The Role of Cardiac Magnetic Resonance Imaging in Hypertrophic Cardiomyopathy
Marco Marchesini, MD,1,2 Lucia Uguccioni, MD,1 Rosario Parisi, MD,1 Gianmaria Mattioli, MD,3 Francesca Terzi, MD,1 Roberto Olivieri, MD,1 Alessandro Capucci, MD,2 Rossella Fattori, MD1
1Cardiology and Interventional Cardiology Unit, Ospedali Riuniti Marche Nord, Pesaro, Italy; 2Cardiology Clinic, Ospedali Riuniti di Ancona, Università Politecnica delle Marche, Ancona, Italy; 3Radiology Department, Ospedali Riuniti Marche Nord, Pesaro, Italy
Until recently, the only imaging technique for the diagnosis and management of hypertrophic cardiomyopathy (HCM) was two-dimensional echocardiography, and the use of cardiac magnetic resonance imaging (cMRI) was limited to patients with poor acoustic windows. Now, cMRI has gained an essential role in the diagnosis of HCM, providing superior visualization of myocardial hypertrophy—even in remote zones of the left ventricle—and visualization of subtle changes in thickness and contractility over time. The morphologic accuracy of cMRI allows for the differentiation of HCM from other pathologic conditions with hypertrophic phenotype. Moreover, cMRI sheds light on the in vivo fibrotic changes in cardiac ultrastructure, offering an important advantage in the understanding of pathologic mechanisms of the disease, allowing early identification, risk stratification, and timely therapeutic management.
[Rev Cardiovasc Med. 2016;17(1/2):57-64 doi: 10.3909/ricm0811]
© 2016 MedReviews®, LLC
The Role of Cardiac Magnetic Resonance Imaging in Hypertrophic Cardiomyopathy
Marco Marchesini, MD,1,2 Lucia Uguccioni, MD,1 Rosario Parisi, MD,1 Gianmaria Mattioli, MD,3 Francesca Terzi, MD,1 Roberto Olivieri, MD,1 Alessandro Capucci, MD,2 Rossella Fattori, MD1
1Cardiology and Interventional Cardiology Unit, Ospedali Riuniti Marche Nord, Pesaro, Italy; 2Cardiology Clinic, Ospedali Riuniti di Ancona, Università Politecnica delle Marche, Ancona, Italy; 3Radiology Department, Ospedali Riuniti Marche Nord, Pesaro, Italy
Until recently, the only imaging technique for the diagnosis and management of hypertrophic cardiomyopathy (HCM) was two-dimensional echocardiography, and the use of cardiac magnetic resonance imaging (cMRI) was limited to patients with poor acoustic windows. Now, cMRI has gained an essential role in the diagnosis of HCM, providing superior visualization of myocardial hypertrophy—even in remote zones of the left ventricle—and visualization of subtle changes in thickness and contractility over time. The morphologic accuracy of cMRI allows for the differentiation of HCM from other pathologic conditions with hypertrophic phenotype. Moreover, cMRI sheds light on the in vivo fibrotic changes in cardiac ultrastructure, offering an important advantage in the understanding of pathologic mechanisms of the disease, allowing early identification, risk stratification, and timely therapeutic management.
[Rev Cardiovasc Med. 2016;17(1/2):57-64 doi: 10.3909/ricm0811]
© 2016 MedReviews®, LLC
The Role of Cardiac Magnetic Resonance Imaging in Hypertrophic Cardiomyopathy
Marco Marchesini, MD,1,2 Lucia Uguccioni, MD,1 Rosario Parisi, MD,1 Gianmaria Mattioli, MD,3 Francesca Terzi, MD,1 Roberto Olivieri, MD,1 Alessandro Capucci, MD,2 Rossella Fattori, MD1
1Cardiology and Interventional Cardiology Unit, Ospedali Riuniti Marche Nord, Pesaro, Italy; 2Cardiology Clinic, Ospedali Riuniti di Ancona, Università Politecnica delle Marche, Ancona, Italy; 3Radiology Department, Ospedali Riuniti Marche Nord, Pesaro, Italy
Until recently, the only imaging technique for the diagnosis and management of hypertrophic cardiomyopathy (HCM) was two-dimensional echocardiography, and the use of cardiac magnetic resonance imaging (cMRI) was limited to patients with poor acoustic windows. Now, cMRI has gained an essential role in the diagnosis of HCM, providing superior visualization of myocardial hypertrophy—even in remote zones of the left ventricle—and visualization of subtle changes in thickness and contractility over time. The morphologic accuracy of cMRI allows for the differentiation of HCM from other pathologic conditions with hypertrophic phenotype. Moreover, cMRI sheds light on the in vivo fibrotic changes in cardiac ultrastructure, offering an important advantage in the understanding of pathologic mechanisms of the disease, allowing early identification, risk stratification, and timely therapeutic management.
[Rev Cardiovasc Med. 2016;17(1/2):57-64 doi: 10.3909/ricm0811]
© 2016 MedReviews®, LLC
KEY WORDS
Hypertrophic cardiomyopathy • Cardiac magnetic resonance • Differential diagnosis
KEY WORDS
Hypertrophic cardiomyopathy • Cardiac magnetic resonance • Differential diagnosis
HCM is defined by the presence of increased LV wall thickness that is not solely explained by abnormal loading conditions.
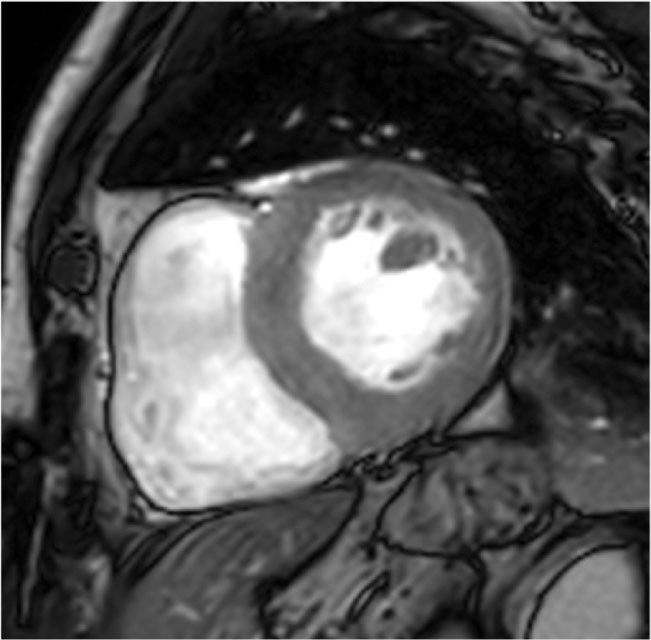
Figure 1. Steady-state free precession image, end-diastolic frame, left ventricular (LV) short-axis view at the papillary muscle level. Focal form with hypertrophied segment confined to basal interventricular septum just below the LV outflow tract.

Figure 1. Steady-state free precession image, end-diastolic frame, left ventricular (LV) short-axis view at the papillary muscle level. Focal form with hypertrophied segment confined to basal interventricular septum just below the LV outflow tract.
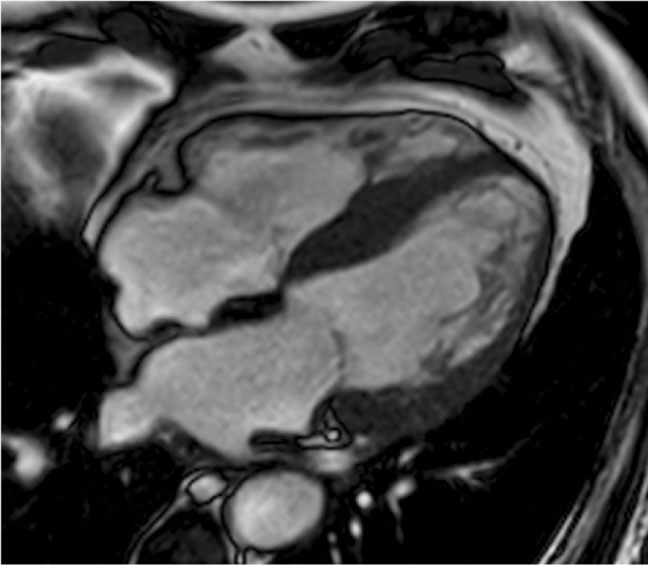
Figure 2. Steady-state free precession image, end-diastolic frame, four-chamber view. Hypertrophic cardiomyopathy with large areas of noncompaction.
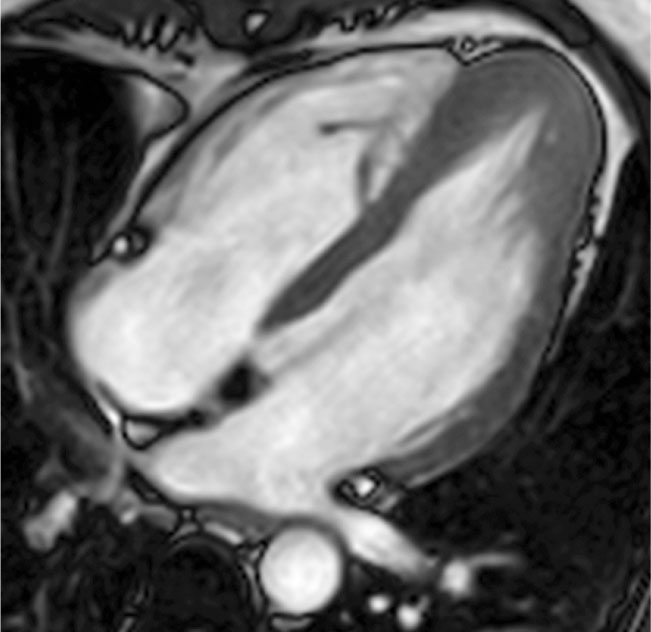
Figure 3. Steady-state free precession image, end-diastolic frame, four-chamber view. Apical variant of hypertrophic cardiomyopathy, sparing the interventricular septum and free wall of the left ventricle.
cMRI is able to accurately quantify ventricular volume and function as the gold standard for ejection fraction measurement.

cMRI is particularly useful for characterizing location and extent of LV hypertrophy, offering a superior visualization and a higher diagnostic accuracy compared with 2D echocardiography…
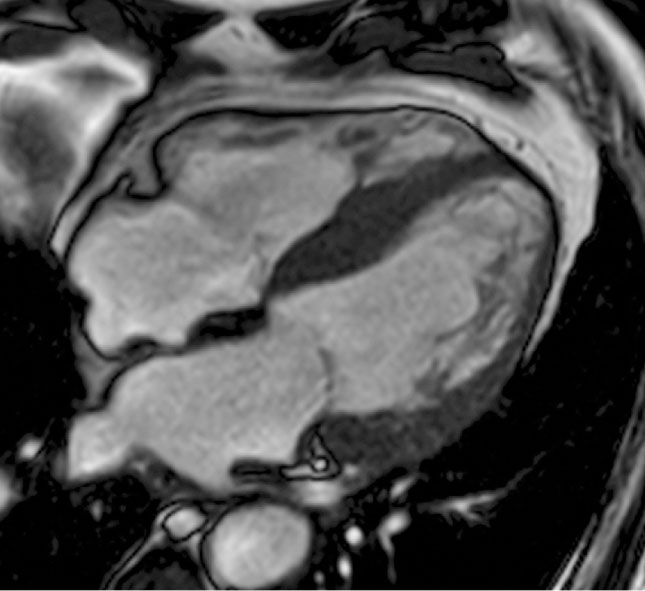
Figure 4. Steady-state free precession image, end-diastolic frame, four-chamber view with left ventricular outflow tract. Apical displacement of posterolateral papillary muscle insertion, localized in the distal third of the lateral wall.

Figure 4. Steady-state free precession image, end-diastolic frame, four-chamber view with left ventricular outflow tract. Apical displacement of posterolateral papillary muscle insertion, localized in the distal third of the lateral wall.
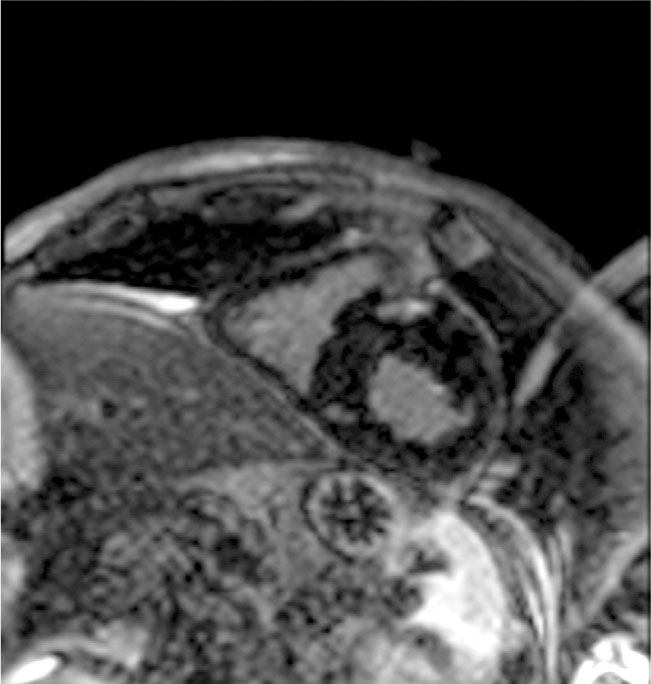
Figure 5. Two-dimensional phase-sensitive inversion-recovery, end-diastolic frame, short-axis view. Inversion recovery sequence showing intramyocardial late gadolinium enhancement at the level of interventricular septum insertion points.
In HCM, contrast-enhanced cMRI can detect areas of focal abnormality in approximately 50% to 80% of patients.
Main Points
• Hypertrophic cardiomyopathy (HCM) is responsible for sudden cardiac death in a subset of young individuals, often athletes; therefore, its timely recognition is of paramount importance. Advances in imaging techniques and genomic analysis have made possible early preclinical recognition of patients at risk of developing overt hypertrophic phenotype, and allow more accurate risk stratification.
• he key histologic feature of HCM is myocyte and myofibrillar disarray. In addition, three other nonspecific findings are generally noted in HCM: myocyte hypertrophy, a marked increase in interstitial space due to myocyte apoptosis with fibrotic replacement, and dysplasia of small arteries.
• Until recently, the only imaging technique for the diagnosis and management of HCM was two-dimensional echocardiography; the use of cardiac magnetic resonance imaging (cMRI) was limited to patients with poor acoustic windows. Now, cMRI has gained an essential role in the diagnosis of HCM, providing superior visualization of myocardial hypertrophy—even in remote zones of the left ventricle—and visualization of subtle changes over time.
• A unique ability of cMRI is tissue characterization with the use of gadolinium (Gd) contrast medium administration. Gd shortens the T1 relaxation time within the surrounding tissue and increases the signal intensity of regions with high Gd concentration in T1-weighted imaging.
Main Points
• Hypertrophic cardiomyopathy (HCM) is responsible for sudden cardiac death in a subset of young individuals, often athletes; therefore, its timely recognition is of paramount importance. Advances in imaging techniques and genomic analysis have made possible early preclinical recognition of patients at risk of developing overt hypertrophic phenotype, and allow more accurate risk stratification.
• he key histologic feature of HCM is myocyte and myofibrillar disarray. In addition, three other nonspecific findings are generally noted in HCM: myocyte hypertrophy, a marked increase in interstitial space due to myocyte apoptosis with fibrotic replacement, and dysplasia of small arteries.
• Until recently, the only imaging technique for the diagnosis and management of HCM was two-dimensional echocardiography; the use of cardiac magnetic resonance imaging (cMRI) was limited to patients with poor acoustic windows. Now, cMRI has gained an essential role in the diagnosis of HCM, providing superior visualization of myocardial hypertrophy—even in remote zones of the left ventricle—and visualization of subtle changes over time.
• A unique ability of cMRI is tissue characterization with the use of gadolinium (Gd) contrast medium administration. Gd shortens the T1 relaxation time within the surrounding tissue and increases the signal intensity of regions with high Gd concentration in T1-weighted imaging.
Hypertrophic cardiomyopathy (HCM) is a common inherited cardiomyopathy that occurs in approximately 1 in 500 individuals.1 Morphologic features typically consist of left ventricular (LV) hypertrophy in the absence of abnormal loading conditions. HCM is responsible for sudden cardiac death (SCD) in a subset of young individuals, often athletes; therefore, its timely recognition is of paramount importance. Depending on the severity and location of hypertrophy, dynamic obstruction of the LV outflow tract can occur, generating the majority of symptoms of an otherwise asymptomatic condition. With advancing age, other consequences become apparent, such as atrial tachyarrhythmias and consequent embolic risk, mitral regurgitation, and progression to the dilated end-stage phase with manifest heart failure.
Advances in imaging techniques and genomic analysis have made early preclinical recognition of patients at risk of developing overt hypertrophic phenotype possible, and allow more accurate risk stratification. Recent development of additional prognostic strategies in HCM have prompted the need for more extensive knowledge, as well as a clear view of the diagnostic potential of cardiac magnetic resonance imaging (cMRI).
Diagnostic Panorama
The most recent expert consensus on cardiomyopathies2 has adopted a new classification system; cardiomyopathies are defined by specific morphologic and functional features as they present for the first time to the observer: hypertrophic, dilated, arrhythmogenic, and restrictive phenotypes. Moreover, cardiomyopathies are grouped into familial/genetic and nonfamilial/nongenetic subtypes, irrespective of the presence of extracardiac disease.
Definition
HCM is defined by the presence of increased LV wall thickness that is not solely explained by abnormal loading conditions.3 Secondary causes of hypertrophy should be considered in the differential diagnosis, such as athlete’s heart, hypertensive cardiomyopathy, valve diseases imposing increased afterload (mainly aortic stenosis), and isolated basal septal hypertrophy in elderly people.
A variable grade and distribution of ventricular wall thickening may represent a common picture of different pathologic conditions, such as infiltrative disorders due to inborn errors of metabolism (eg, Pompe disease, Fabry disease),4 or deposition of anomalous misfolded proteins (a different type of amyloidosis), or other genetic causes such as mitochondrial diseases, neuromuscular disorders (Friederich ataxia), or malformative syndromes such as Noonan or LEOPARD syndromes.
HCM is a genetic disease with an autosomal dominant trait caused by mutations in cardiac sarcomere protein genes. Typically, patients with a sarcomere protein mutation have an earlier clinical presentation and a higher prevalence of family history of HCM or SCD than those without a mutation. They also tend to have more severe hypertrophy, microvascular dysfunction, and myocardial fibrosis.
A number of studies worldwide report a prevalence of HCM, ranging from 0.02% to 0.23% in adults.1,3,5 In pediatric registries, the prevalence of HCM in children is unknown, but population-based studies report an annual incidence of 0.3 to 0.5 per 100,000 children. Most studies report a small preponderance in boys, although the prevalence in different racial groups is similar.
In adults, HCM is defined by a wall thickness ≥15 mm in one or more LV myocardial segments—as measured by any imaging technique such as echocardiography, cMRI or computed tomography (CT)—that is not explained solely by loading conditions. In children, the diagnosis of HCM requires an LV wall thickness > two standard deviations over the predicted mean z score. The clinical diagnosis of HCM in first-degree relatives of patients with unequivocal disease (LV hypertrophy ≥15 mm) is based on the presence of otherwise unexplained increased LV wall thickness ≥13 mm in one or more LV myocardial segments.
Clinical Variants
Many clinical variants have been described; focal forms with exclusive involvement of the anterior septum are called 1 o’clock hypertrophy because of their aspect in basal short-axis view. Other focal forms are localized at the basal interventricular septum just below the LV outflow tract (Figure 1), at the inferolateral wall, or exclusively at the papillary muscles. There are patterns of noncontiguous hypertrophy, in which the thickened segments alternate with normal ones. There are forms of HCM in which the hypertrophy coexists with noncompacted areas (Figure 2) or regional abnormalities such as aneurysm, mainly in the apical segment.
A particular variant is apical hypertrophic cardiomyopathy (AHCM),6 a rare form of HCM, that usually involves the apex of the LV (Figure 3) and rarely involves the right ventricular (RV) apex. Historically, this condition was thought to be confined to the Japanese population. However, in Japan the prevalence of AHCM is 15%, whereas in the United States and Europe the prevalence is 3%. The diagnostic criteria for AHCM include demonstration of asymmetric LV hypertrophy, predominantly localized in the LV apex, with an apical wall thickness ≥15 mm and a ratio of maximal apical to posterior wall thickness ≥1.5 mm. In contrast to the common variant of HCM, up to 54% of patients with AHCM are symptomatic. This is a case in which the role of cMRI is indubitable, because the apical position of hypertrophic segments represents a well-known limitation for two-dimensional (2D) echocardiography.7,8 In this HCM subtype, diagnosis must not be missed because of the high prevalence of complications such as atrial fibrillation, myocardial infarction, apical aneurysm, embolic events, and congestive heart failure.
Clues to Differential Diagnosis
Genetic and nongenetic disorders causing hypertrophic phenotype can present with lesser degrees of wall thickening (13-14 mm); in these cases, the diagnosis of true HCM requires evaluation of other features including family history, noncardiac symptoms and signs, electrocardiogram (ECG) abnormalities, laboratory tests, and multimodal cardiac imaging. In any situation, the age of presentation is a fundamental clue to the differential diagnosis. Severe (maximal thickness > 30 mm or equivalent in children) and concentric ventricular hypertrophy in a child, adolescent, or young adult should raise suspicion of metabolic or storage disorders, in particular Pompe disease in the infantile period and Danon disease in adolescent boys.2 A different degree of concentric hypertrophy with LV systolic impairment is a clue to infiltrative diseases, because the hypokinetic end-stage phase of HCM is often LV dilation.
cMRI in HCM
cMRI offers several advantages in the diagnosis and management of HCM (Table 1).5,9-11 An accurate biventricular morphofunctional study allows a precise measurement of wall thickness of all ventricular segments, independent of location or acoustic window; diastolic dysfunction and outflow tract obstruction can be identified using phase contrast sequences or myocardial tagging. It can be used in screening and preclinical diagnosis of first-degree relatives. However, the most important feature is its emerging role in risk stratification, identifying myocardial fibrosis with late gadolinium (Gd) enhancement.12
Biventricular Morphologic and Functional Study
cMRI is able to accurately quantify ventricular volume and function as the gold standard for ejection fraction (EF) measurement.13 Frequently, in patients with HCM, the ventricular volumes are reduced and the hyperkinetic appearance of systolic contraction translates into a supernormal EF. cMRI is an excellent tool for following even subtle changes in LV systolic performance. Diastolic dysfunction related to myocardial abnormal hypertrophy is an early marker of the disease; for its assessment, Doppler echocardiography is common, but phase-contrast (PC) cMRI is able to quantify transmitral flow with all its parameters (E wave, A wave, and deceleration time), or transpulmonary vein flow parameters (S, D, and Ar). Despite the similar pattern, the overall measurement of E and A velocity values using cMRI is lower than with echocardiography, probably due to lower temporal resolution of the breath-hold PC cMRI technique. New semiautomated three-dimensional (3D) model-based analysis of the LV fillings curve is a promising technique for evaluation of diastolic function in patients with HCM, and demonstrates a good correlation between 2D PC and echocardiography in the assessment of E/A ratio.14
cMRI can analyze and quantify regional myocardial function due to a noninvasive imaging method for tracking myocardial motion: myocardial tissue tagging.15 Noninvasive markers, called tags, are created within the tissue by locally induced perturbations of the magnetic field with selective radiofrequency. These perturbations produce regions of reduced signal intensity, which appear as 3D dark lines in the acquired images, allowing the construction of a tagging grid. Initially designed to analyze myocardial contraction during systole, tags are typically created upon detection of the QRS complex of the ECG. The resulting tags then follow myocardial motion during the whole cardiac cycle, thus reflecting the underlying myocardial deformation of each segment. Moreover, providing persistent tags allows quantification of cardiac strain evolution during late diastole, which can be used to assess diastolic myocardial dysfunction.14
Distribution and Precise Quantification of Hypertrophy
Diffuse hypertrophy, involving > 50% of the LV and eight or more segments, is present in 54% of patients with HCM, whereas only 10% of patients present with single-segment involvement. cMRI is particularly useful for characterizing location and extent of LV hypertrophy, offering a superior visualization and a higher diagnostic accuracy compared with 2D echocardiography, particularly if the involved segments are the basal anterolateral free wall or the apex. Moreover, cMRI can help in the diagnostic process, revealing specific features of genetic HCM, such as anomalies in papillary muscles or RV involvement.
Papillary muscle anomalies in HCM consist of apical displacement, multiple or bifid insertion, and increased mass (Figures 4 and 5).5 These variants favor systolic mitral anterior movement and outflow tract obstruction, perturbing the normal activity of the mitral valve.16
In one-third of patients with HCM, RV wall thickness and/or mass is increased, including approximately 10% of patients with extreme RV wall hypertrophy (> 10 mm).17 The magnitude of maximum RV wall thickness and mass correlates significantly with LV wall thickness and mass. Hypertrophied crista supraventricularis and moderator bands are evident in approximately one-half of all patients with HCM. With regard to the distribution and pattern of hypertrophy, most patients with HCM (53%) have diffuse RV hypertrophy, involving all three segments of the RV (superior, anterior, and inferior), but a conspicuous proportion (47%) demonstrates RV hypertrophy, confined to only the segments contiguous with the ventricular septum; this suggests a “spill over” of the primary LV hypertrophic process from septum into adjacent segments of the RV wall.17
Outflow Tract Obstruction
In a three-chamber view with cine imaging, cMRI can elucidate the precise mechanism of outflow tract obstruction, demonstrating turbulent flow generated by systolic movement of the anterior mitral leaflet, chordae, and papillary muscle toward the interventricular septum.18,19 With specific sequences, cMRI can also identify medioventricular obstruction, a condition characterized by LV hourglass appearance in the end systolic frame and turbulent flow in cine gradient sequences. Moreover, cMRI can also identify the presence of apical aneurysms and thrombi, often missed by echocardiography,20 in patients with an apical variant of HCM.
Role in Screening and Preclinical Diagnosis
In patients with HCM, international guidelines recommend screening of first-degree relatives due to the high risk of SCD.3,21 cMRI should be considered a first-line imaging technique in the evaluation of at-risk family members given its superiority in identifying LV hypertrophy in respect to 2D transthoracic echocardiography.
In preclinical (genotype[+]/phenotype[–]) patients with HCM, cMRI may show the presence of subtle abnormalities such as mitral valve leaflet elongation, fibrosis, diastolic dysfunction, and crypts. Myocardial crypts are deep fissurings of the muscle orthogonal to the endocardial border localized predominantly in the inferior septum. The etiology of these structural abnormalities remains uncertain, but could be related to the pathologic process of myocyte necrosis with fibrotic replacement at the base of HCM. They are present in 61% of patients with genotype[+]/phenotype[–] and only in 4% of patients with overt hypertrophic cardiomyopathy.22,23 Additional investigations are necessary to confirm the clinical significance of such morphologic abnormalities, as some of these findings may also be present in normal individuals.
Contrast-enhanced cMRI and New Techniques
The key histologic feature of HCM is myocyte and myofibrillar disarray. In addition, three other nonspecific findings are generally noted in HCM: myocyte hypertrophy, a marked increase in interstitial space due to myocyte apoptosis with fibrotic replacement, and dysplasia of small arteries, leading to focal ischemia and scarring.
A unique ability of cMRI is tissue characterization with the use of Gd contrast medium administration. Gd shortens the T1 relaxation time within the surrounding tissue and increases the signal intensity of regions with high Gd concentration in T1-weighted imaging. Normal myocardium is typically characterized by a rapid wash-in and wash-out. Conversely, in abnormal myocardium, such as necrotic or fibrotic myocardium, the concentration of Gd increases over time due to an increased extracellular volume distribution with decreased wash-out. These regions are typically hyperintense (ie, bright) 10 minutes after Gd injection. This is typically referred to as late Gd enhancement (LGE). In HCM, contrast-enhanced cMRI can detect areas of focal abnormality in approximately 50% to 80% of patients. Areas of LGE can be measured and the amount quantified and expressed as a percentage of total LV mass (on average 10% of the overall LV myocardial volume).24 There is no specific pattern of LGE that is characteristic of HCM, although the distribution of LGE in HCM does not correspond to a coronary vascular territory. LGE is most often located in the most hypertrophied segment with an intramyocardial distribution (focal spot or linear deposit). Sometimes LGE is located in the ventricular septum but, not uncommonly, can be confined to the LV free wall or insertion points of the RV free wall and ventricular septum. The magnitude of LGE is greatest in patients with HCM in the end-stage phase of the disease (EF < 50%). However, LGE sequences cannot reliably evaluate the more diffuse patterns of myocardial fibrosis observed histologically in explanted hearts of HCM patients. Recent studies evaluate T1 mapping,25,26 a novel technique to detect and quantify such diffuse myocardial fibrosis, and several methods have been validated with myocardial collagen content measured in histologic specimens. Characterization of the native T1 of myocardial tissue can be used to detect and assess various cardiomyopathies, and measurement of T1 with extracellular Gd-based contrast agents provides additional information about the extracellular volume fraction. Compared with healthy subjects, those with HCM manifest lower postcontrast myocardial T1 times, suggestive of more diffuse myocardial fibrosis.
Fibrosis involvement, visually perceivable as specific hyperenhanced foci in HCM, appears inconstantly on T1 maps. The imperfect concordance between native T1 mapping and LGE imaging for detecting focal replacement fibrosis may be explained by the distinct significance of both imaging methods, because LGE (as well as postcontrast T1 mapping) reflects only extracellular space, whereas native T1 mapping reflects a composite of both intra- and extracellular compartments.
LGE has a potential role in risk stratification23,27-30; it is correlated to areas of myocardial fibrosis as arrhythmogenic substrate for ventricular tachyarrhythmias. Christiaans and colleagues29 showed a significant association between the presence of LGE and ventricular tachyarrhythmias on ambulatory 24-hour Holter ECG, whereas Bruder and associates28 demonstrated a significant correlation between the percentage of LGE and risk factors for SCD, with an increased risk (up to 7-fold) of SCD among HCM patients with LGE compared with those without LGE.
There are few prospective (short-term) outcome studies31,32 in patients with implantable cardiac defibrillators (ICDs); the results are so conflicting that the last European Society of Cardiology guidelines, published in 2014,3 do not include LGE in their risk stratification algorithm. However, given the association of LGE with ventricular nonsustained arrhythmias on 24-hour ECG monitoring, LGE is considered an adjunct factor in decision making for prophylactic ICD implantation, particularly in ambiguous cases.
T2-weighted Sequences in HCM
High T2 signal intensity areas have been found in HCM patients, frequently corresponding to LGE regions.33 A high T2 signal is related to increased water content of the tissue, indicating the possible presence of edema that may accompany focal ischemia due to dysplasia of small intramural arteries. The relationship between high T2-signal intensity and LGE can be explained by the pathologic progression of fibrosis; this may start with an acute process (high T2 signal) and end with chronic fibrous tissue.
Conclusions
cMRI has gained an important role in understanding cardiomyopathies. cMRI shows a superior definition of myocardial hypertrophy, particularly in remote zones of the LV, allowing a precise quantification and distribution of hypertrophy; the morphologic accuracy of cMRI is essential in familial screening, and in the differential diagnosis of other pathologic conditions presenting with a hypertrophic phenotype. cMRI is a useful tool in understanding the mechanism of LV tract obstruction, and in planning and monitoring interventional therapies. LGE and T1 mapping have made in vivo recognition of myocardial fibrosis possible, facilitating risk stratification in SCD. ![]()
References
- Maron B, McKenna WJ, Danielson GK, et al. American College of Cardiology/European Society of Cardiology Clinical Expert Consensus Document on Hypertrophic Cardiomyopathy. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the European Society of Cardiology Committee for Practice Guidelines. Eur Heart J. 2003;24:1965-1991.
- Rapezzi C, Arbustini E, Caforio AL, et al. Diagnostic work-up in cardiomyopathies: bridging the gap between clinical phenotypes and final diagnosis. A position statement from the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:1448-1458.
- Elliott PM, Anastasakis A, Borger MA, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733-2779.
- Nagueh SF. Anderson-Fabry disease and other lysosomal storage disorders. Circulation. 2014;130: 1081-1090.
- Nagueh SF, Bierig SM, Budoff MJ, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with hypertrophic cardiomyopathy: Endorsed by the American Society of Nuclear Cardiology, Society for Cardiovascular Magnetic Resonance, and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2011;24:473-498.
- Yusuf SW, Bathina JD, Banchs J, et al. Apical hypertrophic cardiomyopathy. World J Cardiol. 2011;3:256-259.
- Parisi R, Mirabella F, Secco GG, Fattori R. Multimodality imaging in apical hypertrophic cardiomyopathy. World J Cardiol. 2014;6:916-923.
- Fattori R, Biagini E, Lorenzini M, et al. Significance of magnetic resonance imaging in apical hypertrophic cardiomyopathy. Am J Cardiol. 2010;105:1592-1596.
- Maron MS. The current and emerging role of cardiovascular magnetic resonance imaging in hypertrophic cardiomyopathy. J Cardiovasc Transl Res. 2009;2: 415-425.
- To AC, Dhillon A, Desai MY. Cardiac magnetic resonance in hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. 2011;4:1123-1137.
- Pedrotti P. La risonanza magnetica cardiaca nella cardiomiopatia ipertrofica. Cardiol Sci. 2013;11:70-81.
- Noureldin RA, Liu S, Nacif MS, et al. The diagnosis of hypertrophic cardiomyopathy by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2012;14:17.
- Hundley WG, Bluemke DA, Finn JP, et al. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;121:2462-2508.
- Wu V, Chyou JY, Chung S, et al. Evaluation of diastolic function by three-dimensional volume tracking of the mitral annulus with cardiovascular magnetic resonance: comparison with tissue Doppler imaging. J Cardiovasc Magn Reson. 2014;16:71.
- Shehata ML, Cheng S, Osman NF, et al. Myocardial tissue tagging with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2009;11:55.
- Ibrahim M, Rao C, Ashrafian H, et al. Modern management of systolic anterior motion of the mitral valve. Eur J Cardiothorac Surg. 2012;41:1260-1270.
- Maron MS, Hauser TH, Dubrow E, et al. Right ventricular involvement in hypertrophic cardiomyopathy. Am J Cardiol. 2007;100:1293-1298.
- Walker CM, Reddy GP, Mohammed TLH, Chung JH. Systolic anterior motion of the mitral valve. J Thoracic Imaging. 2012;27:W87.
- Biagini E, Lorenzini M, Olivotto I, et al. Effects of myocardial fibrosis assessed by MRI on dynamic left ventricular outflow tract obstruction in patients with hypertrophic cardiomyopathy: a retrospective database analysis. BMJ Open. 2012;2:e001267.
- Gersh BJ, Maron BJ, Bonow RO, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58: e212-e260.
- Christiaans I, van Engelen K, van Langen IM, et al. Risk stratification for cardiac death in hypertrophic cardiomyopathy: systematic review of clinical risk markers. Europace. 2010;12:313-321.
- Maron MS, Rowin EJ, Lin D, et al. Prevalence and clinical profile of myocardial crypts in hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. 2012;5: 441-447.
- Maron MS. Clinical utility of cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J Cardiovasc Magn Reson. 2012;14:13.
- Ellims AH, Iles LM, Ling LH, et al. A comprehensive evaluation of myocardial fibrosis in hypertrophic cardiomyopathy with cardiac magnetic resonance imaging: linking genotype with fibrotic phenotype. Eur Heart J Cardiovasc Imaging. 2014;15:1108-1116.
- Kellman P, Hansen MS. T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson. 2014;16:2.
- Kramer CM, Barkhausen J, Flamm SD, et al; Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized Protocols. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson. 2013;15:91.
- Maron MS, Appelbaum E, Harrigan CJ, et al. Clinical profile and significance of delayed enhancement in hypertrophic cardiomyopathy. Circ Heart Fail. 2008;1:184-191.
- Bruder O, Wagner A, Jensen CJ, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:875-887.
- Christiaans I, van Engelen K, van Langen IM, et al. Risk stratification for sudden cardiac death in hypertrophic cardiomyopathy: systematic review of clinical risk markers. Europace. 2010;12:313-321.
- Shiozaki AA, Kim RJ, Parga JR, et al. Cardiovascular magnetic resonance in hypertrophic cardiomyopathy [Article in Portuguese]. Arq Bras Cardiol. 2007;88:243-248.
- Rubinshtein R, Glockner JF, Ommen SR, et al. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail. 2010;3:51-58.
- O’Hanlon R, Grasso A, Roughton M, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010;56:867-874.
- Kolman L, Stirrat J, Rajchl M, et al. Myocardial T2 signal enhancement in hypertrophic cardiomyopathy: prevalence, clinical profile and pathologic correlation. J Cardiovasc Magn Reson. 2014;16(suppl 1):O85.