Targeting the Papillary Muscles in Mitral Valve Repair for Ischemic Mitral Regurgitation
Christos G. Mihos, DO, Andres M. Pineda, MD, Orlando Santana, MD
Columbia University Division of Cardiology at Mount Sinai Heart Institute, Miami Beach, FL
Ischemic mitral regurgitation due to left ventricular remodeling and leaflet tethering is associated with decreased survival, and the optimal management remains unknown. Restrictive mitral annuloplasty is the current treatment of choice, but it is associated with a 15% to 30% incidence of late recurrent mitral regurgitation, which confers a poor prognosis. A pathophysiology-guided approach to surgical repair is preferable, with a goal of alleviating leaflet tethering and restoring proper subvalvular mechanics. In patients with preoperative predictors of annuloplasty failure, combining a papillary muscle repositioning technique with conventional annuloplasty repair allows for complete geometric repair of the ventriculomitral unit.
[Rev Cardiovasc Med. 2015;16(3):182-188 doi: 10.3909/ricm0772]
© 2015 MedReviews®, LLC
Targeting the Papillary Muscles in Mitral Valve Repair for Ischemic Mitral Regurgitation
Christos G. Mihos, DO, Andres M. Pineda, MD, Orlando Santana, MD
Columbia University Division of Cardiology at Mount Sinai Heart Institute, Miami Beach, FL
Ischemic mitral regurgitation due to left ventricular remodeling and leaflet tethering is associated with decreased survival, and the optimal management remains unknown. Restrictive mitral annuloplasty is the current treatment of choice, but it is associated with a 15% to 30% incidence of late recurrent mitral regurgitation, which confers a poor prognosis. A pathophysiology-guided approach to surgical repair is preferable, with a goal of alleviating leaflet tethering and restoring proper subvalvular mechanics. In patients with preoperative predictors of annuloplasty failure, combining a papillary muscle repositioning technique with conventional annuloplasty repair allows for complete geometric repair of the ventriculomitral unit.
[Rev Cardiovasc Med. 2015;16(3):182-188 doi: 10.3909/ricm0772]
© 2015 MedReviews®, LLC
Targeting the Papillary Muscles in Mitral Valve Repair for Ischemic Mitral Regurgitation
Christos G. Mihos, DO, Andres M. Pineda, MD, Orlando Santana, MD
Columbia University Division of Cardiology at Mount Sinai Heart Institute, Miami Beach, FL
Ischemic mitral regurgitation due to left ventricular remodeling and leaflet tethering is associated with decreased survival, and the optimal management remains unknown. Restrictive mitral annuloplasty is the current treatment of choice, but it is associated with a 15% to 30% incidence of late recurrent mitral regurgitation, which confers a poor prognosis. A pathophysiology-guided approach to surgical repair is preferable, with a goal of alleviating leaflet tethering and restoring proper subvalvular mechanics. In patients with preoperative predictors of annuloplasty failure, combining a papillary muscle repositioning technique with conventional annuloplasty repair allows for complete geometric repair of the ventriculomitral unit.
[Rev Cardiovasc Med. 2015;16(3):182-188 doi: 10.3909/ricm0772]
© 2015 MedReviews®, LLC
KEY WORDS
Ischemic mitral regurgitation • Mitral annuloplasty • Papillary muscle intervention • Subvalvular repair
KEY WORDS
Ischemic mitral regurgitation • Mitral annuloplasty • Papillary muscle intervention • Subvalvular repair
… restrictive mitral annuloplasty is the current surgical procedure of choice in the treatment of IMR, which involves reducing the septolateral diameter of the mitral annulus with an undersized annuloplasty ring.
… innovative papillary muscle procedures performed at the time of annuloplasty, which aim to relieve leaflet tethering and improve the function of the subvalvular apparatus, may confer an improved durability of valve repair in IMR.
Combining annuloplasty with a papillary muscle intervention results in reduced septolateral annular dilatation, moves the anterolateral papillary muscle closer to the annulus, and corrects both lateral displacement and apical restriction of the posterior papillary muscle.
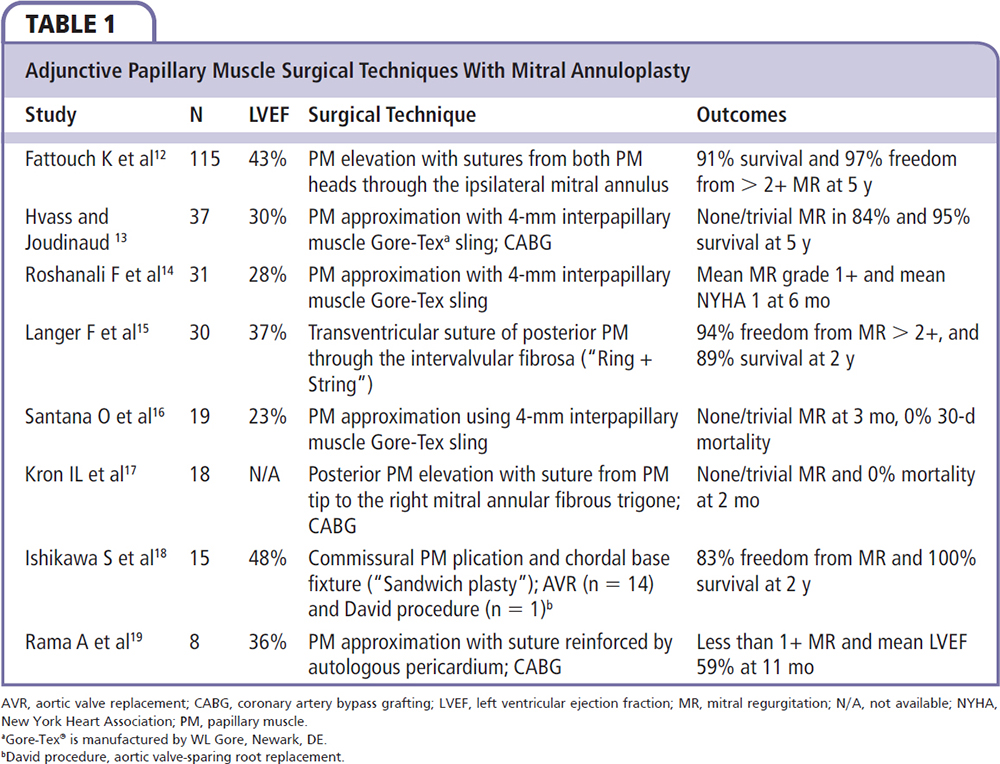
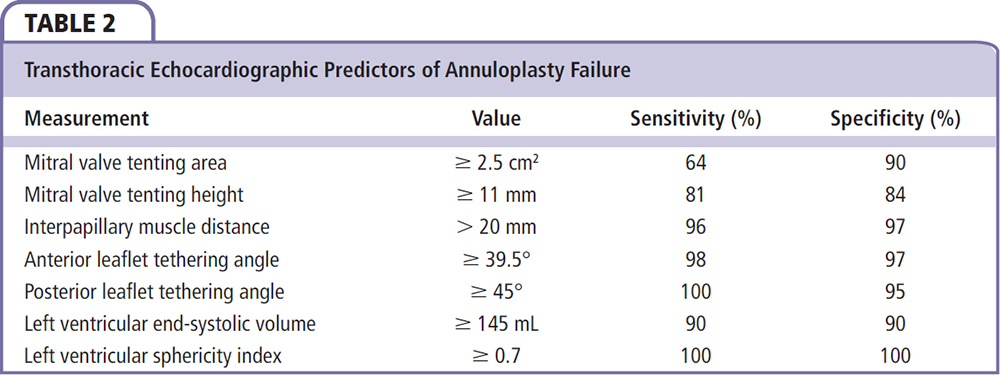
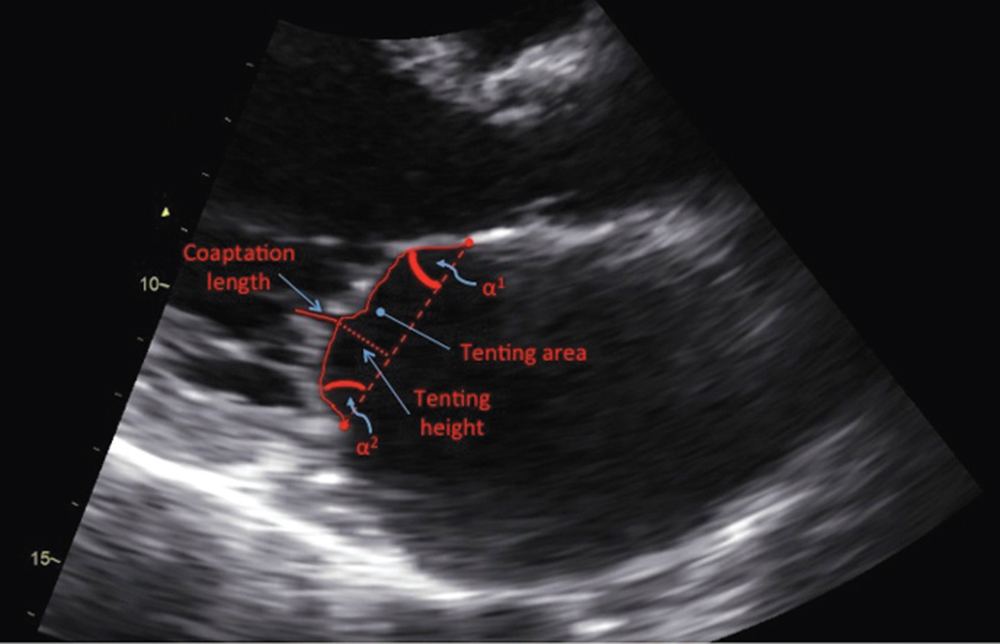
Figure 1. Parasternal long-axis view of the mitral valve in mid-systole on transthoracic echocardiogram. α1, anterior leaflet tethering angle; α2, posterior leaflet tethering angle; tenting area, area enclosed by the mitral leaflets and annular plane.
In patients undergoing isolated CABG with moderate preoperative IMR, up to 50% will have persistence or worsening of the mitral regurgitation grade after revascularization alone…
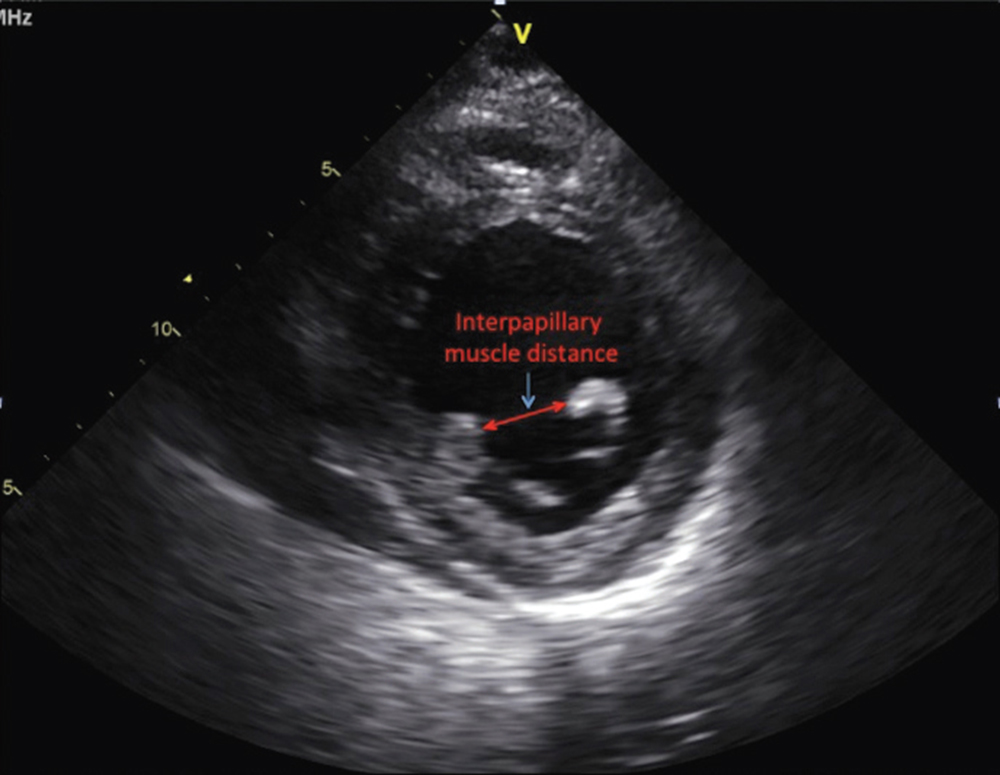
Figure 2. Parasternal short-axis view at the level of the papillary muscles at end-systole on transthoracic echocardiogram.
Main Points
• Ischemic mitral regurgitation (IMR) is a common comorbidity seen in more than half of patients with ischemic cardiomyopathy. Chronic IMR leads to further deterioration of ventricular systolic function, worsening of the mitral regurgitation, and increased adverse cardiovascular events and mortality.
• Restrictive mitral annuloplasty is the current surgical procedure of choice in the treatment of IMR. The 1-year and 5-year survival has been estimated at 82% and 52%, respectively, with significant improvements reported in quality of life and left ventricular (LV) remodeling. However, there is a 15% to 30% incidence of late recurrent mitral regurgitation after annuloplasty, which increases the risk of required reoperation for clinically significant mitral regurgitation, when compared with valve replacement.
• The decision to replace the mitral valve must be balanced against the risks of thromboembolic complications of metallic valves, or the long-term durability of bioprosthetic valves.
• Innovative papillary muscle procedures performed at the time of annuloplasty, which aim to relieve leaflet tethering and improve the function of the subvalvular apparatus, may confer an improved durability of valve repair in IMR.
• Techniques aimed at restoring proper anatomic papillary muscle alignment appear to improve ventricular geometry and the durability of valve repair in the setting of IMR, in patients with preoperative echocardiographic predictors for annuloplasty failure. Several preoperative predictors of persistent or worsened mitral regurgitation after isolated coronary artery bypass grafting (CABG) have been identified, which can help risk stratify patients and select candidates who may benefit from combined CABG and mitral valve repair.
Main Points
• Ischemic mitral regurgitation (IMR) is a common comorbidity seen in more than half of patients with ischemic cardiomyopathy. Chronic IMR leads to further deterioration of ventricular systolic function, worsening of the mitral regurgitation, and increased adverse cardiovascular events and mortality.
• Restrictive mitral annuloplasty is the current surgical procedure of choice in the treatment of IMR. The 1-year and 5-year survival has been estimated at 82% and 52%, respectively, with significant improvements reported in quality of life and left ventricular (LV) remodeling. However, there is a 15% to 30% incidence of late recurrent mitral regurgitation after annuloplasty, which increases the risk of required reoperation for clinically significant mitral regurgitation, when compared with valve replacement.
• The decision to replace the mitral valve must be balanced against the risks of thromboembolic complications of metallic valves, or the long-term durability of bioprosthetic valves.
• Innovative papillary muscle procedures performed at the time of annuloplasty, which aim to relieve leaflet tethering and improve the function of the subvalvular apparatus, may confer an improved durability of valve repair in IMR.
• Techniques aimed at restoring proper anatomic papillary muscle alignment appear to improve ventricular geometry and the durability of valve repair in the setting of IMR, in patients with preoperative echocardiographic predictors for annuloplasty failure. Several preoperative predictors of persistent or worsened mitral regurgitation after isolated coronary artery bypass grafting (CABG) have been identified, which can help risk stratify patients and select candidates who may benefit from combined CABG and mitral valve repair.
Carpentier type IIIb mitral valve dysfunction, which is more commonly referred to as functional or ischemic mitral regurgitation (IMR), is a common comorbidity seen in more than half of patients with ischemic cardiomyopathy.1 It is defined as new mitral regurgitation present ≥ 1 week after a diagnosed myocardial infarction, with the following three requirements: development of a left ventricular (LV) wall motion abnormality, significant coronary artery disease in the territory of the wall motion abnormality, and structurally normal mitral valve leaflets.2 The presence of IMR in this setting is associated with a twofold increase in mortality and new or worsening heart failure, and is commensurate to the degree of mitral regurgitation present.3 The mechanism of IMR is centered on the complex interplay involving LV remodeling and distortion of the subvalvular apparatus. As the left ventricle dilates, there is an apicolateral displacement of the papillary muscles, which leads to chordal and leaflet tethering, with resultant leaflet malapposition and regurgitation. Chronic IMR leads to further deterioration of ventricular systolic function, worsening of the mitral regurgitation, and increased adverse cardiovascular events and mortality.1
Mitral Valve Repair Versus Replacement
First popularized by Boiling and colleagues,4 a restrictive mitral annuloplasty is the current surgical procedure of choice in the treatment of IMR, which involves reducing the septolateral diameter of the mitral annulus with an undersized annuloplasty ring.4 The 1-year and 5-year survival has been estimated at 82% and 52%, respectively, with significant improvements reported in quality of life and LV remodeling.5 However, there is a 15% to 30% incidence of late recurrent mitral regurgitation after annuloplasty, which increases the risk of required reoperation for clinically significant mitral regurgitation, when compared with valve replacement.6,7 Thus, the optimal surgical approach to IMR remains controversial, with most of the reported data obtained from non-randomized, retrospective studies.
In the only randomized trial comparing mitral valve repair versus replacement for IMR, the Cardiothoracic Surgical Trials Network allocated 251 patients with severe IMR to mitral valve repair with restrictive annuloplasty (n = 126) or chordal-sparing mitral valve replacement (n = 125).8 The baseline comorbidities, as well as the LV ejection fraction (repair group = 42% ± 10, replacement group = 40% ± 11; P = .10), were similar between the cohorts, and the majority of patients underwent concomitant coronary artery bypass grafting, tricuspid valve repair, and/or atrial maze procedures. There were no differences between the surgical approaches in mortality at 30 days or 1 year, echo-cardiographic parameters of LV remodeling, or clinical outcomes. However, there was a significantly higher rate of recurrence of moderate to severe mitral regurgitation at 1 year in patients undergoing valve repair as opposed to replacement (32.6% vs 2.3%; P < .001).
Despite the reported benefits in perioperative outcomes, LV reverse remodeling, and New York Heart Association functional class in patients undergoing valve repair for IMR, no proven survival benefit has been shown over valve replacement, with a higher incidence of recurrent mitral regurgitation in repair cohorts observed at follow-up.6-8 Thus, significant equipoise exists regarding the optimal approach to correcting severe IMR. In instances of advanced ventricular remodeling and subvalvular dysfunction, mitral valve replacement with chordal preservation may be considered, which decreases the risk of ventricular-annular disruption and LV functional impairment.9 However, the decision to replace the mitral valve must be balanced against the risks of thromboembolic complications of metallic valves, or the long-term durability of bioprosthetic valves. Furthermore, innovative papillary muscle procedures performed at the time of annuloplasty, which aim to relieve leaflet tethering and improve the function of the subvalvular apparatus, may confer an improved durability of valve repair in IMR.
Papillary Muscle Intervention to Improve Repair Durability in IMR
To minimize the recurrence of mitral regurgitation after valve repair for IMR, techniques aimed at the entire ventriculomitral unit maybe of benefit. Restrictive mitral annuloplasty corrects annular dilatation by reducing the septolateral diameter, without addressing concomitant subvalvular dysfunction. Due to a sufficiently redundant anterior leaflet along with active leaflet remodeling processes that occur as LV function deteriorates, a 1.8-fold increase in annular size can be tolerated before mitral regurgitation develops, suggesting a greater burden from complex ventricular remodeling rather than annular dilatation in IMR.10,11 In the trial by the Cardiothoracic Surgical Trials Network,8 which utilized downsized rigid or semirigid complete annuloplasty rings for valve repair, the patients with recurrent mitral regurgitation had no evidence of reverse remodeling, when compared with the successful annuloplasty procedures (LV end-systolic volume index 64.1 ± 23.9 mL/m2 vs 47.3 ± 23 mL/m2).8 This supports the theory that IMR is a disease of the ventricular myocardium and subvalvular apparatus, as opposed to the mitral valve itself, and continued ventricular remodeling after restrictive mitral annuloplasty leads to progressive displacement of the papillary muscles, which may cause valve incompetence and potential annuloplasty failure.
A pathophysiology-guided approach to surgical repair may be preferable, with the goal of alleviating leaflet tethering forces and ventricular geometric distortions that contribute to annuloplasty failure. One such target has been the reestablishment of physiologic papillary muscle positioning through several innovative subvalvular techniques that can be performed at the time of annuloplasty repair. Such procedures include papillary muscle approximation or elevation, as well as commissural plication and chordal base fixture (Table 1).12-19 Combining annuloplasty with a papillary muscle intervention results in reduced septolateral annular dilatation, moves the anterolateral papillary muscle closer to the annulus, and corrects both lateral displacement and apical restriction of the posterior papillary muscle.20,21 Furthermore, these techniques may help attenuate the augmented posterior leaflet tethering that often occurs after restrictive annuloplasty, which itself is associated with the development of recurrent mitral regurgitation.22,23 In patients with preoperative echocardiographic predictors of annuloplasty failure (Table 2), combining annuloplasty repair with a papillary muscle procedure allows for a complete geometric repair of the ventriculomitral unit (Figures 1 and 2).24
Should Moderate IMR be Treated?
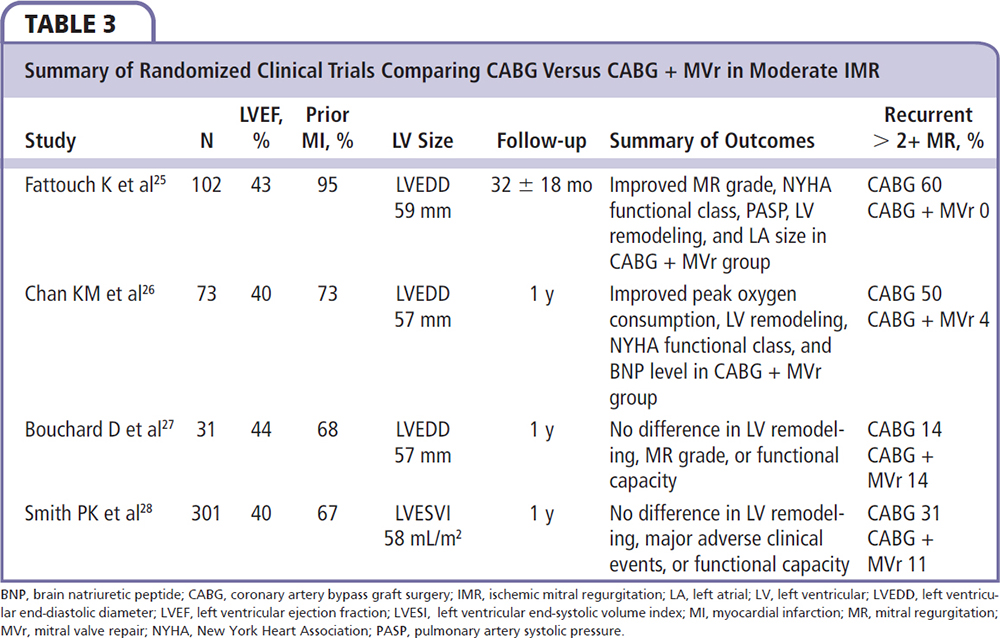
To date, there have been four randomized controlled trials comparing revascularization with coronary artery bypass graft (CABG) surgery alone versus CABG plus mitral valve repair utilizing a restrictive annuloplasty in patients with coronary artery disease and moderate IMR.25-28 The studies yielded equivocal results, with two trials reporting improved functional capacity, LV reverse remodeling, pulmonary hemodynamics, and less mitral regurgitation recurrence with concomitant valve repair,25,26 whereas two later trials found no difference in clinical outcomes or incidence of significant recurrent mitral regurgitation (Table 3).27,28 However, several important limitations were noted among these trials, including lack of consistency in defining IMR, suboptimal analysis of the impact of recurrent mitral regurgitation on functional status and survival, heterogeneity in the types of annuloplasty rings utilized, and limited follow-up of 1 year in three of the four trials.
In patients undergoing isolated CABG with moderate preoperative IMR, up to 50% will have persistence or worsening of the mitral regurgitation grade after revascularization alone, which portends a poor prognosis.29,30 Several preoperative predictors of persistent or worsened mitral regurgitation after isolated CABG have been identified, which can help risk stratify patients and select candidates who may benefit from combined CABG and mitral valve repair. These include (1) extensively infarcted myocardium with ≤ 5 viable myocardial segments; (2) infarcted myocardium subtending or adjacent to one or both papillary muscles; (3) dys-synchronous papillary muscles, defined as > 60 ms by echocardiographic tissue Doppler imaging; and (4) exercise intolerance or worsening mitral regurgitation on exercise stress testing.8,25,30-34 A concomitant papillary muscle intervention may be considered in these patients.
Conclusions
In the 2014 American College of Cardiology/American Heart Association, and the 2012 European Society of Cardiology/European Association for Cardiothoracic Surgery guidelines for the management of valvular heart disease, surgical correction holds a class IIb recommendation for patients with symptomatic severe IMR refractory to optimal guideline-directed management of ischemic heart disease and heart failure.35,36 This reflects the uncertainty regarding the optimal surgical approach to treating IMR. Addressing the mechanisms underlying IMR is critical to applying the optimal surgical strategy. Techniques aimed at restoring proper anatomic papillary muscle alignment appear to improve ventricular geometry and the durability of valve repair in the setting of IMR in patients with preoperative echocardiographic predictors for annuloplasty failure. However, most reports are from small, single-center experiences with short-term follow-up. Randomized trials and multicenter registries are needed to validate the efficacy and long-term outcomes of these promising procedures. ![]()
References
- Levine RA, Schwammenthal E. Ischemic mitral regurgitation on the threshold of a solution: from paradoxes to unifying concepts. Circulation. 2005;112:745-758.
- Borger MA, Alam A, Murphy PM, et al. Chronic ischemic mitral regurgitation: repair, replace or rethink? Ann Thorac Surg. 2006;81:1153-1161.
- Rossi A, Dini FL, Faggiano P, et al. Independent prognostic value of functional mitral regurgitation in patients with heart failure. A quantitative analysis of 1256 patients with ischaemic and non-ischaemic dilated cardiomyopathy. Heart. 2011;97:1675-1680.
- Bolling SF, Deeb GM, Brunsting LA, Bach DS. Early outcome of mitral valve reconstruction in patients with end-stage cardiomyopathy. J Thorac Cardiovasc Surg. 1995;109:676-682.
- Romano MA, Bolling SF. Mitral valve repair as an alternative treatment for heart failure patients. Heart Fail Monit. 2003;4:7-12.
- Dayan V, Soca G, Cura L, Mestres CA. Similar survival after mitral valve replacement or repair for ischemic mitral regurgitation: a meta-analysis. Ann Thorac Surg. 2014;97:758-765.
- Vassileva CM, Boley T, Markwell S, Hazelrigg S. Meta-analysis of short-term and long-term survival following repair versus replacement for ischemic mitral regurgitation. Eur J Cardiothorac Surg. 2011;39:295-303.
- Acker MA, Parides MK, Perrault LP, et al; CTSN. Mitral valve repair versus replacement for severe ischemic mitral regurgitation. N Engl J Med. 2014;370:23-32.
- Reardon MJ, David TE. Mitral valve replacement with preservation of the subvalvular apparatus. Curr Opin Cardiol. 1999;14:104-110.
- He S, Lemmon JD Jr, Weston MW, et al. Mitral valve compensation for annular dilatation: in vitro study into the mechanisms of functional mitral regurgitation with an adjustable annulus model. J Heart Valve Dis. 1999;8:294-302.
- Grande-Allen KJ, Borowski AG, Troughton RW, et al. Apparently normal mitral valves in patients with heart failure demonstrate biochemical and structural derangements: an extracellular matrix and echocardiographic study. J Am Coll Cardiol. 2005;45:54-61.
- Fattouch K, Castrovinci S, Murana G, et al. Papillary muscle relocation and mitral annuloplasty in ischemic mitral valve regurgitation: midterm results. J Thorac Cardiovasc Surg. 2014;148:1947-1950.
- Hvass U, Joudinaud T. The papillary muscle sling for ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2010;139:418-423.
- Roshanali F, Vedadian A, Shoar S, et al. Efficacy of papillary muscle approximation in preventing functional mitral regurgitation recurrence in high-risk patients with ischaemic cardiomyopathy and mitral regurgitation. Acta Cardiol. 2013;68:271-278.
- Langer F, Kunihara T, Hell K, et al. RING+STRING: successful repair technique for ischemic mitral regurgitation with severe leaflet tethering. Circulation. 2009;120(11 suppl):S85-S91.
- Santana O, Solenkova NV, Pineda AM, et al. Minimally invasive papillary muscle sling placement during mitral valve repair in patients with functional mitral regurgitation. J Thorac Cardiovasc Surg. 2014;147:496-499.
- Kron IL, Green GR, Cope JT. Surgical relocation of the posterior papillary muscle in chronic ischemic mitral regurgitation. Ann Thorac Surg. 2002;74:600-601.
- Ishikawa S, Kugawa S, Abe K, et al. Papillary muscle head approximation for the treatment of mitral valve regurgitation combined with aortic valve disease. Int Heart J. 2010;51:47-50.
- Rama A, Praschker L, Barreda E, Gandjbakhch I. Papillary muscle approximation for functional ischemic mitral regurgitation. Ann Thorac Surg. 2007;84:2130-2131.
- Tibayan FA, Rodriguez F, Langer F, et al. Annular or subvalvular approach to chronic ischemic mitral regurgitation? J Thorac Cardiovasc Surg. 2005;129:1266-1275.
- Manabe S, Shimokawa T, Fukui T, et al. Impact of papillary muscle approximation on mitral valve configuration in the surgical correction of ischemic mitral regurgitation. Thorac Cardiovasc Surg. 2012;60:269-274.
- Kuwahara E, Otsuji Y, Iguro Y, et al. Mechanism of recurrent/persistent ischemic/functional mitral regurgitation in the chronic phase after surgical annuloplasty: importance of augmented posterior leaflet tethering. Circulation. 2006;114(1 suppl):I529-I534.
- Green GR, Dagum P, Glasson JR, et al. Restricted posterior leaflet motion after mitral ring annuloplasty. Ann Thorac Surg. 1999;68:2100-2106.
- Bouma W, van der Horst IC, Wijdh-den Hamer IJ, et al. Chronic ischaemic mitral regurgitation. Current treatment results and new mechanism-based surgical approaches. Eur J Cardiothorac Surg. 2010;37:170-185.
- Fattouch K, Guccione F, Sampognaro R, et al. POINT: Efficacy of adding mitral valve restrictive annuloplasty to coronary artery bypass grafting in patients with moderate ischemic mitral valve regurgitation: a randomized trial. J Thorac Cardiovasc Surg. 2009;138:278-285.
- Chan KM, Punjabi PP, Flather M, et al; RIME Investigators. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation: final results of the Randomized Ischemic Mitral Evaluation (RIME) trial. Circulation. 2012;126:2502-2510.
- Bouchard D, Jensen H, Carrier M, et al. Effect of systematic downsizing rigid ring annuloplasty in patients with moderate ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2014;147:1471-1477.
- Smith PK, Puskas JD, Ascheim DD, et al; Cardiothoracic Surgical Trials Network Investigators. Surgical treatment of moderate ischemic mitral regurgitation. N Engl J Med. 2014;371:2178-2188.
- Schroder JN, Williams ML, Hata JA, et al. Impact of mitral valve regurgitation evaluated by intraoperative transesophageal echocardiography on long-term outcomes after coronary artery bypass grafting. Circulation. 2005;112(9 suppl):I293-I298.
- Campwala SZ, Bansal RC, Wang N, et al. Mitral regurgitation progression following isolated coronary artery bypass surgery: frequency, risk factors, and potential prevention strategies. Eur J Cardiothorac Surg. 2006;29:348-353.
- Penicka M, Linkova H, Lang O, et al. Predictors of improvement of unrepaired moderate ischemic mitral regurgitation in patients undergoing elective isolated coronary artery bypass graft surgery. Circulation. 2009;120:1474-1481.
- Cieślikowski D, Baron, T, Grodzicki T. Exercise echocardiography in the evaluation of functional mitral regurgitation: a systematic review of the literature. Cardiol J. 2007;14:436-446.
- Lancelloti P, Troisfontaines P, Toussaint AC, Pierard LA. Prognostic importance of exercise-induced changes in mitral regurgitation in patients with chronic ischemic left ventricular dysfunction. Circulation. 2003;108:1713-1717.
- Szymanski C, Levine RA, Tribouilloy C, et al. Impact of mitral regurgitation on exercise capacity and clinical outcomes in patients with ischemic left ventricular dysfunction. Am J Cardiol. 2011;108:1714-1720.
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451-2496.
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2014;63:e57-e118.