Cardiotoxicity of Anticancer Therapies
Raghunandan Muppidi, MD,1 Laura Spranklin, DO,2 William Scialla, DO,2 Nauman Islam, MD,1 Ronald Freudenberger, MD,1 Robert Malacoff, MD1
1Department of Cardiology, Lehigh Valley Health Network, Allentown, PA; 2Department of Hematology-Oncology, Lehigh Valley Health Network, Allentown, PA
Cardiovascular diseases and cancer continue to remain major causes of mortality and morbidity. However, overall cancer death rates have declined 20% from their peak in 1991. These declines reflect changing patterns in smoking, prevention, earlier diagnosis, and better treatment options in chemotherapy. It is recognized that this improved survival with better cancer therapies has put patients at risk for cardiovascular disease later in life; this may be secondary to risk factors for developing cardiovascular disease or the effect of anticancer therapies. Earlier detection, identifying patients at risk of developing cardiotoxicity, and early institution of treatment are paramount to decreasing morbidity associated with cardiotoxicity. Adverse cardiac effects have been observed and reported with a wide variety of chemotherapeutic agents. Herein we review cardiac effects of some of the common agents used in oncology.
[ Rev Cardiovasc Med. 2015;16(4):225-234 doi: 10.3909/ricm0779 ]
© 2016 MedReviews®, LLC
Cardiotoxicity of Anticancer Therapies
Raghunandan Muppidi, MD,1 Laura Spranklin, DO,2 William Scialla, DO,2 Nauman Islam, MD,1 Ronald Freudenberger, MD,1 Robert Malacoff, MD1
1Department of Cardiology, Lehigh Valley Health Network, Allentown, PA; 2Department of Hematology-Oncology, Lehigh Valley Health Network, Allentown, PA
Cardiovascular diseases and cancer continue to remain major causes of mortality and morbidity. However, overall cancer death rates have declined 20% from their peak in 1991. These declines reflect changing patterns in smoking, prevention, earlier diagnosis, and better treatment options in chemotherapy. It is recognized that this improved survival with better cancer therapies has put patients at risk for cardiovascular disease later in life; this may be secondary to risk factors for developing cardiovascular disease or the effect of anticancer therapies. Earlier detection, identifying patients at risk of developing cardiotoxicity, and early institution of treatment are paramount to decreasing morbidity associated with cardiotoxicity. Adverse cardiac effects have been observed and reported with a wide variety of chemotherapeutic agents. Herein we review cardiac effects of some of the common agents used in oncology.
[ Rev Cardiovasc Med. 2015;16(4):225-234 doi: 10.3909/ricm0779 ]
© 2016 MedReviews®, LLC
Cardiotoxicity of Anticancer Therapies
Raghunandan Muppidi, MD,1 Laura Spranklin, DO,2 William Scialla, DO,2 Nauman Islam, MD,1 Ronald Freudenberger, MD,1 Robert Malacoff, MD1
1Department of Cardiology, Lehigh Valley Health Network, Allentown, PA; 2Department of Hematology-Oncology, Lehigh Valley Health Network, Allentown, PA
Cardiovascular diseases and cancer continue to remain major causes of mortality and morbidity. However, overall cancer death rates have declined 20% from their peak in 1991. These declines reflect changing patterns in smoking, prevention, earlier diagnosis, and better treatment options in chemotherapy. It is recognized that this improved survival with better cancer therapies has put patients at risk for cardiovascular disease later in life; this may be secondary to risk factors for developing cardiovascular disease or the effect of anticancer therapies. Earlier detection, identifying patients at risk of developing cardiotoxicity, and early institution of treatment are paramount to decreasing morbidity associated with cardiotoxicity. Adverse cardiac effects have been observed and reported with a wide variety of chemotherapeutic agents. Herein we review cardiac effects of some of the common agents used in oncology.
[ Rev Cardiovasc Med. 2015;16(4):225-234 doi: 10.3909/ricm0779 ]
© 2016 MedReviews®, LLC
KEY WORDS
Cardiotoxicity • Anticancer therapy • Coronary artery disease • Cardiomyopathy
KEY WORDS
Cardiotoxicity • Anticancer therapy • Coronary artery disease • Cardiomyopathy
The molecular pathogenesis of anthracycline cardiotoxicity remains highly controversial, although the oxidative stress-based hypothesis involving intramyocardial production of reactive oxygen species has gained the widest acceptance.
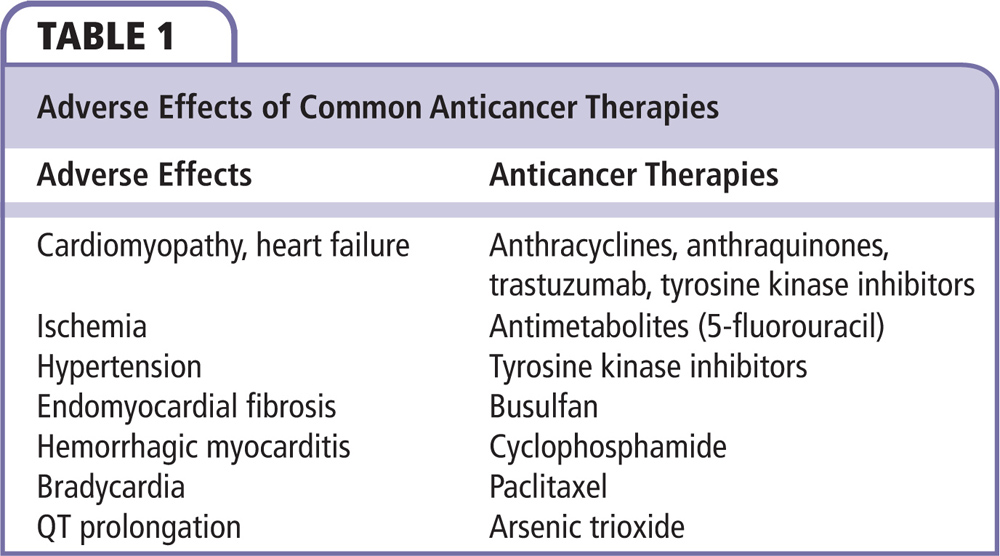

Figure 1. Mechanism of anthracycline-induced cardiomyopathy. ATP, adenosine triphosphate.

Figure 1. Mechanism of anthracycline-induced cardiomyopathy. ATP, adenosine triphosphate.

Figure 2. The probability of developing clinical heart failure at cumulative doses of doxorubicin.

Figure 2. The probability of developing clinical heart failure at cumulative doses of doxorubicin.
Cardiac MRI can be used as a primary modality to evaluate LV function or in cases in which there is discrepancy between the MUGA scan and echocardiographic evaluation of LV function.
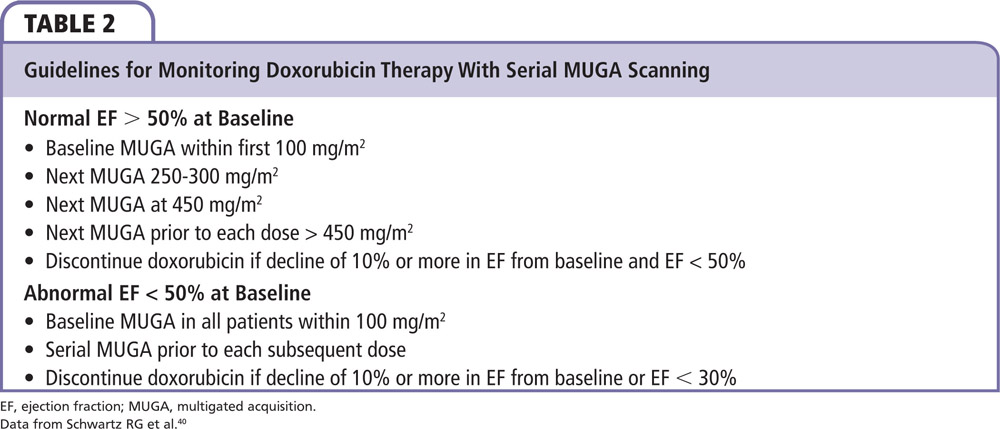

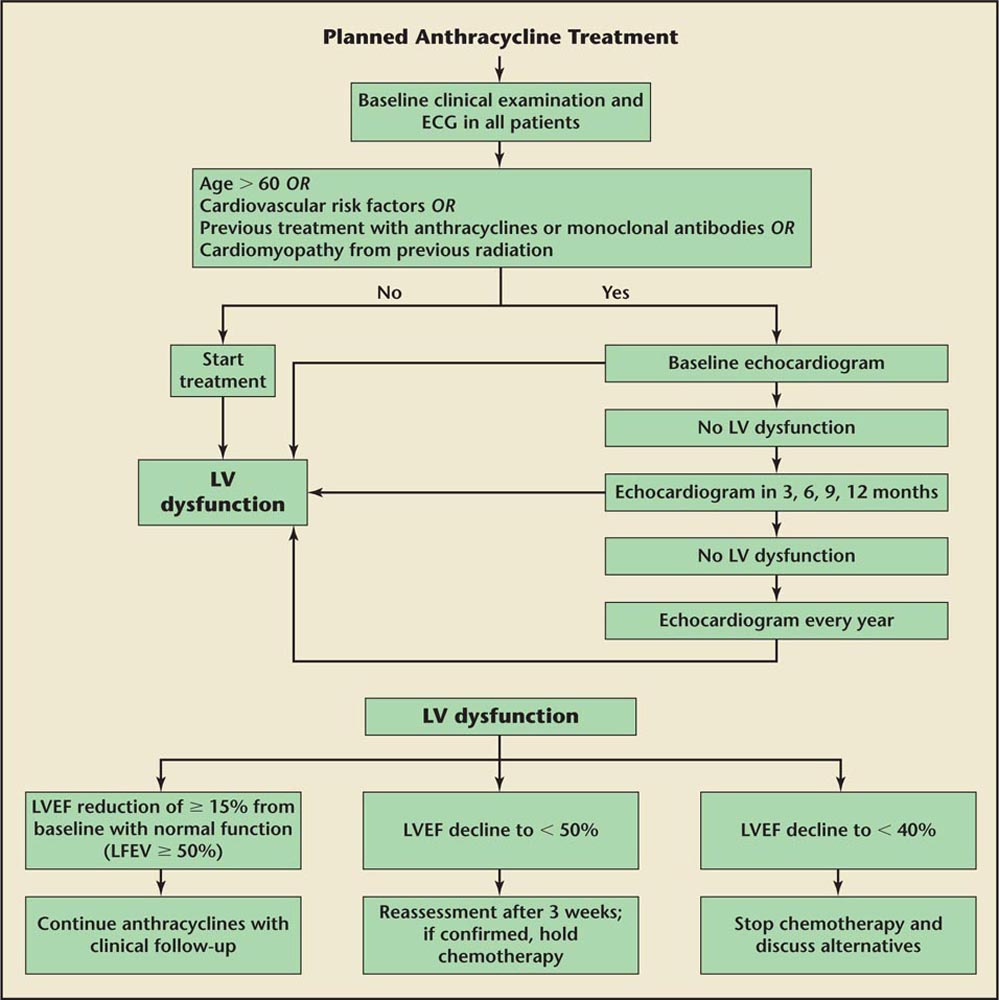
Figure 3. Algorithm for screening echocardiography and recommendations for stopping therapy with anthracyclines. ECG, electrocardiogram; LV, left ventricular; LVEF, left ventricular ejection fraction.

Figure 3. Algorithm for screening echocardiography and recommendations for stopping therapy with anthracyclines. ECG, electrocardiogram; LV, left ventricular; LVEF, left ventricular ejection fraction.
The strongest predictor of cardiotoxicity has been shown to be cumulative dose. Once cumulative doses of anthracyclines exceed 400 mg/m2 the incidence of cardiac toxicity is dramatically increased.
Main Points
• Cardiovascular diseases and cancer continue to remain major causes of mortality and morbidity; however, overall cancer death rates have declined. It is recognized that this improved survival with better cancer therapies has put patients at risk for cardiovascular disease later in life, secondary to risk factors for developing cardiovascular disease or the effects of anticancer therapies.
• Anthracycline agents are a group of highly effective anticancer agents used for treatment of breast cancer, Hodgkin lymphoma, leukemia, and other cancers. The risk of cardiotoxicity is clearly increased with increasing cumulative doses of anthracyclines. Anthracycline toxicity can be classified as acute, subacute, or chronic, and can present acutely within a week of administration, and subacutely within 1 year, or even after 1 year.
• Unlike anthracycline cardiotoxicity, trastuzumab-associated cardiotoxicity is not dose dependent and is believed to be reversible on withdrawal of therapy. In patients with trastuzumab-associated cardiac dysfunction, myocytes appear histologically normal; changes may be seen only by using electron microscopy, in keeping with a reversible cardiomyopathy.
• Antitumor antibiotics, including mitomycin C and bleomycin, have also been associated with cardiotoxic effects, ranging from heart failure to coronary artery disease. The risk of heart failure increases substantially when cumulative doses . 30 mg/m2 of mitomycin C are given, or when given in combination with an anthracycline.
• It is postulated that the mechanism of action of some tyrosine kinase inhibitors, including sunitinib and sorafenib, cause heart failure by blocking the signaling pathway that is involved in developing healthy cardiomyocytes, leading to cardiomyocyte dysfunction and death.
• Understanding the risk factors that are associated with cardiotoxicity should aid in earlier identification of cardiac manifestations of therapy. For example, factors associated with increased risk of cardiotoxicity with anthracycline use include older age at the time of exposure, cumulative dose of anthracyclines, concomitant administration of other agents, prior or ongoing radiation therapy, and known cardiovascular disease.
Main Points
• Cardiovascular diseases and cancer continue to remain major causes of mortality and morbidity; however, overall cancer death rates have declined. It is recognized that this improved survival with better cancer therapies has put patients at risk for cardiovascular disease later in life, secondary to risk factors for developing cardiovascular disease or the effects of anticancer therapies.
• Anthracycline agents are a group of highly effective anticancer agents used for treatment of breast cancer, Hodgkin lymphoma, leukemia, and other cancers. The risk of cardiotoxicity is clearly increased with increasing cumulative doses of anthracyclines. Anthracycline toxicity can be classified as acute, subacute, or chronic, and can present acutely within a week of administration, and subacutely within 1 year, or even after 1 year.
• Unlike anthracycline cardiotoxicity, trastuzumab-associated cardiotoxicity is not dose dependent and is believed to be reversible on withdrawal of therapy. In patients with trastuzumab-associated cardiac dysfunction, myocytes appear histologically normal; changes may be seen only by using electron microscopy, in keeping with a reversible cardiomyopathy.
• Antitumor antibiotics, including mitomycin C and bleomycin, have also been associated with cardiotoxic effects, ranging from heart failure to coronary artery disease. The risk of heart failure increases substantially when cumulative doses . 30 mg/m2 of mitomycin C are given, or when given in combination with an anthracycline.
• It is postulated that the mechanism of action of some tyrosine kinase inhibitors, including sunitinib and sorafenib, cause heart failure by blocking the signaling pathway that is involved in developing healthy cardiomyocytes, leading to cardiomyocyte dysfunction and death.
• Understanding the risk factors that are associated with cardiotoxicity should aid in earlier identification of cardiac manifestations of therapy. For example, factors associated with increased risk of cardiotoxicity with anthracycline use include older age at the time of exposure, cumulative dose of anthracyclines, concomitant administration of other agents, prior or ongoing radiation therapy, and known cardiovascular disease.
Cardiovascular diseases and cancer remain major causes of mortality and morbidity in the United States and across Europe. From 1935 to 2010, heart disease and cancer were the first and second most common causes of death in the United States.1 More than 1.6 million new cancer cases and 580,350 cancer-related deaths were projected in 2013.2 However, overall cancer death rates have declined 20% from their peak in 1991 and death rates continue to decline for all four major cancer sites (lung, colon/rectum, breast, and prostate).2 These declines reflect changing patterns in smoking, prevention, earlier diagnosis, and better treatment options in chemotherapy. It is, however, recognized that this improved survival with better cancer therapies has put patients at risk for cardiovascular disease later in life; this may be secondary to risk factors for developing cardiovascular disease or the effects of anticancer therapies.3 In some studies, the risk of cardiovascular disease among patients surviving cancer has been equal to or higher than the risk of developing recurrent cancer, which underscores the scope of this problem.4 Additionally, because of newer modalities for detecting cardiac injury, including biomarkers, strain imaging, and cardiac magnetic resonance imaging (MRI), the effects of these anticancer therapies are recognized more frequently. Earlier detection, identification of patients at risk of developing cardiotoxicity, and early institution of treatment are paramount to decreasing morbidity associated with cardiotoxicity. Collaboration between oncologists and cardiologists is crucial throughout treatment.5
Adverse cardiac effects have been observed and reported with a wide variety of chemotherapeutic agents (Table 1). We review cardiac effects of some of the common agents used in oncology.
Anthracycline Agents
Anthracycline agents are a group of highly effective anticancer agents used for treatment of breast cancer, Hodgkin lymphoma, leukemia, and other cancers. The risk of cardiomyopathy and heart failure from anthracycline agents was recognized in retrospective studies in the 1970s.6,7 The risk of cardiotoxicity is clearly increased with increasing cumulative doses of anthracyclines.6 In the Surveillance, Epidemiology, and End Results (SEER) database, which studied 43,338 women who were diagnosed with breast cancer, risk factors for development of congestive heart failure included older age, hypertension, diabetes, use of trastuzumab, and pre-existing coronary artery disease (CAD).8
Mechanisms of Cardiotoxicity
Anthracyclines act by two different mechanisms: (1) intercalation between base pairs of DNA and prevention of the replication of cancer cells, and (2) inhibition of type II topoisomerase, which prevents uncoiling of the DNA strand and thereby prevents replication of cancer cells. The molecular pathogenesis of anthracycline cardiotoxicity remains highly controversial, although the oxidative stress-based hypothesis involving intramyocardial production of reactive oxygen species (ROS) has gained the widest acceptance. Anthracyclines may promote the formation of ROS through redox cycling of their aglycones, as well as their anthracycline-iron complexes.9–11 The heart is thought to be particularly vulnerable to toxicity due to an abundance of mitochondria present in cardiomyocytes; mitochondria are thought to be key players in the development of cardiotoxicity.9,12 There are other theories that attempt to explain the mechanism of anthracycline-induced cardiotoxicity, including cardiac apoptosis,13 inhibition of transcription and translation, and production of vasoactive amines.14 By uncoupling the electron transport chain, anthracyclines create highly ROS, which can impair oxidative phosphorylation and adenosine triphosphate (ATP) synthesis.9,15 Anthracyclines can also impair mitochondrial calcium homeostasis, leading to the loss of stability of the mitochondrial membrane, decreased ATP, and cell death (Figure 1).
Clinical Spectrum of Cardiotoxicity
Anthracycline toxicity can be classified as acute, subacute, or chronic. It can present acutely within a week of administration, and subacutely within 1 year or even after 1 year.1 Cardiac side effects that develop early are typically reversible and include arrhythmias, QTc changes on electrocardiogram (ECG), pericarditis, and, sometimes, myocarditis. It remains uncertain whether patients who have these early cardiac side effects are also more likely to develop chronic effects. Late effects typically cause cardiomyopathy. In severe forms it can present as progressive heart failure and cardiac death.6 Cumulative doses and pre-existing risk factors determine the risk of developing cardiomyopathy. The probability of developing clinical heart failure at cumulative doses of doxorubicin < 300 mg/m2 is > 2%.16 Above this cumulative dose the incidence of heart failure increases exponentially (Figure 2). The incidence of HF was approximately 3.0% in patients receiving a cumulative dose of doxorubicin of 400 mg/m2, 7.5% at doses of 550 mg/m2, and 18.0% at doses of 700 mg/m2.
Mitoxantrone
Mitoxantrone is an anthraquinone that is very similar in structure to anthracyclines. Mitoxantrone was originally developed to reduce cardiotoxicity while still preserving the antitumor effect of the anthracycline agents. Mitoxantrone is associated with fewer cardiotoxic effects than doxorubicin; however, it still has an incidence of subclinical decline in ejection fraction (EF) of 13% and a 2.6% incidence of overt heart failure.17
Monocloncal Antibodies
Trastuzumab
Trastuzumab is a humanized monoclonal antibody that targets epidermal growth factor receptor 2 (ERB2) on the surface of ERB2-overexpressing tumor cells. It is overexpressed in approximately 25% of human breast cancer. Herceptin is a critical component of treatment in both early and advanced breast cancers. It is used in both neoadjuvant and adjuvant settings. Cardiotoxicity as an adverse effect of trastuzumab therapy did not become evident until phase III clinical trials.18 Unlike anthracycline cardiotoxicity, trastuzumab-associated cardiotoxicity is not dose dependent and is believed to be reversible on withdrawal of therapy; trastuzumab possibly can be reinstituted after recovery of systolic function. In patients with trastuzumab-associated cardiac dysfunction, myocytes appear histologically normal; changes may be seen only by using electron microscopy, in keeping with a reversible cardiomyopathy.19 Therefore, it is classified as a type II cardiomyopathy, because there is chance of recovery of cardiac function, as compared with type I cardiomyopathy, in which the cardiac damage is irreversible (as with anthracycline use).20
Mechanisms of Cardiotoxicity. The ErbB/HER family of receptor tyrosine kinases consists of four different proteins: Her1 (EGFR, ErbB1), Her2 (Neu, ErbB2), Her3 (ErbB3), and Her4 (ErbB4). Under normal physiologic conditions, the ErbB receptors play crucial roles in propagating signals regulating cell proliferation, differentiation, motility, and apoptosis. Signal transduction pathways are initiated upon ligand-induced receptor homo- or heterodimerization and activation of tyrosine kinase activity. ErbB signaling is best known for its indispensable role during cardiac and neuronal development; blockage of this pathway has been postulated as the cause of cardiotoxicity.
Clinical Spectrum of Cardiotoxicity. In a review of six phase III clinical trials in patients with metastatic breast cancer, it was noted that patients treated with trastuzumab had an increased incidence of cardiac dysfunction.18 The incidence was greatest in patients receiving concomitant trastuzumab and anthracycline plus cyclophosphamide (27%). The risk was substantially lower in patients receiving paclitaxel and trastuzumab (13%) or trastuzumab alone (3%-7%); however, most of these patients had received prior anthracycline therapy. Most trastuzumab-treated patients who developed cardiac dysfunction were symptomatic (75%), and most improved with standard treatment for congestive heart failure (79%).
Antitumor Antibiotics
Antitumor antibiotics, including mitomycin C and bleomycin, have also been associated with cardiotoxic effects, ranging from heart failure to CAD. The risk of heart failure increases substantially when cumulative doses > 30 mg/m2 of mitomycin C are given or when given in combination with an anthracycline.21
Tyrosine Kinase Inhibitors
Imatinib is a tyrosine kinase inhibitor (TKI) that is commonly used in the treatment of chronic myeloid leukemia and gastrointestinal stromal tumors (GISTs). The mechanism of action is the inhibition of Bcr-Abl, KIT, the platelet-derived growth factor receptor, and the Src family of tyrosine kinases. Early in the treatment of chronic myeloid leukemia (CML) with imatinib, an association with heart failure was discovered; however, further studies have identified the incidence of heart failure to be in the range of 1% to 2%.22 It is likely that the incidence of heart failure was overestimated, as early occurrences were based on adverse events. In the only prospective study to assess left ventricular EF (LVEF), no decline in EF was seen in the first 12 months of treatment.23 Multiple second-generation multitargeted TKIs have been developed since then. These second-generation TKIs have been approved in the treatment of Philadelphia chromosome-positive CML; they include nilotinib, dasatinib, and ponatinib, all of which have cardiotoxic effects. Both nilotinib and dasatinib have been associated with QT prolongation, and dasatinib and ponatinib have been associated with heart failure and LV dysfunction. Patients taking ponatinib have a 4% incidence of developing heart failure while on treatment.24 Sorafenib and sunitinib are both multitargeted agents used in the treatment of renal cell carcinoma and GISTs, and both have been associated with a decline in LVEF. A study published in 2007 described a decline in LVEF in up to 28% and clinical heart failure in 3% to 15% of patients taking these drugs.25,26 The largest review was a meta-analysis that included 6936 patients being treated with sunitinib for a variety of cancers. The results recorded an incidence of 4.1% for grade 1-4 heart failure and 1.5% in grade 3-4 heart failure.27 Although data have been published, unfortunately, none of these studies had cardiac endpoints and incidence is based on reports of clinical symptoms. LVEF returned to baseline and clinical symptoms of heart failure resolved upon discontinuation of the drug.
The mechanism of action of imatinib-induced heart failure is mediated by the inhibition of c-Abl. A study published in 2006 by Kerkelä and colleagues28 identified that mice with an imatinib-resistant mutant of c-Abl were protected from the cardiotoxic effects of imatinib, suggesting that c-Abl has a protective function in myocytes. Pathways that induce the pathologic survival and proliferation of cancer cells also play a role in regulating the survival of normal cells, including those found in the heart. When these pathways are blocked by TKIs the cardiomyocytes are also inhibited, causing cardiomyopathy. It is postulated that the mechanism of action of other TKIs, including sunitinib and sorafenib, causes heart failure by blocking the signaling pathway that is involved in developing healthy cardiomyocytes, leading to cardiomyocyte dysfunction and death.29 TKIs have also been linked to the development of proteinuria and hypertension.30 Hypertensive crises after initiation of TKI therapy have been described.
Detection of Cardiotoxicity. Given the multitude of cardiovascular effects of these agents, a baseline comprehensive cardiovascular assessment is warranted in all patients undergoing treatment. Depending on their risk profile, decisions about therapy can then be made on a case-by-case basis. Presence of hypertension, hyperlipidemia, thrombosis, peripheral vascular disease, diabetes, and CAD should be investigated because they can increase risk of cardiotoxicity. An ECG at baseline may identify occult CAD and, on follow-up with serial monitoring, may also show signs of cardiac toxicity and/or arrhythmias. A baseline evaluation of LVEF is imperative with agents that may cause cardiomyopathy. On follow-up echocardiograms diastolic function assessment of the left ventricle with pulse wave Doppler of mitral inflow and tissue Doppler indices of the mitral annulus should be evaluated to detect any early signs of LV dysfunction before reduction of LVEF.31 Heart failure with preserved EF may be the presenting symptom in these patients with evidence of decreased tissue Doppler indices. Contrast echocardiography can be used in patients in whom the endocardial border is not well defined to make an accurate evaluation of LV function; it has been shown to have better intra- and interobserver agreement.11,32 In patients in whom a determination of LV function cannot be accurately done secondary to poor acoustic windows, radionuclide angiography using multigated blood pool imaging (multigated acquisition [MUGA] scan) is an established technique.33 However, the test involves exposure to radiation and diastolic properties of the left ventricle cannot be adequately evaluated. Two- and three-dimensional speckle tracking echocardiography is an area of interest that may be used to detect early changes in the myocardium and identify patients at risk of cardiotoxicity.34 Cardiac MRI can be used as a primary modality to evaluate LV function or in cases in which there is discrepancy between the MUGA scan and echocardiographic evaluation of LV function. However, availability and cost may preclude their use in all patients. In a recent study of patients with postanthracycline MRI, myocardial T1 and extracellular volume were found to be early tissue markers of ventricular remodeling. These changes may represent diffuse fibrosis in children with normal EF after anthracycline therapy.35 Endomyocardial biopsy may be helpful in patients in whom it is important to exclude cardiac toxicity with greater specificity, or in whom noninvasive tests are inconclusive.36 The invasive nature of the procedure and the risk of complications, including but not limited to bleeding, arrhythmias, and perforation, restricts its use to high-risk patients.
Serum biomarkers are also being increasingly recognized as having value in diagnosing subclinical cardiovascular disease, and for monitoring for cardiotoxicity. Serum B-type natriuretic peptide (BNP) and troponin measurements at baseline and serial monitoring with therapy can diagnose subclinical cardiomyopathy.37-39 However, further studies are warranted in order to follow these patients for an extended amount of time after chemotherapy and to confirm whether these biomarkers are accurate enough to be able to stratify patients based on the early results.
Guidelines for monitoring doxorubicin therapy with serial MUGA scanning are listed in Table 2.40
The European Society for Medical Oncology clinical practice guidelines31 provide a broad framework for cardiotoxicity monitoring and management. Some of the recommendations are summarized below.
Baseline clinical and ECG evaluation are recommended in all patients undergoing anthracycline therapy. Assessment of baseline systolic and diastolic cardiac function with Doppler echocardiography should be conducted before treatment with monoclonal antibodies or anthracyclines and their derivates in patients older than 60 years, or those with cardiovascular risk factors such as hypertension, hypercholesterolemia, diabetes, obesity, or previous treatment with 5-hydroxytryptamine-2B agonists (in patients with Parkinson disease and obese patients) potentially inducing cardiac valvulopathy, or documented cardiopathy or previous thoracic radiotherapy.
Further evaluations of LVEF are recommended, even in asymptomatic patients, according to the following schedule: after administration of half the planned dose of anthracycline, or after administration of a cumulative dose of doxorubicin of 300 mg/m2, epirubicin of 450 mg/m2, or mitoxantrone of 60 mg/m2, or after administration of a cumulative dose of doxorubicin of 240 mg/m2 or epirubicin of 360 mg/m2 in patients younger than 15 years or older than 60 years; before every next administration of anthracycline; and 3, 6, and 12 months after the end of therapy with anthracycline (Figure 3).
Periodic monitoring (every 12 weeks) of cardiac function is also suggested for those patients receiving monoclonal antibodies, especially if they were previously treated with anthracycline. Assessment of cardiac function is recommended 4 and 10 years after anthracycline therapy in patients who were treated when they were 15 years of age, or over age 15 years with a cumulative dose of doxorubicin > 240 mg/m2 or epirubicin > 360 mg/m2. LVEF reduction of ≥ 20% from baseline despite normal function or LVEF decline < 50% necessitates reassessment or discontinuation of therapy and further frequent clinical and echocardiographic checks.
A predictive role for biomarkers of cardiotoxicity caused by cancer therapy is not well defined enough to include them as routine screening measurements. However, persistent increases in cardiac troponin I or BNP concentrations seem to identify patients at risk of cardiotoxicity.
Prevention of Cardiotoxicity. Close surveillance and early detection of cardiac manifestations are paramount to avoiding serious complications of cardiac toxicity. The effects of anticancer therapy can arise acutely or subacutely during administration, or may arise even chronically several years after treatment. Although the average time to diagnosis of LV dysfunction is 2.2 years, it may develop as late as 10 to 20 years following chemotherapy treatment. Often it is asymptomatic; therefore, diagnosis is often delayed until the onset of signs and symptoms of heart failure. In a study by Ammon and colleagues,41 timing of LV dysfunction was unknown in the majority of patients; only 25% had a screening echocardiography within the 12 months preceding diagnosis. Types of cancers included lymphoma (32%), breast cancer (32%), leukemia (22%), and other/unspecified (8%). Chemotherapeutic regimens included anthracyclines (73%), alkylating agents (73%), tubulin-active agents (43%), antimetabolites (33%), and trastuzumab (16%).
Understanding the risk factors that are associated with cardiotoxicity should aid in earlier identification of cardiac manifestations of therapy. Previous studies have identified factors associated with increased risk of cardiotoxicity with anthracyclines. These include older age at the time of exposure, cumulative dose of anthracyclines, concomitant administration of other agents, prior or ongoing radiation therapy (XRT), and known cardiovascular disease.42 The strongest predictor of cardiotoxicity has been shown to be cumulative dose. Once cumulative doses of anthracyclines exceed 400 mg/m2 the incidence of cardiac toxicity is dramatically increased.43 Early treatment of risk factors has been shown to prevent the development of cardiotoxicity. β-blockers have been well studied and have been shown to be protective when started prior to initiation of anthracycline treatment.44-47 The Prevention of Left Ventricular Dysfunction With Enalapril and Carvedilol in Patients Submitted to Intensive Chemotherapy for Malignant Hemopathies (OVERCOME) trial48 enrolled 90 patients with recently diagnosed acute leukemia (n = 36) or patients with malignant hemopathies undergoing autologous hematopoietic stem cell transplantation (n = 54) without LV systolic dysfunction; patients were randomly assigned to a group receiving enalapril and carvedilol (n = 45) or to a control group (n = 45). Echocardiographic and cardiac MRI studies were performed before and at 6 months after randomization. The primary efficacy endpoint was the absolute change from baseline in LVEF. At 6 months, LVEF did not change in the intervention group but significantly decreased in control subjects, resulting in a 3.1% absolute difference by echocardiography (P = .035) and a 3.4% absolute difference (P = .09) in the 59 patients who underwent cardiac MRI. Compared with control subjects, patients in the intervention group had a lower incidence of the combined event of death or heart failure (6.7% vs 22%; P = .036) and of death, heart failure, or a final LVEF < 45% (6.7% vs 24.4%; P= .02).
Other strategies to reduce or prevent cardiotoxicity are to lower doses, use continuous infusions to reduce peak drug levels in plasma, or use different drug formulations.49 The use of cardioprotective agents can reduce cardiotoxicity and should be used when indicated.50 Prior studies have clearly shown that the incidence of cardiotoxicity increases dramatically when doxorubicin and trastuzumab are used concurrently. This problem can be minimized by having a drug-free interval between the administrations of these agents.51 Earlier treatment of patients with angiotensin-converting enzyme (ACE) inhibitors may decrease the progression of disease.52 Dexrazoxane is an iron chelating agent that may prevent ROS-related cardiotoxicity.53 Several randomized controlled trials54-57 have shown effectiveness of dexrazoxane in preventing cardiotoxicity resulting from anthracycline use. This also allows delivery of higher doses of anthracyclines. In their 2008 guidelines on the use of cardioprotectants,50 the American Society of Clinical Oncology suggests the use of dexrazoxane in the following settings:
- Consider using for patients with metastatic breast cancer who have received > 300 mg/m2 of doxorubicin in the metastatic setting and who might benefit from continued doxorubicin therapy.
- Management of patients who received > 300 mg/m2 of doxorubicin in the adjuvant setting and are now initiating doxorubicin-based chemotherapy in the metastatic setting who should be individualized with consideration given to the potential of dexrazoxane to decrease response rates as well as decreasing the risk of cardiac toxicity.
- Not recommended for routine use in patients with metastatic breast cancer receiving doxorubicin-based chemotherapy.
- In other malignancies in adults consider the use of dexrazoxane in patients who have received > 300 mg/m2 of doxorubicin-based therapy. Exercise caution in the use of dexrazoxane in settings in which doxorubicin-based chemotherapy has been shown to improve survival.
Treatment. ACE inhibitors constitute first-line therapy for an asymptomatic drop in LV function or clinical heart failure. The offending agent responsible for drop in LV function or heart failure should be temporarily stopped or discontinued. Guideline-suggested β-blockers, diuretics, and other medications should be used as clinically appropriate. Treatment of clinical heart failure should be based on guidelines for management of heart failure in adults and advanced therapies should be used accordingly.58 The prognosis of heart failure with treatment is better if patients are asymptomatic at the time of diagnosis of a drop in LV function. Guideline-recommended heart failure therapies may improve EF and cardiac outcomes. In one study,41 only 54% of patients received a cardiology consult, yet survival was significantly better for those who were followed in collaboration with a cardiologist. Only 78% of patients received ACE inhibitors or angiotensin receptor blockers, 70% received β-blockers, and 65% received both; 15% of patients who died during follow-up had heart failure listed as the primary cause of death.
Cardiac Toxicity of Radiation Treatment for Malignancy. Radiation injury can result from treatment to the mediastinum and can damage any cardiac structure. Two major cancers that benefit from XRT are breast cancer and Hodgkin disease. XRT is often utilized in concert with chemotherapeutic agents, some of which may have cardiotoxic risks of their own. Structures at risk for damage include heart muscle, pericardium, heart valves, coronary circulation, and electrical system. Valves on the left side of the heart are more commonly affected than those on the right. Pericardial disease can include any and all inflammatory processes, including effusive-constrictive disease. Such risks may be immediate or delayed.
Risk factors for XRT cardiotoxicity include total XRT dose, portals of irradiation, adjuvant chemotherapy, age, and traditional risks for CAD, and can extend for as long as two decades after treatment.59 Pathophysiology may involve vascular damage and inflammatory reactions that ultimately result in fibrosis.60 However, the risk of cardiotoxicity has been reduced by lowering total XRT dose, awareness of other potential cardiotoxic therapies, and shielding. Studies in breast cancer patients61 have found that risk for toxicity was increased in women with left-sided disease, was longitudinal over a time frame of 20 years, and increased in those with risks for CAD. In patients with Hodgkin disease, XRT portals can include lower cardiac structures and thus result in complications that include pericarditis, LV dysfunction, valvular disease, and conduction abnormalities.62 Risk for cardiac morbidity may last decades and, as expected, anthracycline use enhances these risks.62,63 Myocardial damage may result in fibrotic changes, leading to diastolic dysfunction64 and heart failure. Additionally, fibrotic degeneration of specialized conduction tissue may result in heart block as well as ventricular arrhythmias. ECG changes are frequent and include bundle branch block and prolongation of the QT interval.65
XRT treatment of cancers in the region of cardiac structures leads to significant risk for damage to critical cardiac tissue that includes myocardium, pericardium, valves, and specialized conduction pathways. Patients with traditional risks for CAD may be at enhanced risk for complications and should be identified prior to treatment. Younger patients are also at risk for long-term complications and special care should be taken to limit XRT dose and adjuvant chemotherapeutic agents with known cardiotoxicity, as is feasible.
Conclusions
With better survival with newer anticancer therapies and an aging population, more cardiovascular effects of anticancer therapies are being recognized. Detection of underlying cardiovascular disease prior to onset of therapy, close surveillance during and after therapy to monitor for cardiotoxicity, and early treatment all help prevent complications and decrease morbidity. Cardiologists and oncologists should collaborate closely with every patient to minimize the risk of cardiotoxicity and maximize the effect of anticancer therapies. Cardiotoxicity from chemotherapy may not be apparent until years after first chemotherapy treatment. Routine echocardiographic screening allows for detection of LV dysfunction prior to development of clinical signs and symptoms of heart failure. Patients with LV dysfunction should be started on guideline-based heart failure treatment and followed in collaboration with cardiologists in order to optimize outcomes. ![]()
References
- Hoyert DL. 75 years of mortality in the United States, 1935-2010. NCHS Data Brief. 2012:1-8.
- Siegel R, Naishadham D, Jemal A. CA Cancer J Clin. 2013;63:11-30.
- Meacham LR, Chow EJ, Ness KK, et al. Cardiovascular risk factors in adult survivors of pediatric cancer—a report from the childhood cancer survivor study. Cancer Epidemiol Biomarkers Prev. 2010;19:170-181.
- Patnaik JL, Byers T, DiGuiseppi C, et al. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:R64.
- Albini A, Pennesi G, Donatelli F, et al. Cardiotoxicity of anticancer drugs: the need for cardio-oncology and cardio-oncological prevention. J Natl Cancer Inst. 2010;102:14-25.
- Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-lnduced congestive heart failure. Ann Intern Med. 1979;91:710-717.
- Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302-314.
- Pinder MC, Duan Z, Goodwin JS, et al. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25:3808-3815.
- Simůnek T, Stérba M, Popelová O, et al. Anthracycline-induced cardiotoxicity: overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacol Rep. 2009;61:154-171.
- Chen Y, Jungsuwadee P, Vore M, et al. Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues. Mol Interv. 2007;7:147-156.
- Adams MJ, Lipshultz SE. Pathophysiology of anthracycline‐and radiation‐associated cardiomyopathies: implications for screening and prevention. Pediatr Blood Cancer. 2005;44:600-606.
- Thomson HL, Basmadjian AJ, Rainbird AJ, et al. Contrast echocardiography improves the accuracy and reproducibility of left ventricular remodeling measurements: a prospective, randomly assigned, blinded study. J Am Coll Cardiol. 2001;38:867-875.
- Childs AC, Phaneuf SL, Dirks AJ, et al. Doxorubicin treatment in vivo causes cytochrome C release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and Bcl-2:Bax ratio. Cancer Res. 2002;62:4592-4598.
- Ito H, Miller SC, Billingham ME, et al. Doxorubicin selectively inhibits muscle gene expression in cardiac muscle cells in vivo and in vitro. Proc Natl Acad Sci U S A. 1990;87:4275-4279.
- Berthiaume JM, Wallace KB. Adriamycin-induced oxidative mitochondrial cardiotoxicity. Cell Biol Toxicol. 2007;23:15-25.
- Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869-2879.
- Smith LA, Cornelius VR, Plummer CJ, et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: systematic review and meta-analysis of randomised controlled trials. BMC Cancer. 2010;10:337.
- Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215-1221.
- Guarneri V, Lenihan DJ, Valero V, et al. Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: the MD Anderson Cancer Center experience. J Clin Oncol. 2006;24:4107-4115.
- Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol. 2005;23:2900-2902.
- Verweij J, Funke-Küpper AJ, Teule GJ, Pinedo HM. A prospective study on the dose dependency of cardiotoxicity induced by mitomycin C. Med Oncol Tumor Pharmacother. 1988;5:159-163.
- Atallah E, Durand JB, Kantarjian H, Cortes J. Congestive heart failure is a rare event in patients receiving imatinib therapy. Blood. 2007;110:1233-1237.
- Estabragh ZR, Knight K, Watmough SJ, et al. A prospective evaluation of cardiac function in patients with chronic myeloid leukaemia treated with imatinib. Leuk Res. 2011;35:49-51.
- Sprycel [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2006.
- Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011-2019.
- Khakoo AY, Kassiotis CM, Tannir N, et al. Heart failure associated with sunitinib malate: a multitargeted receptor tyrosine kinase inhibitor. Cancer. 2008;112:2500-2508.
- Richards CJ, Je Y, Schutz FA, et al. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J Clin Oncol. 2011;29:3450-3456.
- Kerkelä R, Grazette L, Yacobi R, et al. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med. 2006;12:908-916.
- Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7:332-344.
- Kandula P, Agarwal R. Proteinuria and hypertension with tyrosine kinase inhibitors. Kidney Int. 2011;80:1271-1277.
- Bovelli D, Plataniotis G, Roila F; ESMO Guidelines Working Group. Cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO Clinical Practice Guidelines. Ann Oncol. 2010;21(suppl 5):v277-v282.
- Malm S, Frigstad S, Sagberg E, et al. Accurate and reproducible measurement of left ventricular volume and ejection fraction by contrast echocardiography: a comparison with magnetic resonance. J Am Coll Cardiol. 2004;44:1030-1035.
- Palmeri ST, Bonow RO, Myers CE, et al. Prospective evaluation of doxorubicin cardiotoxicity by rest and exercise radionuclide angiography. Am J Cardiol. 1986;58:607-613.
- Biswas M, Sudhakar S, Nanda NC, et al. Two- and three-dimensional speckle tracking echocardiography: clinical applications and future directions. Echocardiography. 2013;30:88-105.
- Tham EB, Haykowsky MJ, Chow K, et al. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson. 2013;15:48.
- From AM, Maleszewski JJ, Rihal CS. Current status of endomyocardial biopsy. Mayo Clin Proc. 2011;86:1095-1102.
- Lipshultz SE, Miller TL, Scully RE, et al. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: associations with long-term echocardiographic outcomes. J Clin Oncol. 2012;30:1042-1049.
- Sawaya H, Sebag IA, Plana JC, et al. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ Cardiovasc Imaging. 2012;5:596-603.
- Twerenbold R, Jaffe A, Reichlin T, et al. High-sensitive troponin T measurements: what do we gain and what are the challenges? Eur Heart J. 2012;33:579-586.
- Schwartz RG, McKenzie WB, Alexander J, et al. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. Seven-year experience using serial radionuclide angiocardiography. Am J Med. 1987;82:1109-1118.
- Ammon M, Arenja N, Leibundgut G, et al. Cardiovascular management of cancer patients with chemotherapy-associated left ventricular systolic dysfunction in real-world clinical practice. J Card Fail. 2013;19:629-634.
- Pinder MC, Duan Z, Goodwin JS, et al. Congestive heart failure in older women treated with adjuvant anthracycline chemotherapy for breast cancer. J Clin Oncol. 2007;25:3808-3815.
- Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1996;125:47-58.
- Spallarossa P, Garibaldi S, Altieri P, et al. Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. J Mol Cell Cardiol. 2004;37:837-846.
- Oliveira PJ, Bjork JA, Santos MS, et al. Carvedilolmediated antioxidant protection against doxorubicin-induced cardiac mitochondrial toxicity. Toxicol Appl Pharmacol. 2004;200:159-168.
- Wouters KA, Kremer LC, Miller TL, et al. Protecting against anthracycline‐induced myocardial damage: a review of the most promising strategies. Br J Haematol. 2005;131:561-578.
- Kalay N, Basar E, Ozdogru I, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. J Am Coll Cardiol. 2006;48:2258-2262.
- Bosch X, Rovira M, Sitges M, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol. 2013;61:2355-2362.
- van Dalen EC, Michiels EM, Caron HN, Kremer LC. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst Rev. 2006:CD005006.
- Hensley ML, Hagerty KL, Kewalramani T, et al. American Society of Clinical Oncology 2008 clinical practice guideline update: use of chemotherapy and radiation therapy protectants. J Clin Oncol. 2009;27:127-145.
- Suter TM, Procter M, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol. 2007;25:3859-3865.
- Cardinale D, Colombo A, Sandri MT, et al. Prevention of high-dose chemotherapy-induced cardiotoxicity in high-risk patients by angiotensin-converting enzyme inhibition. Circulation. 2006;114:2474-2481.
- Lipshultz SE, Rifai N, Dalton VM, et al. The effect of dexrazoxane on myocardial injury in doxorubicin-treated children with acute lymphoblastic leukemia. N Engl J Med. 2004;351:145-153.
- Speyer JL, Green MD, Zeleniuch-Jacquotte A, et al. ICRF-187 permits longer treatment with doxorubicin in women with breast cancer. J Clin Oncol. 1992;10:117-127.
- Lopez M, Vici P, Di Lauro K, et al. Randomized prospective clinical trial of high-dose epirubicin and dexrazoxane in patients with advanced breast cancer and soft tissue sarcomas. J Clin Oncol. 1998;16:86-92.
- Swain SM, Vici P. The current and future role of dexrazoxane as a cardioprotectant in anthracycline treatment: expert panel review. J Cancer Res Clin Oncol. 2004;130:1-7.
- Swain SM, Whaley FS, Gerber MC, et al. Cardioprotection with dexrazoxane for doxorubicin-containing therapy in advanced breast cancer. J Clin Oncol. 1997;15:1318-1332.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147-e239.
- Moslehi J. The cardiovascular perils of cancer survivorship. N Engl J Med. 2013;368:1055-1056.
- Cuzick J, Stewart H, Rutqvist L, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994;12:447-453.
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987-998.
- Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878-1886.
- Swerdlow AJ, Higgins CD, Smith P, et al. Myocardial infarction mortality risk after treatment for Hodgkin disease: a collaborative British cohort study. J Natl Cancer Inst. 2007;99:206-214.
- Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139-3148.
- Larsen RL, Jakacki RI, Vetter VL, et al. Electrocardiographic changes and arrhythmias after cancer therapy in children and young adults. Am J Cardiol. 1992;70:73-77.