A Review of the Clinical Subgroup Analyses From the RE-LY Trial
Rohit Kumar, MD,1 Aref M. Rahman, MD,2 Brian L. Henry, MD, PhD2
1John H. Stroger, Jr. Hospital of Cook County, Department of Internal Medicine, Chicago, IL; 2University of Pittsburgh Medical Center, Heart and Vascular Institute, and Pittsburgh Veterans Administration Medical Center, Department of Cardiology, Pittsburgh, PA
Dabigatran was the first direct-acting oral anticoagulant approved by the US Food and Drug Administration for prevention of stroke and systemic embolism in people with atrial fibrillation, based on data from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Over 18,000 patients with nonvalvular atrial fibrillation and a moderate-to-high risk of thromboembolic stroke were randomized to warfarin or dabigatran. With respect to the primary endpoints for efficacy and safety, dabigatran was superior to warfarin in the prevention of stroke and thromboembolism and noninferior with respect to major bleeding. Although unified by a common arrhythmia and a similar thromboembolic stroke risk, this large patient population is also significantly heterogeneous with respect to other demographics and comorbidities that raise important questions about the efficacy and safety of dabigatran in specific patient populations. Furthermore, there were significant differences between the warfarin and dabigatran groups with respect to several important secondary endpoints. Understanding the differences in outcomes between specific patient subgroups from the RE-LY trial can better inform the practicing clinician’s ability to offer the best anticoagulation options to individual patients.
[Rev Cardiovasc Med. 2016;17(1/2):40-48 doi: 10.3909/ricm0805]
© 2016 MedReviews®, LLC
A Review of the Clinical Subgroup Analyses From the RE-LY Trial
Rohit Kumar, MD,1 Aref M. Rahman, MD,2 Brian L. Henry, MD, PhD2
1John H. Stroger, Jr. Hospital of Cook County, Department of Internal Medicine, Chicago, IL; 2University of Pittsburgh Medical Center, Heart and Vascular Institute, and Pittsburgh Veterans Administration Medical Center, Department of Cardiology, Pittsburgh, PA
Dabigatran was the first direct-acting oral anticoagulant approved by the US Food and Drug Administration for prevention of stroke and systemic embolism in people with atrial fibrillation, based on data from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Over 18,000 patients with nonvalvular atrial fibrillation and a moderate-to-high risk of thromboembolic stroke were randomized to warfarin or dabigatran. With respect to the primary endpoints for efficacy and safety, dabigatran was superior to warfarin in the prevention of stroke and thromboembolism and noninferior with respect to major bleeding. Although unified by a common arrhythmia and a similar thromboembolic stroke risk, this large patient population is also significantly heterogeneous with respect to other demographics and comorbidities that raise important questions about the efficacy and safety of dabigatran in specific patient populations. Furthermore, there were significant differences between the warfarin and dabigatran groups with respect to several important secondary endpoints. Understanding the differences in outcomes between specific patient subgroups from the RE-LY trial can better inform the practicing clinician’s ability to offer the best anticoagulation options to individual patients.
[Rev Cardiovasc Med. 2016;17(1/2):40-48 doi: 10.3909/ricm0805]
© 2016 MedReviews®, LLC
A Review of the Clinical Subgroup Analyses From the RE-LY Trial
Rohit Kumar, MD,1 Aref M. Rahman, MD,2 Brian L. Henry, MD, PhD2
1John H. Stroger, Jr. Hospital of Cook County, Department of Internal Medicine, Chicago, IL; 2University of Pittsburgh Medical Center, Heart and Vascular Institute, and Pittsburgh Veterans Administration Medical Center, Department of Cardiology, Pittsburgh, PA
Dabigatran was the first direct-acting oral anticoagulant approved by the US Food and Drug Administration for prevention of stroke and systemic embolism in people with atrial fibrillation, based on data from the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Over 18,000 patients with nonvalvular atrial fibrillation and a moderate-to-high risk of thromboembolic stroke were randomized to warfarin or dabigatran. With respect to the primary endpoints for efficacy and safety, dabigatran was superior to warfarin in the prevention of stroke and thromboembolism and noninferior with respect to major bleeding. Although unified by a common arrhythmia and a similar thromboembolic stroke risk, this large patient population is also significantly heterogeneous with respect to other demographics and comorbidities that raise important questions about the efficacy and safety of dabigatran in specific patient populations. Furthermore, there were significant differences between the warfarin and dabigatran groups with respect to several important secondary endpoints. Understanding the differences in outcomes between specific patient subgroups from the RE-LY trial can better inform the practicing clinician’s ability to offer the best anticoagulation options to individual patients.
[Rev Cardiovasc Med. 2016;17(1/2):40-48 doi: 10.3909/ricm0805]
© 2016 MedReviews®, LLC
KEY WORDS
Dabigatran • Atrial fibrillation • Stroke • Bleeding
KEY WORDS
Dabigatran • Atrial fibrillation • Stroke • Bleeding

… dabigatran may offer a mortality benefit over warfarin.
The RE-LY trial represents the first study showing a drug to be superior to warfarin without an increased risk of bleeding.
When the risk of bleeding was stratified on basis of age, dabigatran, 150 mg was associated with lower risk of major bleeding for patients age < 75 when compared with warfarin…
… dabigatran is a reasonable alternative to warfarin in patients requiring cardioversion.
Dabigatran, 150 mg, was superior to warfarin for stroke prevention in all types of AF…
Major GI bleeding events were higher with dabigatran compared with warfarin… in the RE-LY trial, but fatal GI bleeding events were similar between treatment groups…
Main Points
• Dabigatran was the first direct-acting oral anticoagulant approved by the US Food and Drug Administration for prevention of stroke and systemic embolism in people with atrial fibrillation (AF). It was developed as an alternative to warfarin but, unlike warfarin, it does not require laboratory monitoring or dose adjustments because of its predictable pharmacokinetic and pharmacodynamic profile. As the only approved oral direct thrombin inhibitor, it has a mechanism of action that is distinct from all other clinically used anticoagulants.
• The Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial was a randomized study designed to compare two fixed doses of dabigatran versus open-label use of warfarin in patients who had AF and were at increased risk for stroke. The primary outcome of stroke or systemic embolism occurred at a rate of 1.11% per year in patients receiving 150 mg of dabigatran compared with a rate of 1.69% per year in patients receiving warfarin; therefore, the 150-mg dose of dabigatran was determined to be superior to warfarin for preventing the primary endpoint by reducing the annual relative risk of stroke by 34%.
• Increasing age is a nonmodifiable risk factor for both the development of AF and thromboembolism. When the risk of bleeding was stratified on basis of age, dabigatran, 150 mg, was associated with lower risk of major bleeding for patients age < 75 when compared with warfarin.
• Intracerebral hemorrhage is the most feared complication of anticoagulant therapy. When compared with warfarin, patients treated with dabigatran, 150 mg, had fewer fatal and traumatic intracranial hemorrhages. However, there was a significantly higher rate of major gastrointestinal bleeding with dabigatran than with warfarin.
Main Points
• Dabigatran was the first direct-acting oral anticoagulant approved by the US Food and Drug Administration for prevention of stroke and systemic embolism in people with atrial fibrillation (AF). It was developed as an alternative to warfarin but, unlike warfarin, it does not require laboratory monitoring or dose adjustments because of its predictable pharmacokinetic and pharmacodynamic profile. As the only approved oral direct thrombin inhibitor, it has a mechanism of action that is distinct from all other clinically used anticoagulants.
• The Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial was a randomized study designed to compare two fixed doses of dabigatran versus open-label use of warfarin in patients who had AF and were at increased risk for stroke. The primary outcome of stroke or systemic embolism occurred at a rate of 1.11% per year in patients receiving 150 mg of dabigatran compared with a rate of 1.69% per year in patients receiving warfarin; therefore, the 150-mg dose of dabigatran was determined to be superior to warfarin for preventing the primary endpoint by reducing the annual relative risk of stroke by 34%.
• Increasing age is a nonmodifiable risk factor for both the development of AF and thromboembolism. When the risk of bleeding was stratified on basis of age, dabigatran, 150 mg, was associated with lower risk of major bleeding for patients age < 75 when compared with warfarin.
• Intracerebral hemorrhage is the most feared complication of anticoagulant therapy. When compared with warfarin, patients treated with dabigatran, 150 mg, had fewer fatal and traumatic intracranial hemorrhages. However, there was a significantly higher rate of major gastrointestinal bleeding with dabigatran than with warfarin.
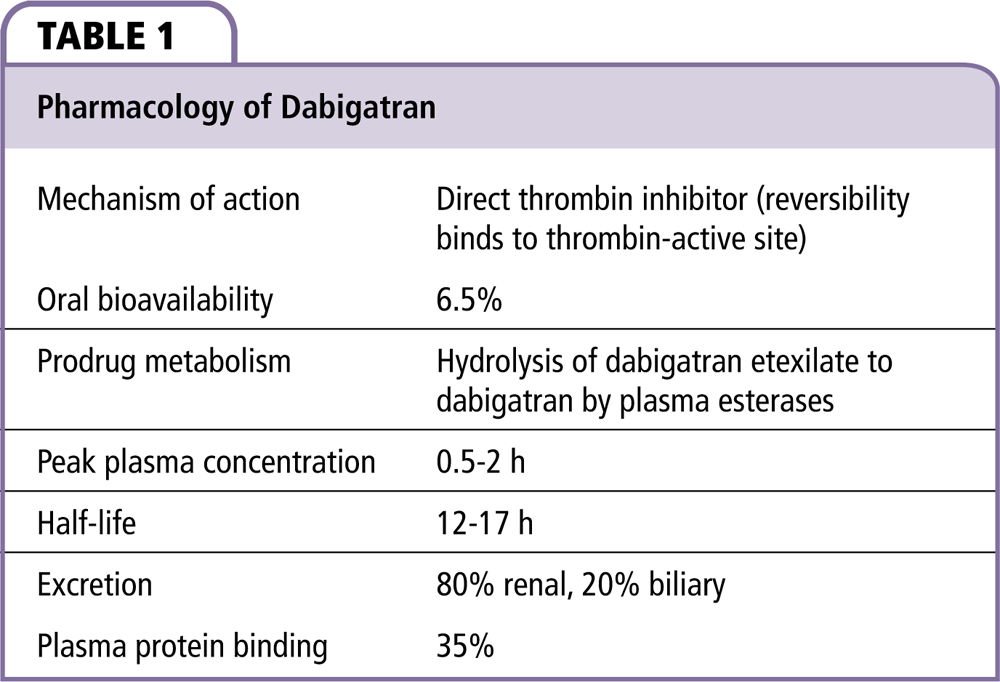
Dabigatran etexilate is a nonpeptide, oral prodrug that is hydrolyzed by plasma esterases to form the competitive direct thrombin inhibitor dabigatran (Table 1).1 Dabigatran is approved to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation (AF), for the treatment of deep venous thrombosis and pulmonary embolism, and to reduce the risk of recurrence of deep venous thrombosis and pulmonary embolism in patients who have been previously treated. After oral administration, dabigatran reaches peak plasma concentration within 0.5 to 2 hours.2 The half-life of dabigatran is 12 to 17 hours, assuming a creatinine clearance (CrCl) of > 60 mL/min2; 35% is protein bound and 80% of the drug is eliminated by the kidneys.2,3 Dabigatran is not recommended for use in dialysis patients or patients with a CrCl < 15 mL/min. Dabigatran was developed as an alternative to warfarin but, unlike warfarin, it does not require laboratory monitoring or dose adjustments because of its predictable pharmacokinetic and pharmacodynamic profile. As the only approved oral direct thrombin inhibitor, it has a mechanism of action that is distinct from all other clinically used anticoagulants.
The RE-LY Trial
The Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial was a randomized study designed to compare two fixed doses of dabigatran versus open-label use of warfarin in patients who had AF and were at increased risk for stroke.4 Patients were randomly assigned dabigatran, 110 mg twice daily; dabigatran, 150 mg twice daily; or warfarin. The dabigatran dose was blinded but the use of warfarin was unblinded. Concomitant use of aspirin (< 100 mg/d) or other antiplatelet agents was permitted. Of note, the 110-mg twice-daily dose is not approved for use in the United States and is not discussed in this review.
To be enrolled in RE-LY, patients had to have nonvalvular AF and at least one risk factor for increased stroke (inclusion criteria closely resemble CHADS2 [congestive heart failure, hypertension, age 75 years, diabetes, stroke] criteria). The exclusion criteria predominantly focus on omitting subjects with comorbidities that increase the patient’s risk of bleeding. Valvular AF, an exclusion criterion, was defined as a prosthetic valve or hemodynamically relevant valve disease.5 More than 18,000 patients were enrolled in the RE-LY trial with a mean age of 71 and a mean CHADS2 score of 2.1.5
The primary outcome of stroke or systemic embolism occurred at a rate of 1.11% per year in patients receiving 150 mg of dabigatran compared with a rate of 1.69% per year in patients receiving warfarin.5 Therefore, the 150-mg dose of dabigatran was determined to be superior to warfarin for preventing the primary endpoint by reducing the annual relative risk (RR) of stroke by 34% (RR 0.66; P < .001). Dabigatran reduced the annual RR of hemorrhagic stroke by 74%; the rates of hemorrhagic stroke were 0.38% per year in the warfarin group compared with 0.10% per year in the group that received 150 mg of dabigatran (RR 0.26; P < .001). The rate of myocardial infarction (MI) was higher in the dabigatran group, at 0.74% per year (RR 1.38; P = .048) compared with 0.53% per year with warfarin. The rates of death from any cause were 4.13% per year with warfarin and 3.64% per year with dabigatran (RR 0.88; P = .051). Although this outcome just missed statistical significance, this strongly suggests that dabigatran may offer a mortality benefit over warfarin.
There was no statistical difference in the rate of major bleeding between warfarin and dabigatran.5 The warfarin group had a 3.36% per year rate of major bleeding as compared with 3.11% per year in the dabigatran group (P = .31). However, subanalyses revealed differences between the types of bleeding. The warfarin group had a statistically higher annual rate of life-threatening bleeding, intracranial bleeding, and major or minor bleeding (1.80%, 0.74%, and 18.15%, respectively) than did patients in the dabigatran group (1.45%, 0.30%, and 16.42%, respectively; all P < .05). Conversely, there was a significantly higher rate of major gastrointestinal (GI) bleeding with dabigatran than with warfarin (1.51% vs 1.02%; P = .007). The RE-LY authors created the “net clinical benefit outcome,” which consisted of major vascular events, major bleeding, and death. The rates of this combined outcome were 7.64% per year with warfarin and 6.91% per year with dabigatran (P = .04). The only adverse medication side effect that was significantly more common with dabigatran than with warfarin was dyspepsia, which occurred in 11.3% of the 150-mg dabigatran group and 5.8% of the warfarin group (P < .001).
The 150-mg dose of dabigatran was associated with lower rates of stroke and systemic embolism and had a similar rate of major hemorrhage compared with warfarin. The RE-LY trial represents the first study showing a drug to be superior to warfarin without an increased risk of bleeding. Previous trials showed that the combination of clopidogrel and aspirin was more effective than aspirin alone but less effective than warfarin.6,7 Another trial demonstrated that subcutaneous idraparinux was more effective than warfarin but was associated with a substantially higher risk of bleeding.8 Since the publication of the RE-LY trial results in 2009, numerous subgroup analyses have been performed on the data to better understand the findings within the RE-LY trial and to better inform clinicians about the risks and benefits of dabigatran relative to warfarin within specific patient populations. This review summarizes and explains some of the clinically important RE-LY subgroup analyses performed to date.
Heart Failure
Heart failure (HF) is a common concomitant illness in patients with AF and increases a patient’s stroke risk. Ferreira and colleagues9 performed a subgroup analysis in RE-LY trial patients with AF and symptomatic HF. Among the 18,113 patients enrolled in the RE-LY trial, 27% (n = 4904) of patients had a history of HF. HF was defined as symptomatic HF (New York Heart Association [NYHA] class II or worse) in the 6 months prior to pretrial screening in patients with history of previous admission for congestive HF. Among this group of HF patients, most were in NYHA class II (74.4%, n = 3645), whereas 23.2% were in NYHA class III (n = 1140), and only 119 patients were in NYHA class IV. The annual rate of stroke or systemic embolism for patients with HF was 1.44% for the 150-mg group compared with 1.92% in the warfarin group, which did not meet statistical significance for superiority (hazard ratio [HR] 0.75; 95% confidence interval [CI], 0.51-1.10). The incidence of major bleeding in patients with HF was 3.10% per year in the 150-mg group compared with 3.9% in the warfarin group, which was not significantly different (HR 0.79; 95% CI, 0.60-1.03). In the same subset of patients, intracranial bleeding occurred more often in the warfarin group (0.65% per year) compared with patients receiving dabigatran, 150 mg, which was 0.26% per year (HR 0.39; 95% CI, 0.17-0.89). The annual rate of vascular death was 4.81% in patients receiving warfarin compared with 4.41% (HR 0.92; 95% CI, 0.73-1.16) in patients on dabigatran, 150 mg, which was not significant. There was no significant effect on the primary efficacy or safety outcomes of dabigatran with respect to reduced or preserved left ventricular ejection fraction or NYHA classification.
Although the annual rate of stroke was numerically higher across all treatment groups in the patient cohort with HF compared with the patient cohort without HF (1.75% vs 1.35% per year), after multivariable adjustment, the difference was not statistically significant (P = .46). This result is counterintuitive given the known association between HF and an increased risk of stroke in patients with AF.10 In this subgroup analysis, the finding that HF was not significantly associated with higher incidence of stroke is probably related to the fact that the RE-LY trial patients with HF were more likely to be younger and less likely to have a history of hypertension or prior stroke. Also, the majority (74.3%) of HF patients were in NYHA class II, which may have further added to the above findings. In patients with NYHA class III/IV HF, there was a higher incidence of stroke (2.34% vs 1.55% per year; P = .012) compared with patients in NYHA class II, which is an expected finding. The authors concluded that the unusual association of HF with the above clinical characteristics might have been due to a selection bias as RE-LY included 5775 patients with only one risk factor for stroke (3396 with hypertension, 1044 with age ≥ 75 years, and 721 with HF).9 HF was not significantly associated with major bleeding (P = .53) or intracranial hemorrhage (ICH; P = .10) on multivariable analysis. However, within the HF patient cohort, patients in NYHA class III/IV had a higher incidence of major bleeding (4.17% vs 3.17% per year; P = .02) compared with those in NYHA class II.
Age
Increasing age is a nonmodifiable risk factor for both the development of AF and thromboembolism.11 The importance of age with respect to stroke risk in AF has been further elevated by the widespread adoption of the CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 y, diabetes, stroke, vascular disease, age 65-74 y, female sex) score, which places greater emphasis on age, especially age ≥ 75, when compared with the older CHADS2 score.12,13 Given the importance of age in AF and stroke, Eikelboom and colleagues14 evaluated the risk of bleeding in patients enrolled in the RE-LY trial with respect to age. In the study cohort, 40% of patients (n = 7258) were age ≥ 75. When the risk of bleeding was stratified on basis of age, dabigatran, 150 mg, was associated with lower risk of major bleeding for patients age < 75 when compared with warfarin (2.12% vs 3.04% per year; P < .001), but was also associated with a trend toward higher risk of major bleeding in those age ≥ 75 years (5.10% vs 4.37% per year; P = 0.07). The larger increase in the rate of major bleeding with respect to increasing age in the dabigatran group compared with the warfarin group (2.12% up to 5.1% vs 3.04% up to 4.37%) was attributed to the normal age-related decline in renal function. These results also show that the risk of bleeding was greater for patients age ≥ 75 compared with younger patients (P < .001). The efficacy of dabigatran for prevention of stroke compared with warfarin was consistent irrespective of the patient’s age group (RR 0.63; 95% CI, 0.46-0.86 for age < 75 and RR 0.67; 95% CI, 0.49-0.90 for age ≥ 75). The incidence rates of intracranial bleeding and life-threatening bleeding were lower for dabigatran compared with warfarin irrespective of age group. GI bleeding events were higher in the dabigatran group compared with the warfarin group and increased with increasing age (1.22% and 2.80% per year in patient age group < 75 and ≥ 75 years, respectively). Dabigatran has a low oral bioavailability (7.2%) and it is predominantly excreted in the feces.15 The authors hypothesized that the increased risk of GI bleeding could be due to the local effect of dabigatran on the microvasculature within the lumen of the GI tract. Because prevalence of GI pathology, such as angiodysplasia and diverticulosis increases with age, they felt that the bleeding risk could be expected to increase accordingly.
Myocardial Infarction
In the RE-LY trial, the rate of MI was 0.53% per year with warfarin and was higher in the 150-mg dabigatran group (0.74% per year, RR 1.38; P = .048).5 Although the difference was small, it did reach statistical significance. The RE-LY authors did speculate about the potential reasons for this finding including the concept that warfarin provides better protection against coronary ischemic events than dabigatran, as warfarin is known to reduce the risk of MI.16 However, they concluded that “the explanation for this finding is therefore uncertain.”5 Hohnloser and associates17 used data from the RE-LY study to determine the rates of MI, unstable angina, cardiac arrest, and cardiac death in dabigatran versus warfarin. The original RE-LY study first reported in 2009 only included clinical MI data, because data on silent MI and other clinical events typically related to myocardial ischemia had not been centrally reported or adjudicated.17 However, these data were available to Hohnloser and associates.17 Therefore, they were able to perform a more detailed analysis of the RE-LY data and better understand the effects of dabigatran on myocardial ischemic events relative to warfarin. They found that MI occurred at annual rates of 0.81% with twice-daily dabigatran, 150 mg, compared with 0.64% with warfarin (HR 1.27; P = .12). The addition of the “silent MI” data, which were not present in the original RE-LY manuscript, eliminated the statistically significant difference between dabigatran and warfarin with respect to MI, but the trend toward increased risk of MI remained. When the composite endpoint of MI, unstable angina, cardiac arrest, and cardiac death was analyzed, the annual rates were 3.33% per year with dabigatran, 150 mg, and 3.41% per year with warfarin (HR 0.98; P = .77). The authors felt that this composite endpoint represented an aggregate of clinical events typically related to myocardial ischemia, which is more inclusive and representative than the clinical MI data presented in the original reporting of the RE-LY data. Hohnloser and associates17 also compared the risk of myocardial ischemic events in high-risk patients (those with prior MI or coronary artery disease) taking warfarin or dabigatran. There was no increased risk of MI or other ischemic cardiac events in high-risk patients taking dabigatran compared with warfarin.
Cardioversion
Anticoagulation to reduce the risk of stroke is only part of the overall management strategy of patients with AF. Clinicians must also decide how to manage the arrhythmia itself. Many patients, especially those with new-onset or recurrent paroxysmal AF, undergo electrical or chemical cardioversion to restore sinus rhythm. Cardioversion in patients with AF is associated with an increased risk of thromboembolic events.18 Because of this risk, current guidelines recommend that patients remain on anticoagulation for 4 weeks after cardioversion therapy.19 These guidelines predate the approval of dabigatran and, therefore, raise uncertainty about the efficacy of dabigatran for preventing postcardioversion thromboembolism relative to warfarin therapy. Patients in the RE-LY trial were permitted to undergo cardioversion.20 There were 672 cardioversions performed in the dabigatran 150-mg group and 664 cardioversions performed in the warfarin group. The 30-day thromboembolic rates were 0.3% and 0.6% for dabigatran, 150 mg, versus warfarin (P = .40) and the major bleeding rates in both groups were 0.6%. Transesophageal echocardiography (TEE) prior to cardioversion was performed on almost twice as many patients in the dabigatran 150-mg group (24.1%) compared with the warfarin-treated group (13.3%). The rate of left atrial thrombi detected by TEE was 1.2% for the dabigatran 150-mg group and 1.1% for the warfarin treated group. The increased utilization of TEE prior to cardioversion was thought to be due to investigator preference, which is reasonable given that dabigatran was an experimental therapy that was not yet proven efficacious. Because the RE-LY trial was open label with respect to warfarin versus dabigatran, individual investigators were aware that the patient was on dabigatran and were only blinded to dosage.5 This study by Nagarakanti and coworkers20 was the first to evaluate a novel oral anticoagulant in the setting of cardioversion. The frequencies of stroke and major bleeding within 30 days of cardioversion were low in both groups, and comparable. The study authors concluded that dabigatran is a reasonable alternative to warfarin in patients requiring cardioversion. For patients not on anticoagulation, dabigatran offers the advantage of reaching peak anticoagulant effect within 2 hours of administration and does not require concurrent administration of heparin.
Concomitant Antiplatelet Use
Many patients with AF also have indications for concomitant use of antiplatelet agents. Given the increased risk of bleeding for patients on anticoagulation and antiplatelet agents, understanding these risks is important for clinicians so that they can determine the risks and benefits of concomitant anticoagulation and antiplatelet therapy in their patients. The use of antiplatelet agents was allowed in the RE-LY trial at the discretion of the supervising physician. Furthermore, the RE-LY trial was the only novel oral anticoagulant trial that allowed the use of dual antiplatelet therapy (DAPT).21 In a study by Dans and colleagues,22 38.4% (n = 6952) of patients were on antiplatelet drugs at some point during the RE-LY study period; 32% of patients were on aspirin monotherapy, 1.9% patients were on clopidogrel monotherapy, and 4.5% patients received DAPT. In patients not taking antiplatelet agents, dabigatran, 150 mg, reduced the primary outcome of stroke and systemic embolism compared with warfarin (HR 0.52; 95% CI, 0.38-0.72). However, this effect was attenuated among patients who used antiplatelets (HR 0.80; 95% CI, 0.59-1.08, P for interaction = .058). Major bleeding was similar between dabigatran and warfarin in both subgroups of patients. However, with respect to ICH, dabigatran, 150 mg, was superior to warfarin regardless of antiplatelet treatment (HR 0.47; 95% CI, 0.28-0.80) or not (HR 0.36; 95% CI, 0.21-0.63; P for interaction = .526). Not surprisingly, patients on single antiplatelet therapy were at higher risk of major bleeding (HR 1.60; 95% CI, 1.42-1.82) compared with no antiplatelet therapy, and DAPT was associated with an even higher risk (HR 2.31; 95% CI, 1.79-2.98; P < .001 for all comparisons). The study authors concluded that, in patients with AF, concomitant antiplatelet therapy has little effect on the relative advantages of dabigatran in comparison with warfarin. Concomitant use of aspirin or clopidogrel appeared to increase the risks for bleeding to a similar extent in all treatment arms of RE-LY, and more so when two antiplatelet agents were used together.
Dabigatran and DAPT were investigated in greater detail in the Randomised Dabigatran Etexilate Dose Finding Study in Patients With Acute Coronary Syndromes Post Index Event With Additional Risk Factors for Cardiovascular Complications Also Receiving Aspirin and Clopidogrel (RE-DEEM) trial.23 Specifically, the RE-DEEM investigators evaluated the safety and efficacy of dabigatran in patients with a recent history of acute coronary syndrome who were also being treated with DAPT (aspirin and clopidogrel). The RE-DEEM study was a double-blind, placebo-controlled, dose-escalation trial with > 1800 patients who were enrolled after a recent ST-elevation (60%) or non–ST-elevation (40%) MI. Patients on DAPT were randomized to placebo or twice-daily treatment with dabigatran, 50 mg, 75 mg, 110 mg, or 150 mg. The primary outcome was the composite of major or clinically relevant minor bleeding during the 6-month treatment period. During the study, there were 96 primary outcome events. When compared with placebo, there was a dose-dependent increase in bleeding events with dabigatran (50-mg HR = 1.77, 75-mg HR = 2.17, 110-mg HR = 3.92, 150-mg HR = 4.27).23 With respect to efficacy, 14 (3.8%) patients died or had an MI or stroke in the placebo group compared with 17 (4.6%) in the 50-mg, 18 (4.9%) in the 75-mg, 12 (3.0%) in the 110-mg, and 12 (3.5%) in the 150-mg dabigatran groups. Oldgren and associates23 concluded that the concomitant use of dabigatran and DAPT was associated with a dose-dependent increase in bleeding events. Additionally, the composite of cardiovascular death, nonfatal MI, and nonhemorrhagic stroke was lower in higher doses of dabigatran (3% in 110 mg and 3.5% in 150 mg) than in the placebo group (3.8%), but a larger phase III study is needed to establish the net clinical benefit of dabigatran in this specific population.
Previous Stroke
Stroke is the dreaded complication of AF. Patients with prior history of stroke are at greater risk of future stroke and this is reflected in the CHADS2 score, which assigns additional value to a prior history of stroke or transient ischemic attacks (TIA).12 In this subgroup analysis of the RE-LY trial by Diener and colleagues, the effects of dabigatran compared with warfarin in secondary prevention of stroke or TIA was assessed.24 In RE-LY, 20% (n = 3623) of patients had a previous history of stroke or TIA of which 89.6% had a CHADS2 score > 3. Not surprisingly, the annual stroke rate was higher in patients with a previous history of stroke or TIA than in patients with no such history (2.38% vs 1.22% respectively; P < .0001). In patients with a previous history of stroke or TIA, the primary endpoint (stroke or systemic embolism) occurred in 65 patients on warfarin (2.78%/y) compared with 51 on 150 mg dabigatran (2.07%/y, RR 0.75; 95% CI, 0.52-1.08), which was nonsignificant. Dabigatran, 150 mg, had lower rates of intracerebral hemorrhage but major bleeding rates were similar to warfarin. These results are consistent with the overall theme of the RE-LY trial. The lack of superiority for dabigatran with respect to prevention of secondary stroke or TIA is likely a function of a relatively small sample size.
Intracerebral Hemorrhage
If thromboembolic stroke is the most feared complication of AF, intracerebral hemorrhage is the most feared complication of anticoagulant therapy. During the RE-LY trial, 154 ICHs occurred in 153 patients (46% intracerebral, 45% subdural, and 8% subarachnoid).25 A history of trauma was associated with 30% of ICHs (11% intracerebral and 44% subdural) and 28% of patients concomitantly used aspirin. The annual rates of ICH were significantly lower in the dabigatran 150-mg group (0.31%) compared with the warfarin group (0.76%; P < .001). Compared with warfarin, the 150-mg dabigatran group had fewer fatal (13 vs 32 patients; P < .01) and traumatic ICHs (11 vs 25 patients; P < .05). The concomitant aspirin use was an independent risk factor predictive of ICH (RR 1.6; P = .01) making concomitant aspirin use the most important modifiable risk factor for ICH. Hart and colleagues25 concluded that dabigatran is beneficial even though there is no specific antidote, as it is associated with lower rate of all ICHs and there is no increase in mortality from ICH compared with warfarin.
Type of AF
Although the risk of stroke has been previously demonstrated to be similar between paroxysmal and sustained AF, Flaker and coworkers27 analyzed the effects of the type of AF on the primary outcomes from the RE-LY trial. In the RE-LY trial, 5943 patients had paroxysmal AF, 5789 had persistent AF, and 6375 had permanent AF. Dabigatran, 150 mg, was superior to warfarin for stroke prevention in all types of AF (paroxysmal HR 0.61, persistent HR 0.64, permanent HR 0.70). There were no differences in the rates of major bleeding among the different AF subgroups. Overall, the results were consistent with the main RELY study.
GI
In the RE-LY trial, the major finding demonstrated that dabigatran was inferior to warfarin with respect to GI adverse events (AEs).5 Study participants were more likely to suffer a GI bleed despite a similar overall risk of major bleeding and a decreased risk of ICH. Patients were also more likely to discontinue dabigatran when compared with warfarin due to nonbleeding GI side effects. Dyspepsia occurred in 5.8% of patients in the warfarin group and in 11.3% of patients in the 150-mg dabigatran group (P < .001). In a subgroup analysis by Bytzer and coworkers,28 the RE-LY database was utilized to quantify nonbleeding upper GI adverse effects (NB-UGI AEs).28 NB-UGI AEs were divided into four subgroups: gastroesophageal reflux (GERD), upper abdominal pain and dyspepsia, dysmotility, and gastroduodenal injury. Patients taking dabigatran had a higher incidence of NB-UGI AEs compared with warfarin (RR 1.81; P < .001). Specifically, in patients taking dabigatran, 16.9% (n = 2045) reported NB-UGI AEs, whereas only 9.4% (n = 563) of patients in the warfarin group reported similar complaints. With respect to the four NB-UGI AE subgroups, dabigatran-treated patients had a significantly higher incidence of all four of the NB-UGI AE subgroups. With respect to the qualitative severity of the symptoms, 46.3% reported their symptoms as mild, 44.8% reported moderate symptoms, and 8.9% reported severe symptoms, which were similar in distribution to the warfarin group. Discontinuation of drug due to NB-UGI AEs was higher with dabigatran (4%) compared with warfarin (1.7%, RR 2.34; P < .001). The majority of patients who discontinued dabigatran due to NB-UGI AEs did so within 3 months of drug initiation, and the GERD subgroup had the highest rate of drug discontinuation (P < .001).
Major GI bleeding events were higher with dabigatran compared with warfarin (RR 1.30; P = .01) in the RE-LY trial, but fatal GI bleeding events were similar between treatment groups (4.5% in the dabigatran 150-mg group, 4.2% in the warfarin group). In patients with a major GI bleed on dabigatran, 34.5% reported NB-UGI AEs before or during the episode, compared with 31.1% in the warfarin group. In patients who reported any NB-UGI AEs, the major bleeding rate was comparable between the dabigatran and warfarin groups (6.5% vs 8.2%; P = .15). All the NB-UGI AEs except for upper abdominal pain were associated with an increased risk of a major GI bleed. In dabigatran-treated patients reporting NB-UGI AEs, the rate of major GI bleeding was approximately twice that of those without NB-UGI AEs (RR 2.41), whereas patients taking warfarin who reported NB-UGI AEs had a fourfold higher RR of a major GI bleed (RR 4.37). The study authors concluded that dabigatran is associated with increased NB-UGI AEs compared with warfarin; patient symptoms are generally mild to moderate, usually appear within 30 days of drug initiation, and most patients do not require permanent drug discontinuation. All NB-UGI AEs except for upper abdominal pain were associated with increased risk of a major GI bleed in both the dabigatran and warfarin groups, and the majority of symptoms appear to be esophageal in origin.
Reversal of Dabigatran
Lack of a specific antidote for reversing dabigatran has been a major concern for clinicians and patients. Strategies for managing patients with life-threatening dabigatran-associated hemorrhage are based on small case series or extrapolation from pharmacology data.29-33 These bleeding management strategies have included the use of dialysis, recombinant factor VIIa, and prothrombin complex concentrates.29,30,34 Fortunately, the results of an extremely promising clinical trial, Reversal Effects of Idarucizumab on Active Dabigatran (REVERSE-AD), describe the efficacy of idarucizumab, which is a specific antidote for dabigatran.35 Idarucizumab is a monoclonal antibody fragment that binds dabigatran with 350 times greater affinity than thrombin.36 Consequently, idarucizumab binds dabigatran and neutralizes its activity.36
REVERSE-AD is an ongoing prospective cohort study designed to determine the safety of intravenous idarucizumab and its capacity to reverse the anticoagulant effects of dabigatran.35,37 The trial consists of two groups: patients who have serious bleeding while taking dabigatran (group A), and patients taking dabigatran who require an urgent procedure (group B).35,37 The primary endpoint is the maximum percentage reversal of the anticoagulant effect of dabigatran within 4 hours after administration of idarucizumab, based on dilute thrombin time (dTT) or ecarin clotting time (ECT). An interim analysis recently published in the New England Journal of Medicine included 90 patients who received idarucizumab (51 patients in group A and 39 in group B).35 There were 68 patients with an elevated dTT and 81 with an elevated ECT; after administration of idarucizumab, 5 g, the median maximum percentage reversal was 100%. Within minutes after the administration of idarucizumab, 88% to 98% of patients had normalization of their dTTs and/or ECTs. Furthermore, the duration of the complete reversal effect was still present 24 hours after administration in 79% of patients. As a secondary endpoint, restoration of hemostasis was also reported in REVERSE-AD. Among 35 group A patients, hemostasis, as determined by local investigators, was restored at a median of 11.4 hours. Among 36 group B patients who underwent a procedure, normal intraoperative hemostasis was reported in 33 patients, and mildly or moderately abnormal hemostasis was reported in 2 patients and 1 patient, respectively. One thrombotic event occurred within 72 hours after idarucizumab administration in a patient in whom anticoagulants had not been reinitiated.35
Conclusions
From a “big picture” perspective, the RE-LY trial demonstrates that twice-daily dabigatran, 150 mg, is superior to warfarin for the prevention of stroke without increasing the risk of major bleeding. However, RE-LY also contains a large amount of information about various important clinical subgroups that can be extracted through subgroup analysis. Understanding of the results from these subgroup analyses can better inform clinical decision making with respect to anticoagulation and enhance the ability of the clinician to individualize anticoagulation therapy for a specific patient. ![]()
References
- Hankey GJ, Eikelboom JW. Dabigatran etexilate: a new oral thrombin inhibitor. Circulation. 2011;123: 1436-1450.
- Stangier J, Clemens A. Pharmacology, pharmacokinetics, and pharmacodynamics of dabigatran etexilate, an oral direct thrombin inhibitor. Clin Appl Thromb Hemost. 2009;15(suppl 1):9S-16S.
- Stangier J, Rathgen K, Stähle H, et al. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64:292-303.
- Ezekowitz MD, Connolly S, Parekh A, et al. Rationale and design of RE-LY: randomized evaluation of long-term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J. 2009;157:805-810.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
- Connolly SJ, Pogue J, Hart RG, et al. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009;360:2066-2078.
- Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367:1903-1912.
- Bousser MG, Bouthier J, Büller HR, et al. Comparison of idraparinux with vitamin K antagonists for prevention of thromboembolism in patients with atrial fibrillation: a randomised, open-label, non-inferiority trial. Lancet. 2008;371:315-321.
- Ferreira J, Ezekowitz MD, Connolly SJ, et al; RE-LY Investigators. Dabigatran compared with warfarin in patients with atrial fibrillation and symptomatic heart failure: a subgroup analysis of the RE-LY trial. Eur J Heart Fail. 2013;15:1053-1061.
- Camm AJ, Kirchhof P, Lip GY, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369-2429.
- Krahn AD, Manfreda J, Tate RB, et al. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98:476-484.
- Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263-272.
- Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864-2870.
- Eikelboom JW, Wallentin L, Connolly SJ, et al. Risk of bleeding with 2 doses of dabigatran compared with warfarin in older and younger patients with atrial fibrillation: an analysis of the randomized evaluation of long-term anticoagulant therapy (RE-LY) trial. Circulation. 2011;123:2363-2372.
- Blech S, Ebner T, Ludwig-Schwellinger E, et al. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. 2008;36:386-399.
- Hurlen M, Abdelnoor M, Smith P, et al. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347:969-974.
- Hohnloser SH, Oldgren J, Yang S, et al. Myocardial ischemic events in patients with atrial fibrillation treated with dabigatran or warfarin in the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) trial. Circulation. 2012;125:669-676.
- Lown B, Perlroth MG, Kaidbey S, et al. “Cardioversion” of atrial fibrillation. A report on the treatment of 65 episodes in 50 patients. N Engl J Med. 1963;269:325-331.
- Fuster V, Rydén LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257-e354.
- Nagarakanti R, Ezekowitz MD, Oldgren J, et al. Dabigatran versus warfarin in patients with atrial fibrillation: an analysis of patients undergoing cardioversion. Circulation. 2011;123:131-136.
- Henry BL, Jentzer JC, Holtz JE, et al. A clinically oriented review of the landmark clinical trials comparing warfarin and aspirin to novel oral anticoagulants in atrial fibrillation. J Cardio Vasc Med. 2014;2:1-10.
- Dans AL, Connolly SJ, Wallentin L, et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial. Circulation. 2013;127:634-640.
- Oldgren J, Budaj A, Granger CB, et al; RE-DEEM Investigators. Dabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double-blind, phase II trial. Eur Heart J. 2011;32:2781-2789.
- Diener HC, Connolly SJ, Ezekowitz MD, et al; RE-LY study group. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol. 2010;9:1157-1163.
- Hart RG, Diener HC, Yang S, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke. 2012;43:1511-1517.
- Hohnloser SH, Pajitnev D, Pogue J, et al; ACTIVE W Investigators. Incidence of stroke in paroxysmal versus sustained atrial fibrillation in patients taking oral anticoagulation or combined antiplatelet therapy: an ACTIVE W Substudy. J Am Coll Cardiol. 2007;50:2156-2161.
- Flaker G, Ezekowitz M, Yusuf S, et al. Efficacy and safety of dabigatran compared to warfarin in patients with paroxysmal, persistent, and permanent atrial fibrillation: results from the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) study. J Am Coll Cardiol. 2012;59:854-855.
- Bytzer P, Connolly SJ, Yang S, et al. Analysis of upper gastrointestinal adverse events among patients given dabigatran in the RE-LY trial. Clin Gastroenterol Hepatol. 2013;11:246-252.e1-e5.
- Singh T, Maw TT, Henry BL, et al. Extracorporeal therapy for dabigatran removal in the treatment of acute bleeding: a single center experience. Clin J Am Soc Nephrol. 2013;8:1533-1539.
- Kumar R, Smith RE, Henry BL. A review of and recommendations for the management of patients with life-threatening dabigatran-associated hemorrhage: a single-center university hospital experience. J Intensive Care Med. 2015;30:462-472.
- Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet. 2010;49:259-268.
- Khadzhynov D, Wagner F, Formella S, et al. Effective elimination of dabigatran by haemodialysis. A phase I single-centre study in patients with end-stage renal disease. Thromb Haemost. 2013;109:596-605.
- Warkentin TE, Margetts P, Connolly SJ, et al. Recombinant factor VIIa (rFVIIa) and hemodialysis to manage massive dabigatran-associated postcardiac surgery bleeding. Blood. 2012;119:2172-2174.
- Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573-1579.
- Pollack CV Jr, Reilly PA, Eikelboom J, et al. Idarucizumab for dabigatran reversal. N Engl J Med. 2015;373:511-520.
- Schiele F, van Ryn J, Canada K, et al. A specific antidote for dabigatran: functional and structural characterization. Blood. 2013;121:3554-3562.
- Pollack CV Jr, Reilly PA, Bernstein R, et al. Design and rationale for RE-VERSE AD: a phase 3 study of idarucizumab, a specific reversal agent for dabigatran. Thromb Haemost. 2015;114:198-205.