ Physiol. 2008;295:H2321-H2327.
- Nagatsu M, Spinale FG, Koide M, et al. Bradycardia and the role of beta-blockade in the amelioration of left ventricular dysfunction. Circulation. 2000;101:653-659.
- Hankes GH, Ardell JL, Tallaj J, et al. Beta1-adrenoceptor blockade mitigates excessive norepinephrine release into cardiac interstitium in mitral regurgitation in dog. Am J Physiol Heart Circ Physiol. 2006;291: H147-H151.
- Dell’italia LJ, Balcells E, Meng QC, et al. Volume-overload cardiac hypertrophy is unaffected by ACE inhibitor treatment in dogs. Am J Physiol. 1997;273: H961-H970.
- Tallaj J, Wei CC, Hankes GH, et al. Beta1-adrenergic receptor blockade attenuates angiotensin II-mediated catecholamine release into the cardiac interstitium in mitral regurgitation. Circulation. 2003;108:225-230.
- Nagatsu M, Zile MR, Tsutsui H, et al. Native beta-adrenergic support for left ventricular dysfunction in experimental mitral regurgitation normalizes indexes of pump and contractile function. Circulation. 1994;89:818-826.
- Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation. 1998;98:1329-1334.
- Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215-262.
- Swedberg K, Hjalmarson A, Waagstein F, Wallentin I. Prolongation of survival in congestive cardiomyopathy by beta-receptor blockade. Lancet. 1979;1:1374-1376.
- Foody JM, Farrell MH, Krumholz HM. Beta-blocker therapy in heart failure: scientific review. JAMA. 2002;287:883-889.
- Ahmed MI, Aban I, Lloyd SG, et al. A randomized controlled phase IIb trial of beta(1)-receptor blockade for chronic degenerative mitral regurgitation. J Am Coll Cardiol. 2012;60:833-838.
- Stewart RA, Raffel OC, Kerr AJ, et al. Pilot study to assess the influence of beta-blockade on mitral regurgitant volume and left ventricular work in degenerative mitral valve disease. Circulation. 2008;118:1041-1046.
- Varadarajan P, Joshi N, Appel D, et al. Effect of Beta-blocker therapy on survival in patients with severe mitral regurgitation and normal left ventricular ejection fraction. Am J Cardiol. 2008;102:611-615.
- Oh SH, Meyers DG. Afterload reduction may halt and beta-adrenergic blockade may worsen progression of left ventricular dysfunction in patients with chronic compensated mitral regurgitation: a retrospective cohort study. Angiology. 2007;58:196-202.
- Sarnoff SJ, Braunwald E, Welch GH Jr, et al. Hemodynamic determinants of oxygen consumption of the heart with special reference to the tension-time index. Am J Physiol. 1958;192:148-156.
- Reiken S, Wehrens XH, Vest JA, et al. Beta-blockers restore calcium release channel function and improve cardiac muscle performance in human heart failure. Circulation. 2003;107:2459-2466.
- Spoladore R, Fragasso G, Perseghin G, et al. Beneficial effects of beta-blockers on left ventricular function and cellular energy reserve in patients with heart failure. Fundam Clin Pharmacol. 2013;27:455-464.
- Hall SA, Cigarroa CG, Marcoux L, et al. Time course of improvement in left ventricular function, mass and geometry in patients with congestive heart failure treated with beta-adrenergic blockade. J Am Coll Cardiol. 1995;25:1154-1161.
- Tom B, Dendorfer A, de Vries R, et al. Bradykinin potentiation by ACE inhibitors: a matter of metabolism. Br J Pharmacol. 2002;137:276-284.
- Brown NJ, Vaughan DE. Angiotensin-converting enzyme inhibitors. Circulation. 1998;97:1411-1420.
- Marcotte F, Honos GN, Walling AD, et al. Effect of angiotensin-converting enzyme inhibitor therapy in mitral regurgitation with normal left ventricular function. Can J Cardiol. 1997;13:479-485.
- Tischler MD, Rowan M, LeWinter MM. Effect of enalapril therapy on left ventricular mass and volumes in asymptomatic chronic, severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol. 1998;82:242-245.
- Sampaio RO, Grinberg M, Leite JJ, et al. Effect of enalapril on left ventricular diameters and exercise capacity in asymptomatic or mildly symptomatic patients with regurgitation secondary to mitral valve prolapse or rheumatic heart disease. Am J Cardiol. 2005;96:117-121.
- Tunaoğlu FS, Olguntürk R, Kula S, Oğuz D. Effective regurgitant orifice area of rheumatic mitral insufficiency: response to angiotensin converting enzyme inhibitor treatment. Anadolu Kardiyol Derg. 2004;4:3-7.
- Gupta DK, Kapoor A, Garg N, et al. Beneficial effects of nicorandil versus enalapril in chronic rheumatic severe mitral regurgitation: six months follow up echocardiographic study. J Heart Valve Dis. 2001;10:158-165.
- Wisenbaugh T, Sinovich V, Dullabh A, Sareli P. Six month pilot study of captopril for mildly symptomatic, severe isolated mitral and isolated aortic regurgitation. J Heart Valve Dis. 1994;3:197-204.
- Malev E, Zemtsovsky E, Omelchenko M, Vasina L. Effects of renin-angiotensin system blockade in chronic degenerative mitral regurgitation. Eur Heart J Cardiovasc Imaging. 2014;15:(ii167).
- Geirsson A, Singh M, Ali R, et al. Modulation of transforming growth factor-beta signaling and extracellular matrix production in myxomatous mitral valves by angiotensin II receptor blockers. Circulation. 2012;126:S189-S197.
- Strauss CE, Duval S, Pastorius D, Harris KM. Pharmacotherapy in the treatment of mitral regurgitation: a systematic review. J Heart Valve Dis. 2012;21:275-285.
- Borgenhagen DM, Serur JR, Gorlin R, et al. The effects of left ventricular load and contractility on mitral regurgitant orifice size and flow in the dog. Circulation. 1977;56:106-113.
- Yoran C, Yellin EL, Becker RM, et al. Mechanism of reduction of mitral regurgitation with vasodilator therapy. Am J Cardiol. 1979;43:773-777.
- Chatterjee K, Parmley WW, Swan HJ, et al. Beneficial effects of vasodilator agents in severe mitral regurgitation due to dysfunction of subvalvar apparatus. Circulation. 1973;48:684-690.
- Weiland DS, Konstam MA, Salem DN, et al. Contribution of reduced mitral regurgitant volume to vasodilator effect in severe left ventricular failure secondary to coronary artery disease or idiopathic dilated cardiomyopathy. Am J Cardiol. 1986;58:1046-1050.
- Roth A, Shotan A, Elkayam U. A randomized comparison between the hemodynamic effects of hydralazine and nitroglycerin alone and in combination at rest and during isometric exercise in patients with chronic mitral regurgitation. Am Heart J. 1993;125:155-163.
- Kelbaek H, Aldershvile J, Skagen K, et al. Pre- and afterload reduction in chronic mitral regurgitation: a double-blind randomized placebo-controlled trial of the acute and 2 weeks’ effect of nifedipine or isosorbide dinitrate treatment on left ventricular function and the severity of mitral regurgitation. Br J Clin Pharmacol. 1996;41:493-497.
- Jeang MK, Petrovich LJ, Adyanthaya AV, Alexander J. Effects of isosorbide dinitrate on rheumatic and non-rheumatic mitral regurgitation. Tex Heart Inst J. 1986;13:453-457.
- Goodman DJ, Rossen RM, Holloway EL, et al. Effect of nitroprusside on left ventricular dynamics in mitral regurgitation. Circulation. 1974;50:1025-1032.
- Grayburn PA. Vasodilator therapy for chronic aortic and mitral regurgitation. Am J Med Sci. 2000;320:202-208.
- Ibrahim M, Rao C, Ashrafian H, et al. Modern management of systolic anterior motion of the mitral valve. Eur J Cardiothorac Surg. 2012;41:1260-1270.
- Shepherd AM, Irvine NA. Differential hemodynamic and sympathoadrenal effects of sodium nitroprusside and hydralazine in hypertensive subjects. J Cardiovasc Pharmacol. 1986;8:527-533.
- Greenberg BH, Massie BM, Brundage BH, et al. Beneficial effects of hydralazine in severe mitral regurgitation. Circulation. 1978;58:273-279.
- Greenberg BH, DeMots H, Murphy E, Rahimtoola SH. Arterial dilators in mitral regurgitation: effects on rest and exercise hemodynamics and long-term clinical follow-up. Circulation. 1982;65:181-187.
- Rivera IR, Moisés VA, Carvalho AC, de Paola AA. Mildly symptomatic chronic mitral regurgitation. Analysis of left ventricular systolic function and mitral regurgitation fraction under pharmacological influence. Echocardiographic study. Arq Bras Cardiol. 2003;80:144-149, 138-143.
- Mehta J, Feldman RL, Nichols WW, et al. Acute haemodynamic effects of oral prazosin in severe mitral regurgitation. Br Heart J. 1980;43:556-560.
- Hachenberg T, Möllhoff T, Holst D, et al. Cardiopulmonary effects of enoximone or dobutamine and nitroglycerin on mitral valve regurgitation and pulmonary venous hypertension. J Cardiothorac Vasc Anesth. 1997;11:453-457.
- Capomolla S, Pozzoli M, Opasich C, et al. Dobutamine and nitroprusside infusion in patients with severe congestive heart failure: hemodynamic improvement by discordant effects on mitral regurgitation, left atrial function, and ventricular function. Am Heart J. 1997;134:1089-1098.
- Stevenson LW, Bellil D, Grover-McKay M, et al. Effects of afterload reduction (diuretics and vasodilators) on left ventricular volume and mitral regurgitation in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1987;60:654-658.
- Looney Y, Quinton P. Mitral valve surgery. Contin Educ Anaesth Crit Care Pain. 2005;5:199-202.
- Grewal KS, Malkowski MJ, Piracha AR, et al. Effect of general anesthesia on the severity of mitral regurgitation by transesophageal echocardiography. Am J Cardiol. 2000;85:199-203.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62: e147-e239.
- Rihal CS, Sorajja P, Booker JD, et al. Principles of percutaneous paravalvular leak closure. JACC Cardiovasc Interv. 2012;5:121-130.
- Dujardin KS, Enriquez-Sarano M, Bailey KR, et al. Effect of losartan on degree of mitral regurgitation quantified by echocardiography. Am J Cardiol. 2001;87:570-576.
- Harris KM, Aeppli DM, Carey CF. Effects of angiotensin-converting enzyme inhibition on mitral regurgitation severity, left ventricular size, and functional capacity. Am Heart J. 2005;150:1106.
- Høst U, Kelbaek H, Hildebrandt P, et al. Effect of ramipril on mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol. 1997;80:655-658.
- Knirsch W, Tlach L, Stambach D, Bauersfeld U. Angiotensin-converting enzyme inhibitors in pediatric patients with mitral valve regurgitation-case-control study and review of the literature. Congenital Heart Disease. 2010;5:278-284.
- Sekuri C, Utuk O, Bayturan O, et al. Effect of losartan on exercise tolerance and echocardiographic parameters in patients with mitral regurgitation. J Renin Angiotensin Aldosterone Syst. 2008;9:107-111.
- Wong GC, Marcotte F, Rudski LG. Impact of chronic lisinopril therapy on left atrial volume versus dimension in chronic organic mitral regurgitation. Can J Cardiol. 2006;22:125-129.
- Mirabel M, Iung B, Baron G, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. 2007;28:1358-1365.