Tricuspid Regurgitation, the Forgotten Valvular Lesion—A Contemporary Review of Etiology, Prevalence, and Management Options
Richard Cheng, MD,1 Amir Azarbal, MD,2 Jesse Currier, MD,3 Louise E. J. Thomson, MBChB, FRACP,1 Michele A. Hamilton, MD,1 Fardad Esmailian, MD,1 Babak Azarbal, MD1,3
1Cedars-Sinai Heart Institute, Los Angeles, CA; 2Department of Medicine, University of Alabama Medical Center, Huntsville, AL; 3Division of Cardiology, VA Greater Los Angeles Healthcare System, Los Angeles, CA
Tricuspid regurgitation (TR) is a common finding. Pathologic TR is an independent risk factor for mortality. TR can be classified by etiology into functional versus organic. Organic TR is caused by structural damage to the tricuspid valve (TV) by a spectrum of etiologies, including pacemaker leads and right heart biopsies, whereas functional TR is predominantly due to elevated pulmonary pressures. Atrial fibrillation and chamber enlargement, among other risk factors, are strong predictors of functional TR. Correction of elevated pulmonary pressures improves TR, and concurrent repair of severe TR at the time of left heart valve surgery improves postoperative heart failure symptoms but does not improve survival. TR repair is associated with less operative and long-term mortality than TV replacement, and demonstrates similar improvements in heart failure symptoms. Substantial residual TR remains after repair, and reoperative mortality for residual TR is considerable. Percutaneous TV replacement may offer a rescue strategy.
[Rev Cardiovasc Med. 2015;16(3):171-181 doi: 10.3909/ricm0766]
© 2015 MedReviews®, LLC
Tricuspid Regurgitation, the Forgotten Valvular Lesion—A Contemporary Review of Etiology, Prevalence, and Management Options
Richard Cheng, MD,1 Amir Azarbal, MD,2 Jesse Currier, MD,3 Louise E. J. Thomson, MBChB, FRACP,1 Michele A. Hamilton, MD,1 Fardad Esmailian, MD,1 Babak Azarbal, MD1,3
1Cedars-Sinai Heart Institute, Los Angeles, CA; 2Department of Medicine, University of Alabama Medical Center, Huntsville, AL; 3Division of Cardiology, VA Greater Los Angeles Healthcare System, Los Angeles, CA
Tricuspid regurgitation (TR) is a common finding. Pathologic TR is an independent risk factor for mortality. TR can be classified by etiology into functional versus organic. Organic TR is caused by structural damage to the tricuspid valve (TV) by a spectrum of etiologies, including pacemaker leads and right heart biopsies, whereas functional TR is predominantly due to elevated pulmonary pressures. Atrial fibrillation and chamber enlargement, among other risk factors, are strong predictors of functional TR. Correction of elevated pulmonary pressures improves TR, and concurrent repair of severe TR at the time of left heart valve surgery improves postoperative heart failure symptoms but does not improve survival. TR repair is associated with less operative and long-term mortality than TV replacement, and demonstrates similar improvements in heart failure symptoms. Substantial residual TR remains after repair, and reoperative mortality for residual TR is considerable. Percutaneous TV replacement may offer a rescue strategy.
[Rev Cardiovasc Med. 2015;16(3):171-181 doi: 10.3909/ricm0766]
© 2015 MedReviews®, LLC
Tricuspid Regurgitation, the Forgotten Valvular Lesion—A Contemporary Review of Etiology, Prevalence, and Management Options
Richard Cheng, MD,1 Amir Azarbal, MD,2 Jesse Currier, MD,3 Louise E. J. Thomson, MBChB, FRACP,1 Michele A. Hamilton, MD,1 Fardad Esmailian, MD,1 Babak Azarbal, MD1,3
1Cedars-Sinai Heart Institute, Los Angeles, CA; 2Department of Medicine, University of Alabama Medical Center, Huntsville, AL; 3Division of Cardiology, VA Greater Los Angeles Healthcare System, Los Angeles, CA
Tricuspid regurgitation (TR) is a common finding. Pathologic TR is an independent risk factor for mortality. TR can be classified by etiology into functional versus organic. Organic TR is caused by structural damage to the tricuspid valve (TV) by a spectrum of etiologies, including pacemaker leads and right heart biopsies, whereas functional TR is predominantly due to elevated pulmonary pressures. Atrial fibrillation and chamber enlargement, among other risk factors, are strong predictors of functional TR. Correction of elevated pulmonary pressures improves TR, and concurrent repair of severe TR at the time of left heart valve surgery improves postoperative heart failure symptoms but does not improve survival. TR repair is associated with less operative and long-term mortality than TV replacement, and demonstrates similar improvements in heart failure symptoms. Substantial residual TR remains after repair, and reoperative mortality for residual TR is considerable. Percutaneous TV replacement may offer a rescue strategy.
[Rev Cardiovasc Med. 2015;16(3):171-181 doi: 10.3909/ricm0766]
© 2015 MedReviews®, LLC
KEY WORDS
Tricuspid regurgitation • Tricuspid valve disease • Cardiac surgery • Valvular heart disease
KEY WORDS
Tricuspid regurgitation • Tricuspid valve disease • Cardiac surgery • Valvular heart disease
The main etiologies of TR with concomitant stenosis include rheumatic disease, congenital abnormalities, and infective endocarditis.
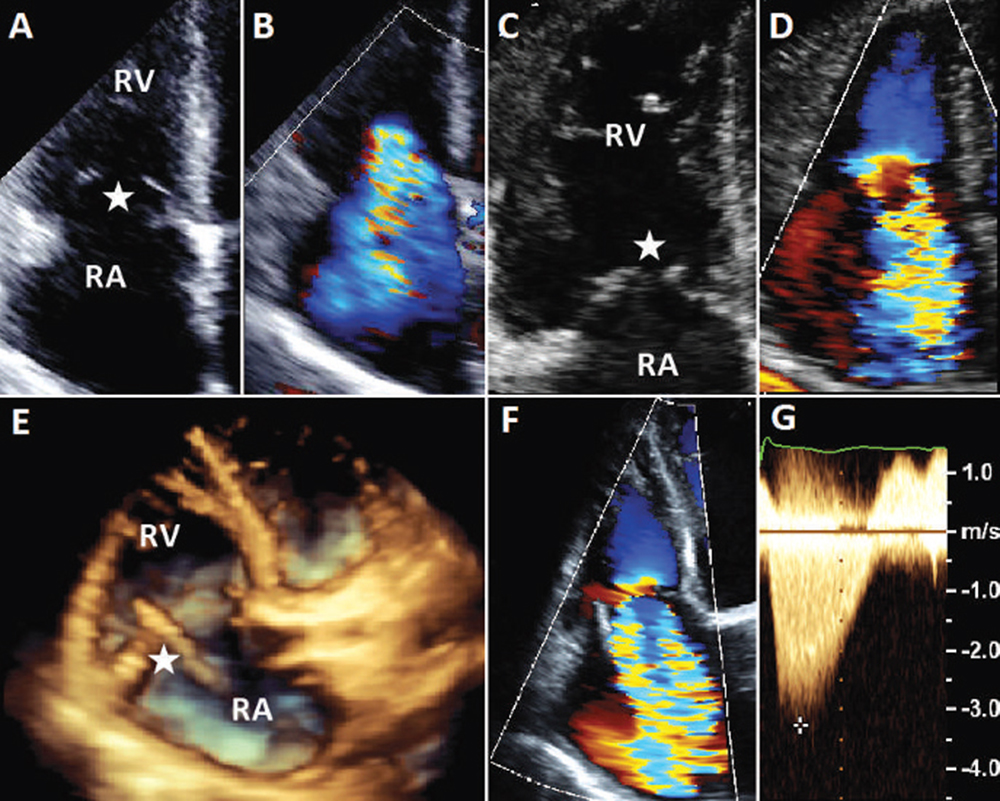
Figure 1. Tricuspid valve disease by transthoracic echocardiography. Transthoracic echocardiogram in patients with severe tricuspid regurgitation. (A, B) Severe functional tricuspid regurgitation with a normal-appearing tricuspid valve (star) in a patient with acute decompensated heart failure. (C, D) Severe organic tricuspid regurgitation with severe thickening, shortening, and retraction of the tricuspid leaflets (star) consistent with carcinoid heart disease. (E, F) Severe iatrogenic tricuspid regurgitation due to a pacemaker lead (star) restricting the anterior leaflet on three-dimensional (E) and two-dimensional (F) transthoracic echocardiogram. (G) A continuous-wave Doppler of severe tricuspid regurgitation showing a characteristic daggershaped pattern of early peak pressure and fast decline. RA, right atrium; RV, right ventricle.
FTR is defined as TR in an otherwise structurally normal TV, usually secondary to elevated pulmonary pressures due to any cause, but most commonly from left-sided heart disease.
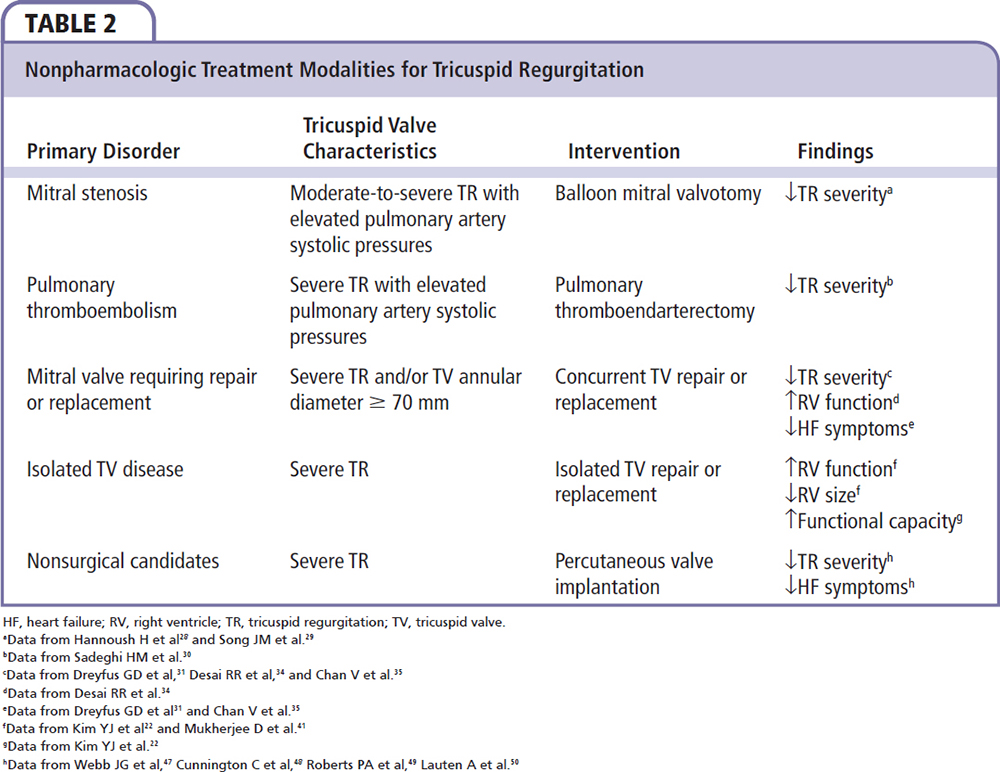
Figure 2. Factors contributing to organic and functional tricuspid regurgitation. dz, disease; PASP, pulmonary artery systolic pressure; RV, right ventricle, TV, tricuspid valve.
Isolated TR is often discovered incidentally on echocardiography, as patients with significant isolated TR often do not have any symptoms until the disease has reached an advanced stage.
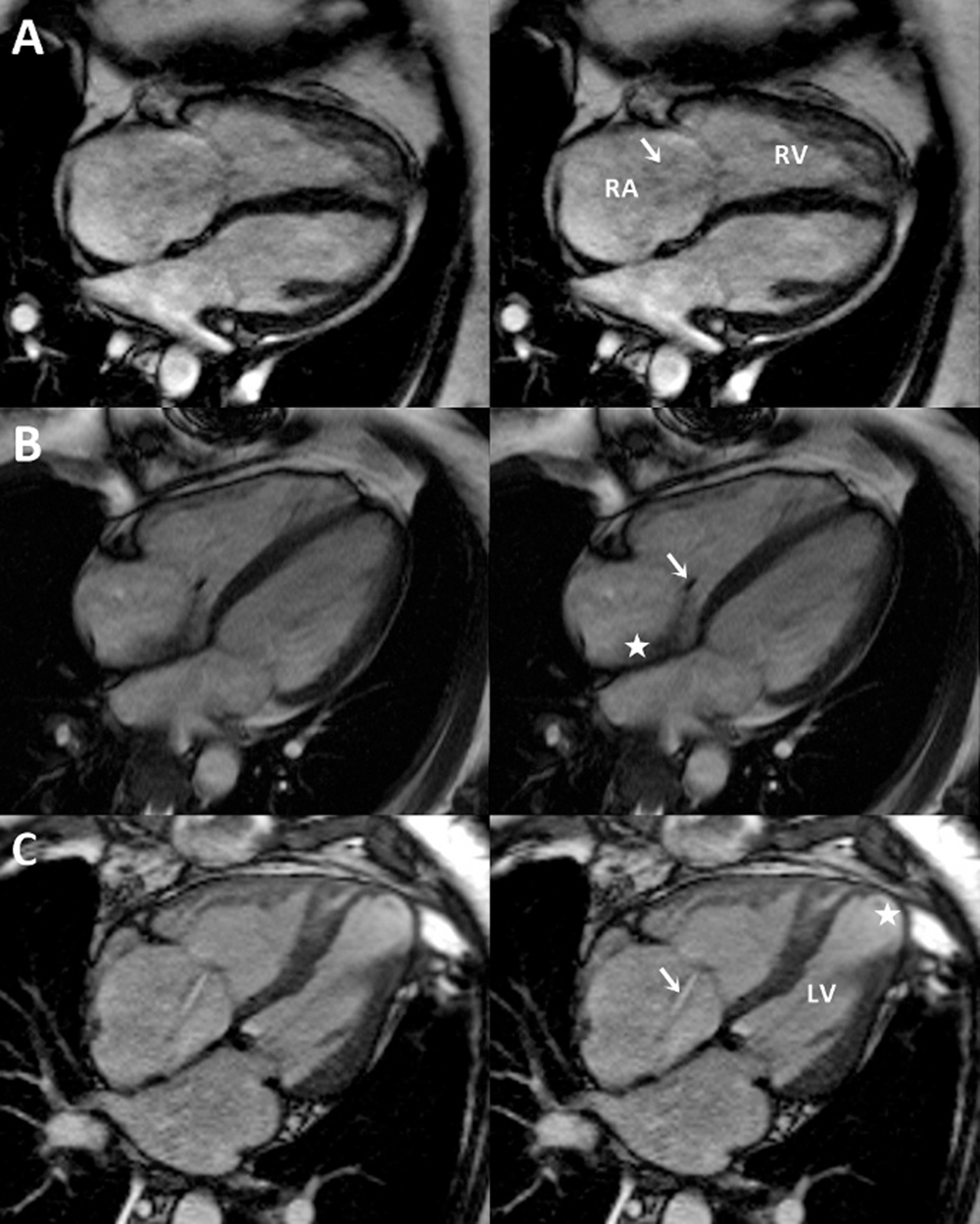
Figure 3. Tricuspid valve disease by cine magnetic resonance imaging. Single representative systolic frames from cine magnetic resonance images of the heart show variable image appearance in patients with severe tricuspid regurgitation. (A) Increased diameter of the tricuspid annulus, with a central broad jet of tricuspid regurgitation (arrow) that, in view of its low velocity, results in minimal turbulence and is relatively difficult to see on cine imaging. (B) A vegetation on the tricuspid valve (arrow) results in a turbulent and eccentric jet of TR that can be easily seen (star). (C) Central TR (arrow) with annular diameter increase in a patient with longstanding mitral valve disease, prior mitral valve surgery, and coronary artery disease. The left ventricle is thinned and dilated at the apex (star). LV, left ventricle; RA, right atrium; RV, right ventricle.
Routine concurrent TV repair at the time of mitral valve surgery when TV annular diameter is $≥70 mm regardless of TR severity has been shown to have favorable results.
Repair is generally recommended over replacement. Although valve replacement typically results in more durable reduction in TR severity, operative mortality is higher…
… the emergence of percutaneous therapies offers a potential rescue strategy for patients at high operative risk who require valve replacement.
Main Points
• Tricuspid regurgitation (TR) is a common echocardiographic finding. Physiologic TR is described as a thin central Doppler jet in early systole in the absence of valve leaflet pathology and dilated chamber sizes. The vast majority of cases are managed conservatively due to the still-evolving understanding of the disease and surgical repair for severe isolated TR is rarely performed.
• Functional TR (FTR) is defined as regurgitation without any identifiable structural damage to the TV, leaflets, or chords. FTR usually occurs in the presence of pulmonary artery hypertension from any cause (most commonly from elevated left-sided pressures), right ventricular enlargement, and tricuspid annular dilatation.
• Physical examination findings specific to severe TR include auscultative findings of TR, evidence of central venous pressure elevation, RV failure, hepatic enlargement and dysfunction, and edema. The TR murmur is soft and holosystolic, increases with inspiration, and is best heard at the upper parasternal border. A diastolic rumble may be present in cases of organic TR with concomitant stenosis.
• Severity of TR is associated with worse liver and kidney function, suggestive of the detrimental effects of elevated central venous pressure and the tricuspid regurgitant jet.
• Current guidelines recommend concurrent TV surgery for severe TR in patients undergoing left heart valve surgery, and less strongly recommends surgery in those with less-than-severe TR in the presence of a dilated annulus. Repair is generally recommended over replacement. Although valve replacement typically results in more durable reduction in TR severity, operative mortality is higher, and the need for reoperation for residual TR is similar between the two strategies.
• Percutaneous pulmonary valves have been implanted valve-in-valve in the tricuspid position with high procedural success. Moreover, percutaneous self-expanding valves have been successfully implanted in the superior and inferior vena cava for treatment of TR. Although long-term data are lacking, the emergence of percutaneous therapies offers a potential rescue strategy for patients at high operative risk who require valve replacement.
Main Points
• Tricuspid regurgitation (TR) is a common echocardiographic finding. Physiologic TR is described as a thin central Doppler jet in early systole in the absence of valve leaflet pathology and dilated chamber sizes. The vast majority of cases are managed conservatively due to the still-evolving understanding of the disease and surgical repair for severe isolated TR is rarely performed.
• Functional TR (FTR) is defined as regurgitation without any identifiable structural damage to the TV, leaflets, or chords. FTR usually occurs in the presence of pulmonary artery hypertension from any cause (most commonly from elevated left-sided pressures), right ventricular enlargement, and tricuspid annular dilatation.
• Physical examination findings specific to severe TR include auscultative findings of TR, evidence of central venous pressure elevation, RV failure, hepatic enlargement and dysfunction, and edema. The TR murmur is soft and holosystolic, increases with inspiration, and is best heard at the upper parasternal border. A diastolic rumble may be present in cases of organic TR with concomitant stenosis.
• Severity of TR is associated with worse liver and kidney function, suggestive of the detrimental effects of elevated central venous pressure and the tricuspid regurgitant jet.
• Current guidelines recommend concurrent TV surgery for severe TR in patients undergoing left heart valve surgery, and less strongly recommends surgery in those with less-than-severe TR in the presence of a dilated annulus. Repair is generally recommended over replacement. Although valve replacement typically results in more durable reduction in TR severity, operative mortality is higher, and the need for reoperation for residual TR is similar between the two strategies.
• Percutaneous pulmonary valves have been implanted valve-in-valve in the tricuspid position with high procedural success. Moreover, percutaneous self-expanding valves have been successfully implanted in the superior and inferior vena cava for treatment of TR. Although long-term data are lacking, the emergence of percutaneous therapies offers a potential rescue strategy for patients at high operative risk who require valve replacement.
Tricuspid regurgitation (TR) is a common echocardiographic diagnosis, and the prevalence of any TR is estimated to be 70% in the general population. Although trace to mild TR is often an incidental finding identified on echocardiography without any adverse clinical consequences, presence of moderate to severe TR is an independent risk factor for mortality.1-4 The prognosis of severe functional TR (FTR) is comparable with that of severe functional mitral regurgitation.1
In the current era, much focus has been devoted to the diagnosis, management, and treatment of aortic and mitral valve pathology. Clear national and international consensus and practice standards guide the management of the various disease conditions associated with dysfunction of these valves.5,6 However, the understanding of underlying disease processes associated with TR is much less complete, and there is a paucity of consensus guidelines on management options for patients with severe TR.
In this article, the etiology, risk factors, associated disease conditions for TR, as well as diagnostic modalities available for the diagnosis of this condition, are explored. Current treatment modalities, including indications for medical versus surgical therapy, as well as emerging surgical and percutaneous options, are discussed.
Prevalence and Etiology
TR is a common echocardiographic finding. Physiologic TR, described as a thin central Doppler jet in early systole in the absence of valve leaflet pathology and dilated chamber sizes, is seen in over 70% of healthy adults.7 Although the precise discrimination between physiologic TR and pathologic TR seen in tricuspid valve (TV) disease is not well established, significant TR is estimated to occur in more than 1.6 million people in the United States. The vast majority of cases are managed conservatively due to the still-evolving understanding of the disease,8,9 and surgical repair for severe isolated TR is rarely performed.4
TV disease can be described as organic due to local destruction of the valve, or as FTR. Organic TR can occur with or without tricuspid stenosis. The main etiologies of TR with concomitant stenosis include rheumatic disease, congenital abnormalities, and infective endocarditis. There also are a variety of metabolic, enzymatic, or pharmacologic abnormalities associated with TR, including carcinoid (Figure 1), Fabry disease, Whipple disease, and methysergide therapy.10 Rheumatic disease, although decreasing in prevalence, remains a major cause, particularly outside the United States.
A wider range of etiologies account for organic TR without concomitant stenosis and are summarized along with other etiologies of TR in Table 1.11 Moreover, pacemaker leads in the right ventricle (RV) and right heart biopsies after cardiac transplantation are also recognized as iatrogenic causes of TR.12-15
FTR is defined as regurgitation without any identifiable structural damage to the TV, leaflets, or chords. FTR usually occurs in the presence of pulmonary artery hypertension from any cause (most commonly from elevated left-sided pressures), RV enlargement, and tricuspid annular dilatation.16-18 With the decreasing prevalence of rheumatic disease, FTR is proportionally more common and accounts for at least 80% of observed cases of severe TR.17
Pacemaker Leads
In a recent review of over 1600 patients, there is near uniform evidence for increased prevalence and severity of TR in patients with pacemaker leads. The thickness of the leads, such as those of implant-able cardioverter defibrillators, and the number of leads passing through the TV, are also associated with severity of TR.14 In one series of 745 patients, the odds ratio for moderate-to-severe TR with a pacemaker lead is increased nearly fivefold.19 The mechanism is thought to be TV leaflet damage due to lead entanglement, impingement, adherence, and/or perforation,15 although scar formation, lead thrombus, and ventricular dyssynchrony from RV pacing are also implicated.14 Transthoracic two-and three-dimensional imaging of severe iatrogenic TR due to a pacemaker lead restricting the anterior TV leaflet is shown in Figure 1.
Right Ventricular Biopsy
The prevalence of moderate-to-severe TR after cardiac transplantation is reported as 34% in 334 patients with mean 4.5-year follow-up,20 and is similar to a 25% prevalence of severe TR described in another cohort.13 TV replacement was required in 35% of patients with severe TR and heart failure, with demonstrable improvement in New York Heart Association (NYHA) class. All excised valves had chordae and/or leaflet damage,20 and the number of biopsies performed in each patient was associated with increased risk of severe TR, such that beyond 31 biopsies, 60% of patients had severe TR compared with none in those who had fewer than 18 biopsies.13
FTR
FTR is defined as TR in an otherwise structurally normal TV, usually secondary to elevated pulmonary pressures due to any cause, but most commonly from left-sided heart disease (Figure 1).7 Elevated pulmonary pressures are recognized as an important cause of FTR. In an echocardiographic study of 2139 patients, severity of TR was stratified according to the level of pulmonary artery systolic pressure (RASP). For patients with a PASP < 50 mm Hg, 96% had no-to-mild TR; higher PASP was associated with increasing proportions of moderate and severe TR. However, even with severely elevated PASP, FTR is not universally seen. In patients with a PASP of 50 to 69 mm Hg, and those with a PASP of ≥ 70 mm Hg, no-to-mild TR was found in 65% and 46% of patients, respectively, suggesting that isolated elevations in PASP do not fully explain the severity of TR.16
FTR has been demonstrated to be associated with adverse clinical outcomes. A recent large prospective observational study of 353 patients with FTR and without significant pulmonary hypertension demonstrated that 10-year survival was significantly lower in patients with severe TR compared with those without: 38% compared with 70% (P < .0001). In this cohort the best predictor of mortality was by the proximal isovelocity surface area method.4 Additional major risk factors for FTR include atrial fibrillation (AF), TV annular dilatation, and RV dilatation and/or dysfunction.1,18,21
Atrial Fibrillation
AF is found to be increased in patients with TR without organic TV disease. In a series of 1421 patients, AF prevalence increased with increasing severity of TR.1 Twice the prevalence of AF is found in patients with moderate-to-severe TR compared with those with mild TR,16 and AF is observed in over 80% of patients with severe TR requiring isolated TR surgery for heart failure.22 In a series of 242 patients with severe TR, 9.5% had FTR without elevated pulmonary pressures, and AF prevalence in this group was 93%.17 In patients with AF, age, female sex, and chronicity of AF were additional risk factors for TR severity.16,23
Annular and Chamber Dilatation
Patients with moderate-to-severe TR had increased TV dilatation,17 and TR severity was associated with increased atrial dimensions.1,16,17 Moreover, increased atrial, ventricular, and TV dimensions predicted the presence of severe TR,23 and the association of chamber sizes with TR has been consistently demonstrated across different patient cohorts.18,21 The relative contributions of structural dilatation and volume status are not clear.
Additional Risk Factors
RV dysfunction due to any cause including RV infarction, left-sided heart failure of any cause, organic mitral valve disease, and mitral regurgitation were additional risk factors for moderate-to-severe TR.16 Significant TR is reported in 15% of patients with arrhythmogenic right ventricular dysplasia (ARVD).24
Summary of Risk Factors for FTR
AF, annular and chamber dilatation, and severity of TR are observed in concert.1,16,17,23 Longstanding AF leads to annular and chamber dilatation that results in TR. Alternatively, elevated pulmonary pressures can lead to development of TR and cause annular and chamber dilatation; these changes can lead to increased atrial pressure and/or volume overload, atrial enlargement, and the genesis of AF. The factors contributing to organic and FTR are summarized in Figure 2.
Clinical Presentation
Clinical presentation is highly variable and dependent on the presence or absence of left-sided structural heart disease or other valvular pathologies, presence or absence of pulmonary hypertension of any cause, or atrial arrhythmias such as AF. Isolated TR is often discovered incidentally on echocardiography, as patients with significant isolated TR often do not have any symptoms until the disease has reached an advanced stage. Symptoms depend on presence or absence of other cardiovascular and valvular disorders, and may include fatigue, liver congestion, and resulting right upper quadrant discomfort, dyspepsia and/or indigestion, and fluid retention and ascites.25 Physical examination findings can also vary based on presence or absence of coexistent cardiac disease and/or pulmonary hypertension. These findings are reviewed elsewhere and are not covered in detail for the purposes of this review.25
Physical examination findings specific to severe TR include auscultative findings of TR, evidence of central venous pressure elevation, RV failure, hepatic enlargement and dysfunction, and edema. The TR murmur is soft and holosystolic, increases with inspiration, and is best heard at the upper parasternal border. A diastolic rumble may be present in cases of organic TR with concomitant stenosis. Wide splitting of S2 with an increased P2 component may be heard in patients with elevated pulmonary pressures. With severe TR, there is distension and elevation of the jugular venous pulsation. The internal jugular vein demonstrates a distinct C-V regurgitant wave during systole, which may be paradoxically more prominent with inspiration as a result of increased venous return. Hepatomegaly is a common finding, often accompanied by tenderness and associated pulsatility. Ascites, peripheral edema, and even anasarca can be present.25
Diagnostic and Imaging Findings
Imaging modalities for the evaluation of TV disease include transthoracic echocardiography (TTE), transesophageal echocardiography (TEE), and cardiac magnetic resonance imaging (MRI).7 Other than intraoperative assessment of tricuspid annular dilatation, TEE does not add significant diagnostic information, and TTE is often adequate for the diagnosis and evaluation of TV disease and grading of TR severity.25
TTE with Doppler allows rapid quantification of presence and severity of TR, presence of any structural or TV disorder, including presence of TV stenosis, vegetations, flail or perforated leaflets, and ruptured chordae. It also allows for assessment of right atrial size and volume, RV size and function, presence and extent of pulmonary hypertension, and presence of other structural heart abnormalities, including associated valvular abnormalities.7 Color-flow jet size in the right atrium is associated with severity of TR, as is vena contracta width. A vena contracta width ≥ 0.65 cm had sensitivity and specificity for severe TR of 89% and 93%, respectively.26 More recently, the stratification of TR as severe using proximal isovelocity surface area method and an effective regurgitant orifice ≥ 40 mm2 predicted increased cardiac events and mortality.4
In younger patients with TR or patients with known congenital abnormalities, such as Ebstein anomaly and ARVD, cardiac MRI may be helpful and is the preferred imaging modality for evaluating the size and function of the RV.5 Moreover, cardiac MRI allows clear visual characterization of complex disease pathologies that may lead to TR, as demonstrated in Figure 3.
Prognosis and Natural History
There are limited data on the prognosis and natural history of TR. Severe TR is associated with mortality (hazard ratio [HR] 1.31) despite correction for age, left ventricular ejection fraction, RV size and function, and inferior vena cava plethora, in a large series of 5223 patients, and is associated with increased mortality regardless of pulmonary pressures.2 Smaller series have also observed the association of severe TR with mortality, with an odds ratio for 1-year mortality with severe TR of 1.55.1 In a cohort with isolated TR with RV systolic pressure < 50 mm Hg, in patients with severe TR compared with those without, 10-year survival was 38% and 70%, respectively.4 In an ARVD cohort significant TR was a strong predictor of death or heart transplant (HR 7.6).24
Furthermore, severity of TR is associated with worse liver and kidney function,21 an observation also found in prior series, suggestive of the detrimental effects of elevated central venous pressure and the tricuspid regurgitant jet. Tellingly, in a series of patients with flail leaflets, the large majority trauma induced, 57% had resultant symptomatic heart failure, and excess mortality was 4.5% yearly compared with a US matched population.27 However, despite these observations, no studies of surgical correction for TR to date have shown a survival benefit. Moreover, the cause for the increased mortality in severe TR is not clear, and its presence may be merely an early marker of RV dysfunction, or mask its presence. Nonetheless, the expected 1-year survival for no TR, mild TR, moderate TR, and severe TR are 92%, 90%, 78%, and 64%, respectively.2
Management
Treatment directed at left-sided valve disease, left ventricular systolic dysfunction, fluid overload, and/or elevated pulmonary pressures may improve the severity of TR. For refractory TR, especially with evidence of local leaflet destruction, it is retrospectively observed that TV surgery improves symptoms of heart failure in severe TR. Current guidelines recommend TV surgery for organic severe TR that is symptomatic despite medical therapy.5,6
FTR With Elevated PASP
TR secondary to left-sided valve disease, fluid status, and/or elevated pulmonary pressures improves with treatment of the primary cause. In a series of 53 patients with significant TR and concomitant mitral stenosis, 51% had improvement of TR severity after mitral balloon valvuloplasty. Those with improvement of TR had a higher prevalence of FTR (85% vs 8%), lower prevalence of AF (7% vs 38%), and a larger decrease in PASP after the procedure (70.2-49.8 mm Hg),28 suggesting FTR rather than organic TR is more likely to improve with medical therapy. A second study of patients with moderate-to-severe TR and concomitant mitral stenosis observed resolution of TR in 32% of patients after mitral balloon valvuloplasty.29 In another illustrative series of patients undergoing pulmonary thromboendarterectomy, 70% of patients had resolution of severe TR after the procedure.30 In the absence of left heart valve surgery (LHVS), current guidelines do not make recommendations for TV surgery for FTR in the presence of elevated pulmonary pressures.5,6
Concurrent TV Surgery With LHVS
Although correction of elevated pulmonary pressures due to mitral stenosis with valvuloplasty and pulmonary hypertension from pulmonary thromboembolism with thromboendarterectomy may result in improvements in TR severity, it has been observed that the absence of concurrent TV repair for TR in patients undergoing LHVS leads to persistent TR and poor outcomes.31 The TV is asymmetric, geometrically more complex than the mitral valve, and dependent on right atrial and ventricular size and geometry. Right heart remodeling from elevated pulmonary pressures causes TV annular dilatation that may not resolve even after surgical treatment of diseased left heart valves. Moreover, reoperation operative mortality for TV surgery after LHVS is observed to be 27% to 37%.32,33 Consequently, concurrent TV repair with LHVS has been increasingly utilized over the past decade.
In a study of 1833 patients undergoing mitral valve surgery with severe preoperative TR, patients who did not undergo concurrent TV surgery had transient postoperative improvements in TR, but TR severity and RV function deteriorated to preoperative levels within 3 years. Those who underwent concurrent TV surgery had durable improvements in TR and RV function. Conversely, in patients with mild-to-moderate preoperative TR who underwent mitral valve surgery without concurrent TV surgery, TR more durably improved, and moderate-to-severe TR developed in less than 20% of patients at follow-up.34 It is additionally observed that heart failure symptoms improved with concurrent repair; however, no difference in long-term mortality has been seen.31,35
Routine concurrent TV repair at the time of mitral valve surgery when TV annular diameter is ≥ 70 mm regardless of TR severity has been shown to have favorable results. Mean TR grade before surgery was 0.9 in patients with annular dilatation ≥ 70 mm, compared with 0.7 in those without, suggesting poor correlation between annular dilatation and TR severity. Patients who received concurrent repair had improved NYHA functional class (1.11 vs 1.59). Moreover, TR severity improved by 0.52 grades in patients who received concurrent TV surgery versus worsening of severity by 2 grades in patients without repair.31 Similarly, only 2% had worsening by 2 grades with surgery, compared with 48% of patients without repair.31 However, it is unclear whether patients should receive TV repair based on tricuspid annular dilatation alone when severe TR is not present; a subsequent study showed preoperative annular diameter only predicted postoperative TR progression if preoperative TR was severe.35
Current guidelines recommend concurrent TV surgery for severe TR in patients undergoing LHVS, and less strongly recommends surgery in those with less than severe TR in the presence of a dilated annulus.56 Nonpharmacologic treatment modalities for TR are summarized in Table 2.
TV Repair Versus Replacement
Repair is generally recommended over replacement. Although valve replacement typically results in more durable reduction in TR severity, operative mortality is higher, and the need for reoperation for residual TR is not different between the two strategies.33,36,37 In a study of TV surgery for organic TV disease, operative mortality was higher in the replacement group (22% vs 4%). It is important to note this was not a randomized trial, and the replacement group may have had more severe underlying disease. Although there was less residual moderate-to-severe TR in the replacement group (5% vs 38%), NYHA functional class and reoperation rates were not different.37 In another study, 30-day mortality in the replacement group was higher (33% vs 14%), but freedom from TV reoperation was not different between the two strategies.33 In a third study of 926 patients, operative mortality was again higher by propensity score analysis in the replacement group (21% vs 13%), as was late mortality. Again, residual postoperative severe TR was lower in the replacement group (12% vs 31%); however, reoperation rates were not worse in the repair group, nor were postoperative NYHA functional classes different.36 The actuarial 10-year survival for replacement and repair are 37% to 50% and 47% to 69%, respectively.33,36,38
Current guidelines favor TV repair, and suggest reserving TV replacement in cases of severe TR with diseased and/or abnormal leaflets not amenable to repair.6
Surgical Techniques in TV Repair
Repair strategy is evolving but favors the placement of rigid or semirigid annuloplasty rings at the time of repair over the placement of flexible rings or suture-only procedures. Although repair has previously been focused on the annular level, multilevel repair involving the commissure and leaflets are increasingly utilized, although limited evidence exists.
In a study of 702 patients undergoing repair, patients either received an annuloplasty ring or a De Vega suture-only procedure. Operatively mortality was similar, but ring placement was associated with less residual moderate-to-severe TR at mean 5.9-year follow-up (30% vs 36%), and improved 15-year survival (49% vs 36%).39 In a second study of 2227 patients, at the annular level, flexible prosthesis (Cosgrove-Edwards band; Edwards Lifesciences, Irvine, CA), rigid prosthesis (Carpentier-Edwards ring or 3-dimensional Edwards MC; Edwards Lifesciences3), Peri-Guard (Synovis Life Technologies, St. Paul, MN), and suture-only De Vega procedures were compared. Residual severe TR was present in 11% of all patients 3 months post-operatively and 17% at final follow-up. Reoperation was uncommon and similar among procedure groups. The placement of rigid rings was associated with the most durable reduction of severe TR, but results are imperfect and residual TR of any grade was 78% despite repair.40 Current guidelines do not make recommendations on repair technique.
Isolated TV Surgery
There is limited evidence for isolated TV surgery. RV volume and function improved with isolated TV surgery in a series of 12 patients.41 In a larger series of 61 patients who underwent surgery for isolated severe TR, functional capacity and RV size improved. In the cohort, 93% of the patients had prior LHVS and 66% had NYHA class III/IV heart failure. Operative mortality was 10%.22 Additional series of 51 and 68 patients had operative mortalities of 2% and 6% for isolated TV surgery, respectively, but did not report on improvement in functional capacity or RV parameters.42,43
European Society of Cardiology guidelines recommend TV surgery for symptomatic severe TR after LHVS in the absence of left-sided myocardial, valve, or RV dysfunction, and without severe pulmonary hypertension. The guidelines additionally recommend surgery for isolated TR with mild or no symptoms with progressive dilatation or deterioration of RV function.5
Timing of Surgery
When performed at time of LHVS, concurrent TV surgery does not appear to increase mortality,31,34,35 compared with substantial mortality if TV surgery was done in a step-wise manner after LHVS,32,33 suggesting repair should be best performed at time of LHVS. However, there is potential benefit in earlier surgery. Patients who undergo TV surgery for isolated severe TR before RV end-systolic area was > 20 cm2 did better than those with larger ventricles, which suggests a benefit in earlier surgery,22 but the timing of surgery remains controversial.
Risk Factors for Residual TR After Surgery
Residual TR after surgery is common, and in patients undergoing mitral balloon valvuloplasty or pulmonary thromboendarterectomy, right atrial area, preoperative TR severity, preoperative AF, and postoperative AF were associated with persistent TR after the procedure.28-30 In patients with mild TR who underwent LHVS without concurrent TV repair, late significant TR developed in 27% with preoperative AF as the major risk factor (odds ratio 5.37). A twofold prevalence of AF was observed in the group who developed late TR (83% vs 47%).44 Furthermore, in a cohort of 216 patients with FTR undergoing TV surgery, preoperative TV leaflet tethering distance on echocardiography was an independent predictor of significant postoperative TR, as was age and preoperative TR severity.45 In another study of 59 patients, tenting volumes and anterior-posterior annulus diameters were independent predictors of residual TR after repair.46 In a third study, severity of preoperative TR, pacemaker leads through the TV, and decreased left ventricular ejection fraction were predictors of residual TR as well.40
Future Directions
Substantial residual TR remains despite concurrent TV surgery with LHVS, and no-to-mild TR not repaired at time of LHVS can progress to significant postoperative TR.36,37,44 In light of high operative mortality, in the range of 27% to 37% in reoperation for residual TR,32,33 percutaneous TV replacement has become an attractive option for residual TR. Balloon expandable bioprosthetic valves have been placed valve-in-valve in the tricuspid position through a hybrid transcatheter approach,47 and in a reverse-mounted fashion via a transfemoral approach for a patient with Ebstein anomaly.48 Likewise, percutaneous pulmonary valves have been implanted valve-in-valve in the tricuspid position with high procedural success, as described in a series of 15 patients with acquired and congenital heart disease.49 Moreover, percutaneous self-expanding valves have been successfully implanted in the superior and inferior vena cava for treatment of TR.50 Although long-term data are lacking, the emergence of percutaneous therapies offers a potential rescue strategy for patients at high operative risk who require valve replacement.
Although much progress has been made in informing TV surgery, current evidence remains at the observational level, and prospective randomized control trials have not been performed. Two studies are listed on the National Institutes of Health clinical trials registry. The first aims to randomize 200 patients to concurrent tricuspid repair for moderate TR during mitral valve surgery,51 and the second study aims to randomize 300 patients to concurrent tricuspid repair with mitral valve surgery when tricuspid annular dilatation is present even in less-than-severe TR.52 Additionally, a third study aims to randomize 200 patients to different pacemaker lead thicknesses and positions and observe their effect on TR severity.53 These results may strengthen the level of evidence for guideline recommendations and better inform TR management.
Conclusions
TR is a common condition, and moderate to severe TR is associated with increased mortality comparable in magnitude with the mortality rate observed in moderate to severe mitral regurgitation, a condition that garners more clinical scrutiny.
As our understanding and recognition of the disease condition has improved, there has been increased utilization of management options. TV surgery has more than doubled over the past decade, and repair rates have increased compared with replacement, reflecting the body of evidence preferentially supporting repair. However, isolated TV surgery remains a small proportion of all repairs (20%) compared with concurrent valve surgery.54 Despite the poor 10-year survival of severe isolated TR of 38% and the body of evidence supporting its repair or replacement, cardiac surgery is still rarely performed for treatment of this condition, and utilized only in 16% of cases at 5 years after diagnosis.4,55 Additionally, despite the increasing rate of TV surgery, there remains an incomplete utilization of concurrent repair with LHVS.8
Although correction of elevated pulmonary pressures may improve TR, concurrent repair of severe TR at the time of LHVS improves postoperative heart failure symptoms. Improved predictive models that incorporate imaging parameters could better identify patients who would most benefit from TV surgery and to inform the timing of surgery. TR repair is associated with less operative and long-term mortality than replacement and demonstrates similar improvements in heart failure symptoms. Because substantial residual TR remains after repair but reoperative mortality for residual TR is considerable, percutaneous TV replacement may offer a rescue strategy Further investigations are warranted to define the timing of TV surgery and to define the role of percutaneous TV replacement. ![]()
References
- Koelling TM, Aaronson KD, Cody RJ, et al. Prognostic significance of mitral regurgitation and tricuspid regurgitation in patients with left ventricular systolic dysfunction. American Heart J. 2002;144:524-529.
- Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43:405-409.
- Neuhold S, Huelsmann M, Pernicka E, et al. Impact of tricuspid regurgitation on survival in patients with chronic heart failure: unexpected findings of a longterm observational study. Eur Heart J. 2013;34:844-852.
- Topilsky Y, Nkomo VT, Vatury O, et al. Clinical outcome of isolated tricuspid regurgitation. JACC Cardiovasc Imaging. 2014;7:1185-1194.
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451-2496.
- Nishimura RA, Otto CM, Bonow RO, et al; ACC/AHA Task Force Members. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440-2492.
- Badano LP, Muraru D, Enriquez-Sarano M. Assessment of functional tricuspid regurgitation. Eur Heart J. 2013;34:1875-1885.
- Agarwal S, Tuzcu EM, Rodriguez ER, et al. Interventional cardiology perspective of functional tricuspid regurgitation. Circ Cardiovasc Interv. 2009;2:565-573.
- Supino PG, Borer JS, Preibisz J, Bornstein A. The epidemiology of valvular heart disease: a growing public health problem. Heart Fail Clin. 2006;2: 379-393.
- Waller BF, Howard J, Fess S. Pathology of tricuspid valve stenosis and pure tricuspid regurgitation— Part I. Clin Cardiol. 1995;18:97-102.
- Waller BF, Howard J, Fess S. Pathology of tricuspid valve stenosis and pure tricuspid regurgitation— Part II. Clin Cardiol. 1995;18:167-174.
- Fiorelli AI, Coelho GH, Aiello VD, et al. Tricuspid valve injury after heart transplantation due to endomyocardial biopsy: an analysis of 3550 biopsies. Transplant Proc. 2012;44:2479-2482.
- Nguyen V, Cantarovich M, Cecere R, Giannetti N. Tricuspid regurgitation after cardiac transplantation: how many biopsies are too many? J Heart Lung Transplant. 2005;24(7 suppl):S227-S231.
- Al-Bawardy R, Krishnaswamy A, Bhargava M, et al. Tricuspid regurgitation in patients with pacemakers and implantable cardiac defibrillators: a comprehensive review. Clin Cardiol. 2013;36:249-254.
- Lin G, Nishimura RA, Connolly HM, et al. Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverterdefibrillator leads. J Am Coll Cardiol. 2005;45:1672-1675.
- Mutlak D, Aronson D, Lessick J, et al. Functional tricuspid regurgitation in patients with pulmonary hypertension: is pulmonary artery pressure the only determinant of regurgitation severity? Chest. 2009;135:115-121.
- Mutlak D, Lessick J, Reisner SA, et al. Echocardiography-based spectrum of severe tricuspid regurgitation: the frequency of apparently idiopathic tricuspid regurgitation. J Am Soc Echocardiogr. 2007;20:405-408.
- Sagie A, Schwammenthal E, Padial LR, et al. Determinants of functional tricuspid regurgitation in incomplete tricuspid valve closure: Doppler color flow study of 109 patients. J Am Coll Cardiol. 1994;24: 446-453.
- Paniagua D, Aldrich HR, Lieberman EH, et al. Increased prevalence of significant tricuspid regurgitation in patients with transvenous pacemakers leads. Am J Cardiol. 1998;82:1130-1132, A9.
- Chan MC, Giannetti N, Kato T, et al. Severe tricuspid regurgitation after heart transplantation. J Heart Lung Transplant. 2001;20:709-717.
- Maeder MT, Holst DP, Kaye DM. Tricuspid regurgitation contributes to renal dysfunction in patients with heart failure. J Card Fail. 2008;14:824-830.
- Kim YJ, Kwon DA, Kim HK, et al. Determinants of surgical outcome in patients with isolated tricuspid regurgitation. Circulation. 2009;120:1672-1678.
- Najib MQ, Vinales KL, Vittala SS, et al. Predictors for the development of severe tricuspid regurgitation with anatomically normal valve in patients with atrial fibrillation. Echocardiography. 2012;29:140-146.
- Pinamonti B, Dragos AM, Pyxaras SA, et al. Prognostic predictors in arrhythmogenic right ventricular cardiomyopathy: results from a 10-year registry. Eur Heart J. 2011;32:1105-1113.
- Shah PM, Raney AA. Tricuspid valve disease. Curr Probl Cardiol. 2008;33:47-84.
- Tribouilloy CM, Enriquez-Sarano M, Bailey KR, et al. Quantification of tricuspid regurgitation by measuring the width of the vena contracta with Doppler color flow imaging: a clinical study. J Am Coll Cardiol. 2000;36:472-478.
- Messika-Zeitoun D, Thomson H, Bellamy M, et al. Medical and surgical outcome of tricuspid regurgitation caused by flail leaflets. J Thorac Cardiovasc Surg. 2004;128:296-302.
- Hannoush H, Fawzy ME, Stefadouros M, et al. Regression of significant tricuspid regurgitation after mitral balloon valvotomy for severe mitral stenosis. Am Heart J. 2004;148:865-870.
- Song JM, Kang DH, Song JK, et al. Outcome of significant functional tricuspid regurgitation after percutaneous mitral valvuloplasty. Am Heart J. 2003;145:371-376.
- Sadeghi HM, Kimura BJ, Raisinghani A, et al. Does lowering pulmonary arterial pressure eliminate severe functional tricuspid regurgitation? Insights from pulmonary thromboendarterectomy. J Am Coll Cardiol. 2004;44:126-132.
- Dreyfus GD, Corbi PJ, Chan KM, Bahrami T. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg. 2005;79:127-132.
- McCarthy PM, Bhudia SK, Rajeswaran J, et al. Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg. 2004;127:674-685.
- Guenther T, Noebauer C, Mazzitelli D, et al. Tricuspid valve surgery: a thirty-year assessment of early and late outcome. Eur J Cardiothorac Surg. 2008;34:402-409; discussion 409.
- Desai RR, Vargas Abello LM, Klein AL, et al. Tricuspid regurgitation and right ventricular function after mitral valve surgery with or without concomitant tricuspid valve procedure. J Thorac Cardiovasc Surg. 2013;146:1126.e10-1132.e10.
- Chan V, Burwash IG, Lam BK, et al. Clinical and echocardiographic impact of functional tricuspid regurgitation repair at the time of mitral valve replacement. Ann Thorac Surg. 2009;88:1209-1215.
- Marquis-Gravel G, Bouchard D, Perrault LP, et al. Retrospective cohort analysis of 926 tricuspid valve surgeries: clinical and hemodynamic outcomes with propensity score analysis. Am Heart J. 2012;163:851. e1-858.e1.
- Singh SK, Tang GH, Maganti MD, et al. Midterm outcomes of tricuspid valve repair versus replacement for organic tricuspid disease. Ann Thorac Surg. 2006;82:1735-1741; discussion 1741.
- Moraca RJ, Moon MR, Lawton JS, et al. Outcomes of tricuspid valve repair and replacement: a propensity analysis. Ann Thorac Surg. 2009;87:83-88; discussion 88-89.
- Tang GH, David TE, Singh SK, et al. Tricuspid valve repair with an annuloplasty ring results in improved long-term outcomes. Circulation. 2006;114(1 suppl):I577-I581.
- Navia JL, Nowicki ER, Blackstone EH, et al. Surgical management of secondary tricuspid valve regurgitation: annulus, commissure, or leaflet procedure? J Thorac Cardiovasc Surg. 2010;139:1473. e5-1482.e5.
- Mukherjee D, Nader S, Olano A, et al. Improvement in right ventricular systolic function after surgical correction of isolated tricuspid regurgitation. J Am Soc Echocardiogr. 2000;13:650-654.
- Topilsky Y, Khanna AD, Oh JK, et al. Preoperative factors associated with adverse outcome after tricuspid valve replacement. Circulation. 2011;123:1929-1939.
- Kim JB, Jung SH, Choo SJ, et al. Clinical and echocardiographic outcomes after surgery for severe isolated tricuspid regurgitation. J Thorac Cardiovasc Surg. 2013;146:278-284.
- Kwak JJ, Kim YJ, Kim MK, et al. Development of tricuspid regurgitation late after left-sided valve surgery: a single-center experience with long-term echocardiographic examinations. Am Heart J. 2008;155:732-737.
- Fukuda S, Song JM, Gillinov AM, et al. Tricuspid valve tethering predicts residual tricuspid regurgitation after tricuspid annuloplasty. Circulation. 2005;111:975-979.
- Min SY, Song JM, Kim JH, et al. Geometric changes after tricuspid annuloplasty and predictors of residual tricuspid regurgitation: a real-time three-dimensional echocardiography study. Eur Heart J. 2010;31:2871-2880.
- Webb JG, Wood DA, Ye J, et al. Transcatheter valvein-valve implantation for failed bioprosthetic heart valves. Circulation. 2010;121:1848-1857.
- Cunnington C, Hoschtitzky JA, Hasan R, et al. Percutaneous tricuspid valve-in-valve implantation in Ebstein’s anomaly: one-year follow-up of valve function. Int J Cardiol. 2014;174:e77-e78.
- Roberts PA, Boudjemline Y, Cheatham JP, et al. Percutaneous tricuspid valve replacement in congenital and acquired heart disease. J Am Coll Cardiol. 2011;58:117-122.
- Lauten A, Doenst T, Hamadanchi A, et al. Percutaneous bicaval valve implantation for transcatheter treatment of tricuspid regurgitation: clinical observations and 12-month follow-up. Circ Cardiovasc Interv. 2014;7:268-272.
- Tricuspid Annuloplasty for Moderate Tricuspid Regurgitation Associated With Mitral Operation. ClinicalTrials.gov website. http://www.clinicaltrials.gov/ct2/show/NCT01246947. Accessed July 13, 2014.
- Prophylactic Tricuspid Valve Annuloplasty in Patients Undergoing Mitral Valve Surgery. ClinicalTrials. gov website. http://www.clinicaltrials.gov/ct2/show/NCT01580436. Accessed July 13, 2014.
- Tricuspid Regurgitation Study. ClinicalTrials.gov website. http://www.clinicaltrials.gov/ct2/show/NCT01093001. Accessed July 13, 2014.
- Vassileva CM, Shabosky J, Boley T, et al. Tricuspid valve surgery: the past 10 years from the Nationwide Inpatient Sample (NIS) database. J Thorac Cardiovasc Surg. 2012;143:1043-1049.
- Pettersson GB, Rodriguez LL, Blackstone EH. Severe tricuspid valve regurgitation is not an innocent finding to be ignored! JACC Cardiovasc Imaging. 2014;7:1195-1197.