Heparin: Physiology, Pharmacology, and Clinical Application
Michael S. Lee, MD, Jeremy Kong, BS
UCLA Medical Center, Los Angeles, CA
Heparin is stored endogenously within the secretory granules of basophils and mast cells, and is only released into the vasculature at sites of injury. At these sites, it helps maintain proper blood flow by balancing the active anticoagulant and procoagulant processes. Pharmaceutical-grade heparin is derived from animal tissue, but one form is made synthetically. Heparin is commonly used in the management of coronary artery disease, deep vein thrombosis, pulmonary embolism, and atrial fibrillation, and in the prevention of thrombosis during cardiopulmonary bypass and extracorporeal membrane oxygenation. Heparin treatment is a key component in elective percutaneous coronary intervention (PCI). It plays an important role in minimizing the risk of thrombotic events during PCI and is one of the most popular anticoagulants used. However, some studies show that higher heparin doses are associated with more frequent bleeding complications, which can increase morbidity and mortality. The optimal heparin dosing regimens are still debated, as well as their efficacy in PCI compared with that of other drugs such as bivalirudin. This review examines the physiology, pharmacology, therapeutic applications, dosing regimens, and efficacy of heparin in the setting of PCI. In addition, included is a review of data on addition of glycoprotein IIb/IIIa inhibitors to heparin and comparison of heparin monotherapy to bivalirudin in PCI.
[Rev Cardiovasc Med. 2015;16(3):189-199 doi: 10.3909/ricm0778]
© 2015 MedReviews®, LLC
Heparin: Physiology, Pharmacology, and Clinical Application
Michael S. Lee, MD, Jeremy Kong, BS
UCLA Medical Center, Los Angeles, CA
Heparin is stored endogenously within the secretory granules of basophils and mast cells, and is only released into the vasculature at sites of injury. At these sites, it helps maintain proper blood flow by balancing the active anticoagulant and procoagulant processes. Pharmaceutical-grade heparin is derived from animal tissue, but one form is made synthetically. Heparin is commonly used in the management of coronary artery disease, deep vein thrombosis, pulmonary embolism, and atrial fibrillation, and in the prevention of thrombosis during cardiopulmonary bypass and extracorporeal membrane oxygenation. Heparin treatment is a key component in elective percutaneous coronary intervention (PCI). It plays an important role in minimizing the risk of thrombotic events during PCI and is one of the most popular anticoagulants used. However, some studies show that higher heparin doses are associated with more frequent bleeding complications, which can increase morbidity and mortality. The optimal heparin dosing regimens are still debated, as well as their efficacy in PCI compared with that of other drugs such as bivalirudin. This review examines the physiology, pharmacology, therapeutic applications, dosing regimens, and efficacy of heparin in the setting of PCI. In addition, included is a review of data on addition of glycoprotein IIb/IIIa inhibitors to heparin and comparison of heparin monotherapy to bivalirudin in PCI.
[Rev Cardiovasc Med. 2015;16(3):189-199 doi: 10.3909/ricm0778]
© 2015 MedReviews®, LLC
Heparin: Physiology, Pharmacology, and Clinical Application
Michael S. Lee, MD, Jeremy Kong, BS
UCLA Medical Center, Los Angeles, CA
Heparin is stored endogenously within the secretory granules of basophils and mast cells, and is only released into the vasculature at sites of injury. At these sites, it helps maintain proper blood flow by balancing the active anticoagulant and procoagulant processes. Pharmaceutical-grade heparin is derived from animal tissue, but one form is made synthetically. Heparin is commonly used in the management of coronary artery disease, deep vein thrombosis, pulmonary embolism, and atrial fibrillation, and in the prevention of thrombosis during cardiopulmonary bypass and extracorporeal membrane oxygenation. Heparin treatment is a key component in elective percutaneous coronary intervention (PCI). It plays an important role in minimizing the risk of thrombotic events during PCI and is one of the most popular anticoagulants used. However, some studies show that higher heparin doses are associated with more frequent bleeding complications, which can increase morbidity and mortality. The optimal heparin dosing regimens are still debated, as well as their efficacy in PCI compared with that of other drugs such as bivalirudin. This review examines the physiology, pharmacology, therapeutic applications, dosing regimens, and efficacy of heparin in the setting of PCI. In addition, included is a review of data on addition of glycoprotein IIb/IIIa inhibitors to heparin and comparison of heparin monotherapy to bivalirudin in PCI.
[Rev Cardiovasc Med. 2015;16(3):189-199 doi: 10.3909/ricm0778]
© 2015 MedReviews®, LLC
KEY WORDS
Heparin • Percutaneous coronary intervention • Bivalirudin • Anticoagulation
KEY WORDS
Heparin • Percutaneous coronary intervention • Bivalirudin • Anticoagulation
Heparin is a highly sulfated glycosaminoglycan with the highest negative charge density of any known biological molecule.
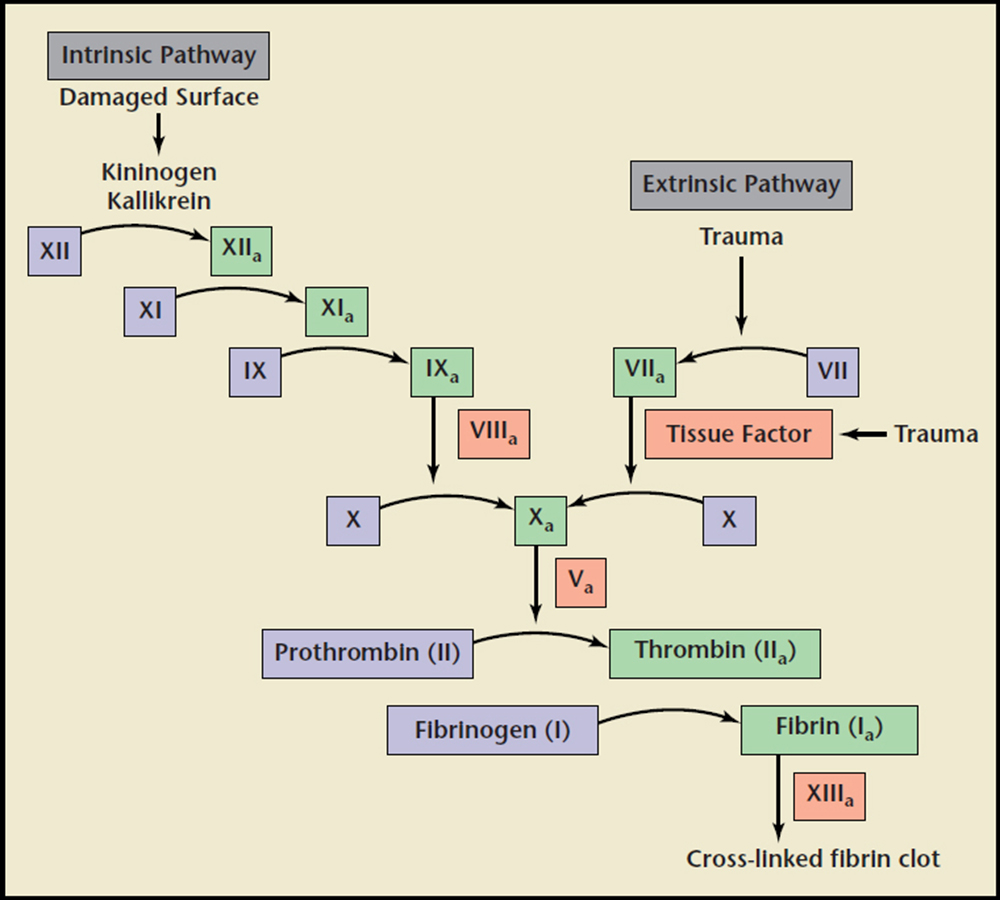
Figure 1. Schematic diagram to represent intrinsic, extrinsic, and common coagulation pathways in the clotting cascade. XII, Inactive Factor XII; XIIa, Active Factor XII protease; XI, Inactive Factor XI; XIa, Active Factor XI protease; IX, Inactive Factor IX; IXa, Active Factor IX protease; VIIIa, Active Factor VIII; VII, Inactive Factor VII; VIIa, Active Factor VII protease; X, Inactive Factor X; Xa, Active Factor X protease; Va, Active Factor V; II, Inactive Factor II (Prothrombin); IIa, Active Factor II (Thrombin); I, Inactive Factor I (Fibrinogen); Ia, Active Factor I (Fibrin); XIIIa, Active Factor XIII.
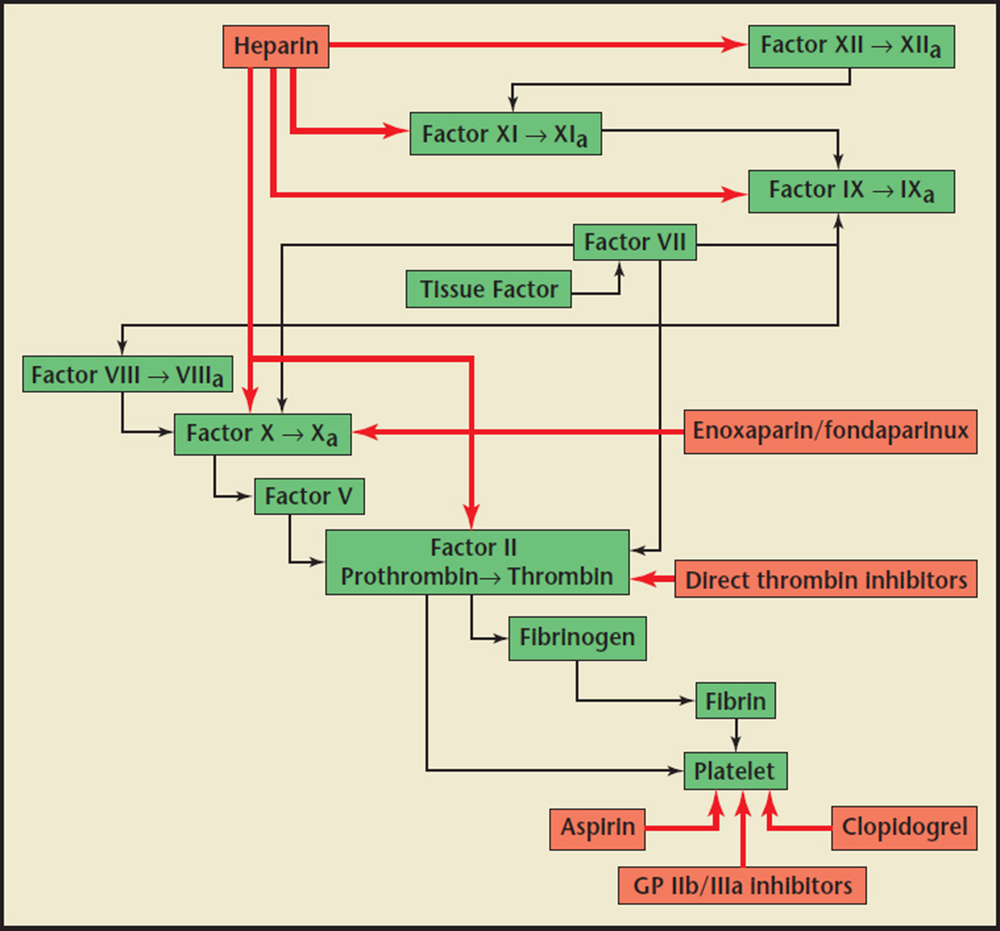
Figure2. Inhibiting interactions (red arrows) of antiplatelet medications (aspirin, glycoprotein [GP] IIb/IIIa inhibitors, clopidogrel) and anticoagulant medications (unfractionated heparin, enoxaparin/fondaparinux, direct thrombin inhibitors) on clotting cascade factors. Adapted from Earnest M, Tadros P. Non-ST segment elevation MI and unstable angina: what role for anticoagulants and antiplatelet agents? Consultant website. http://www.consultantlive.com/urologic-diseases/non-st-segment-elevation-mi-and-unstable-angina-whatrole-anticoagulants-and-antiplatelet-agents. Created March 1, 2007. Accessed September 21, 2015.
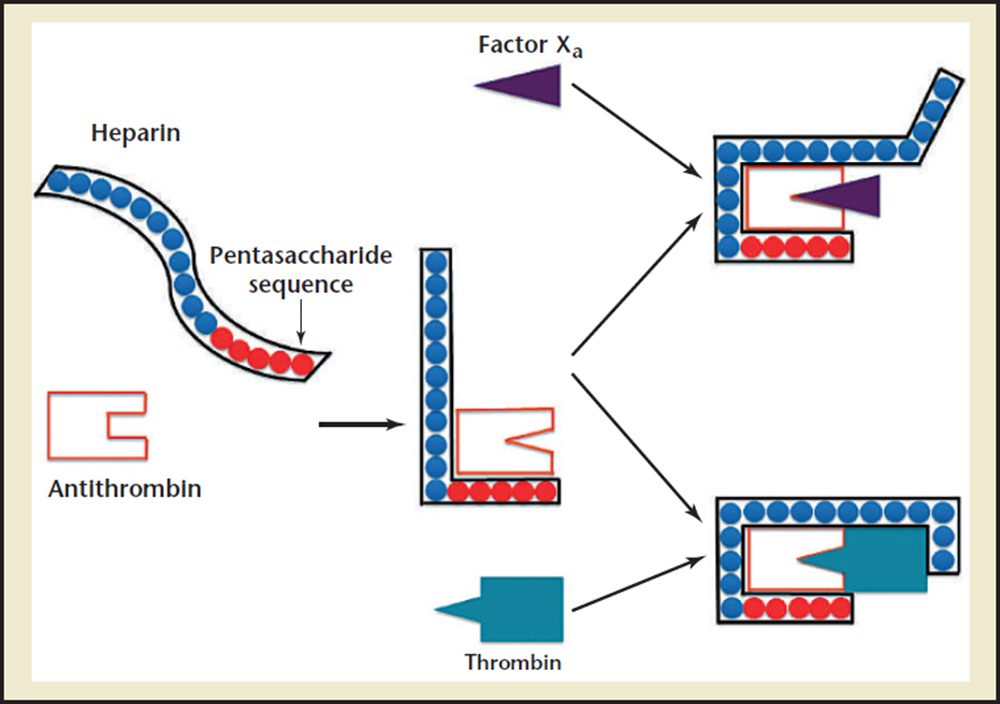
Figure3. Mechanism of action of heparin on antithrombin. Heparin binds to antithrombin via its pentasaccharide sequence, inducing a conformational change at the antithrombin reactive center loop. Both thrombin and Factor Xa can then bind to the heparin-antithrombin complex. To potentiate thrombin inactivation, heparin must bind both thrombin and antithrombin. However, the inactivation of Factor Xa does not require heparin binding to it directly. Adapted from Fauci AS et al.53
Overdosing of heparin has potentially catastrophic bleeding complications.
Although ACT is a quick, easy, and reliable method of anticoagulation testing that is extremely useful in monitoring heparin therapy, its disadvantages are mainly related to differences between commercially available devices and the various ACT values that are calculated.
Lower doses of heparin may be a viable strategy to reduce the risk of bleeding complications.
… conservative dosing of heparin is recommended in patients with severe renal impairment when treating thromboembolism.
… shorter aPTTs are associated with smoking and diabetes in the setting of ACS, whereas longer aPTTs are associated with older age, low body weight, African ancestry, and female sex.
Bivalirudin possesses several pharmacologic advantages over heparin, but no clear evidence suggests bivalirudin has any advantage with regard to ischemic protection.
One clear advantage to using heparin over bivalirudin during PCI is that it is substantially less expensive.
Main Points
• Heparin is a highly sulfated glycosaminoglycan with the highest negative charge density of any known biological molecule. It is a naturally occurring anticoagulant produced by mast cells and basophils that plays an important role in vivo in the fine balance of anticoagulant and procoagulant processes. Although it does not break down preformed clots like tissue plasminogen activator, it instead potentiates the progression of the body’s natural clot lysis mechanisms to prevent the formation of clots.
• Heparin is administered parenterally because it is not absorbed in the gut due to its high negative charge and size; intramuscular injections are avoided because of the risk of developing hematomas. Subcutaneous administration is predominantly given for deep vein thrombosis prophylaxis. In order to maintain its antithrombotic effect throughout the duration of percutaneous coronary intervention (PCI), heparin must be rebolused or continually infused because of its short half-life.
• Heparin is the most commonly used anticoagulant in the world administered to minimize thrombotic complications during PCI; its onset of action is immediate after intravenous administration. In addition to avoiding thrombotic complications, anticoagulation strategies must be designed to avoid major bleeding complications, as they are associated with increased morbidity, mortality, and cost.
• The combination of heparin and glycoprotein (GP) IIb/IIIa inhibitors has a potent anti-ischemic effect, but also has an increased risk of serious bleeding complications that must be balanced carefully. Although randomized trials have demonstrated the reduction of ischemic events with GP IIb/IIIa inhibitors in patients who undergo PCI for non-ST elevation acute coronary syndromes and ST-elevation myocardial infarction, bleeding complications are also increased.
• Although several studies support the use of bivalirudin over heparin and a GP IIb/IIIa inhibitor, study limitations and other data findings question their superiority. Bivalirudin possesses several pharmacologic advantages over heparin, but no clear evidence suggests bivalirudin has any advantage with regard to ischemic protection.
Main Points
• Heparin is a highly sulfated glycosaminoglycan with the highest negative charge density of any known biological molecule. It is a naturally occurring anticoagulant produced by mast cells and basophils that plays an important role in vivo in the fine balance of anticoagulant and procoagulant processes. Although it does not break down preformed clots like tissue plasminogen activator, it instead potentiates the progression of the body’s natural clot lysis mechanisms to prevent the formation of clots.
• Heparin is administered parenterally because it is not absorbed in the gut due to its high negative charge and size; intramuscular injections are avoided because of the risk of developing hematomas. Subcutaneous administration is predominantly given for deep vein thrombosis prophylaxis. In order to maintain its antithrombotic effect throughout the duration of percutaneous coronary intervention (PCI), heparin must be rebolused or continually infused because of its short half-life.
• Heparin is the most commonly used anticoagulant in the world administered to minimize thrombotic complications during PCI; its onset of action is immediate after intravenous administration. In addition to avoiding thrombotic complications, anticoagulation strategies must be designed to avoid major bleeding complications, as they are associated with increased morbidity, mortality, and cost.
• The combination of heparin and glycoprotein (GP) IIb/IIIa inhibitors has a potent anti-ischemic effect, but also has an increased risk of serious bleeding complications that must be balanced carefully. Although randomized trials have demonstrated the reduction of ischemic events with GP IIb/IIIa inhibitors in patients who undergo PCI for non-ST elevation acute coronary syndromes and ST-elevation myocardial infarction, bleeding complications are also increased.
• Although several studies support the use of bivalirudin over heparin and a GP IIb/IIIa inhibitor, study limitations and other data findings question their superiority. Bivalirudin possesses several pharmacologic advantages over heparin, but no clear evidence suggests bivalirudin has any advantage with regard to ischemic protection.
Unfractionated heparin was discovered in 1916 by McLean and Howell, predating the establishment of the US Food and Drug Administration, and was first used in clinical practice in 1935.1 Heparin was originally isolated from canine liver cells, and thus given its name because hepar is the Greek word for liver. Heparin is the most commonly used antithrombotic agent in the hospital. It plays a crucial role in mediating procoagulant and anticoagulant processes to maintain proper blood flow. It is a versatile drug used for the treatment of atrial fibrillation, acute coronary syndrome (ACS), arterial thrombosis, deep vein thrombosis, and pulmonary embolism, and in the prevention of thrombosis during cardiopulmonary bypass and extracorporeal membrane oxygenation. It is also used as adjunctive pharmacotherapy in percutaneous coronary intervention (PCI). Overdosing of heparin can lead to dangerous bleeding complications.2,3 Optimal dosing regimens of heparin during PCI remain unknown and are still highly debated.
This article reviews the physiology, pharmacology, therapeutic applications, and clinical data on heparin in the setting of PCI. In addition, different dosing regimens and the efficacy of heparin monotherapy compared with bivalirudin in the setting of PCI are discussed.
Heparin Physiology
Heparin is a highly sulfated glycosaminoglycan with the highest negative charge density of any known biological molecule.4 It is a mixture of sulfated glycosaminoglycans composed of alternating residues of iduronic acid and D-glucosamine.2 It is a naturally occurring anticoagulant produced by mast cells and basophils that plays an important role in vivo in the fine balance of anticoagulant and procoagulant processes. Although it does not break down preformed clots like tissue plasminogen activator, it instead potentiates the progression of the body's natural clot lysis mechanisms to prevent the formation of clots.5
Heparin plays a vital role in the complex network of serine proteases that convert proenzymes to their active forms. Thrombin, or factor lla, is the final serine protease that cleaves fibrinogen to form fibrin, the foundation of a clot when combined with a platelet plug (Figure 1). Vascular injury exposes these serine proteases to tissue factor and collagen, procoagulant stimuli that activate the coagulation cascade.35 Several endogenous anticoagulant proteins are also present to regulate the formation of thrombin, keeping the clotting process local and cleaning up proteases that stray into the rest of the vasculature. These include antithrombin (AT), heparin cofactor II, and protein C inhibitor, which are members of a class of proteins called serpins (short for serine protease inhibitors).5 Heparin assists these serpins in anticoagulant processes, and additionally stimulates the release of tissue factor pathway inhibitor from endothelial cells.2 Heparin's most significant anticoagulant contribution is through potentiating the action on the serpin AT, which is the major heparin cofactor in the inhibition of thrombin and other coagulation proteases, particularly factor Xa and lla (Figure 2).3,5 Heparin binds to enzyme-inhibitor AT through a high-affinity pentasaccharide sulfation sequence contained within the heparin polymer (Figure 3). Heparin must bind to both the coagulation enzyme and AT to inhibit thrombin.3 A ternary complex formed between thrombin, AT, and heparin results in the inactivation of the procoagulant enzyme. Thus, heparin's activity against thrombin is size dependent and requires at least 18 saccharide units. Heparin molecules with fewer than 18 saccharides lack the chain length to bridge between AT and thrombin. However, binding to the enzyme is not required for the inhibition of factor Xa; only the pentasaccharide binding site is required. AT and the other serpins possess a reactive loop that mimics a serine protease substrate sequence. A procoagulant protease that cleaves this loop is then trapped in an inactive complex with the serpin. When heparin binds to AT, a conformational change in the enzyme inhibitor increases the flexibility at its reactive site loop and activates it.3 This activated AT then binds and inactivates thrombin and other blood clotting proteases. Once the enzyme is inactivated, heparin attached to the AT is released so that it can act again on another free serpin available.5 By inactivating thrombin, heparin not only prevents fibrin formation, but also inhibits thrombin-induced activation factors V and VIII and platelets.
Heparin Pharmacology
Commercial preparation of animal-derived heparin is derived from tissue extract from pig intestines and cow lungs.6 Given its negative charge and size, heparin has a propensity to bind to positively charged surfaces such as platelet proteins, plasma proteins, and endothelial cells, leading to variable anticoagulation responses and heparin resistance.3 Heparin resistance is usually caused by an acute-phase response that leads to high levels of procoagulant proteins such as factor VIII.2 Heparin binding to macrophages and endothelial cells can also result in dose-dependent clearance. In addition, heparin can lead to complications including anaphylaxis, bleeding, thrombocytopenia, and osteopenia.2,3 Long-term therapy in pregnant women, for example, can cause osteoporosis. Radiographic evidence has shown bone loss in approximately 15% of women who received prolonged treatment during pregnancy.2
Heparin is administered parenterally because it is not absorbed in the gut due to its high negative charge and size. Intramuscular injections are avoided because of the risk of developing hematoma. Subcutaneous administration is predominantly given for deep vein thrombosis prophylaxis.2 In order to maintain its antithrombotic effect throughout the duration of PCI, heparin must be rebolused or continually infused because of its short half-life of 1.5 hours.7 However, higher doses of heparin have a longer half-life than lower doses. Heparin is primarily excreted by the reticuloendothelial system in a rapid dose-dependent manner, but the extent of reticuloendothelial saturation is difficult to ascertain. As endothelial cell binding of heparin is saturated, a higher burden is placed on the kidneys as they clear the drug from the bloodstream at a slower rate.7 Renal excretion provides a secondary, slower first-order clearance of heparin that results in nonlinear pharmacokinetics that become increasingly disproportionate at higher doses.8
Overdosing of heparin has potentially catastrophic bleeding complications. Protamine sulfate, a highly cationic peptide drug that is produced primarily through recombinant biotechnology, can reverse the anticoagulant effects of heparin in 30 to 60 seconds after intravenous administration by binding to heparin to form a stable ion pair that does not have anticoagulant activity.9
Monitoring Level of Anticoagulation
The pharmacodynamics of heparin vary among individuals and therefore require close monitoring with either activated partial thromboplastin time (aPTT) or activated clotting time (ACT). aPTT measures the activity of the intrinsic and common coagulation pathways by measuring the clotting time from the activation of factor XII to the fibrin clot formation.10,11 It is commonly used to monitor the anticoagulant effect of heparin, argatroban, and hirudin, although its use is not ideal at high doses of heparin. The aPTT usually becomes prolonged beyond measurable levels at heparin concentrations > 1 U/mL. Thus, aPTT is unsuitable for monitoring heparin dosage during PCI because patients may require heparin levels > 1 U/mL. In addition, the measurement of aPTT is generally performed in a hospital's core laboratory facility and takes approximately 30 to 60 minutes, making the delay in reporting impractical for PCI.10,11
The measurement of ACT first came into clinical use in the mid 1970s to guide the administration and reversal of heparin during cardiopulmonary bypass.10 The measurement of ACT is widely used in the cardiac catheterization laboratory, where immediate results are obtained with a point-of-care assay to monitor the anticoagulant effect of heparin. It measures the number of seconds it takes for whole blood to clot upon exposure to an intrinsic pathway activator. Although it monitors agents that possess both anti-Xa and anti-IIa activities, the ACT is influenced predominantly by anti-IIa activity. It has an advantage over aPTT for PCI because, at high doses of heparin, the dose-response relationship remains linear for ACT.10 The ACT graded response to heparin concentrations is in the range of 1 U to 5 U/mL.10 Although ACT is a quick, easy, and reliable method of anticoagulation testing that is extremely useful in monitoring heparin therapy, its disadvantages are mainly related to differences between commercially available devices and the various ACT values that are calculated.12 For example, a target ACT of 250 to 300 seconds for HemoTec (Medtronic, Minneapolis, MN) is the equivalent of 300 to 350 seconds for Hemochron (Accriva Diagnostics, Piscataway Township, NJ) systems.13
Dosing of Heparin for PCI
Heparin is the most commonly used anticoagulant in the world to minimize thrombotic complications during PCI. The onset of action is immediate after intravenous administration. In addition to avoiding thrombotic complications, anticoagulation strategies must be designed to avoid major bleeding complications, as they are associated with increased morbidity, mortality, and cost.14,15 These strategies must also encompass flexibility and versatility, as patients have a heparin variable response. The American College of Cardiology Foundation/ American Heart Association (AHA)/Society of Coronary Angiography and Interventions guidelines recommend a 70- to 100-IU/kg bolus of heparin to achieve a target ACT of 250 to 300 seconds for HemoTec when glycoprotein (GP) IIb/IIIa inhibitors are not planned for use. The guidelines recommend administering a 50- to 70-IU/kg bolus of heparin when GP IIb/IIIa inhibitors are used in order to maintain an ACT of 200 to 250 seconds for HemoTec systems, or 300 to 350 seconds for Hemochron systems.13 However, these guidelines are mostly based on older studies that predate the use of thienopyridines, thus requiring larger anticoagulation doses. Furthermore, these doses have not been prospectively studied or validated. The AHA additionally recommends the use of various heparin dose-adjustment nomograms and protocols that have been developed, including a weight-based nomogram for heparin dosing.16 These nomograms and protocols monitor levels of anticoagulation with aPTT
The American College of Chest Physicians (ACCP) guidelines also recommend the use of weight-based heparin17; 15 years of internal analyses have shown that heparin dosing using average body weight correlated best with favorable aPTT responses. Average body weight was defined as actual body weight plus ideal body weight divided by 2. It was anticipated that the use of actual weight would result in higher initial aPTT values and potentially expose patients to further bleeding risks, especially in obese patients. Patients received a bolus of 50 U/kg followed by 15 U/kg/h of continuous intravenous infusion based on average body weight. This use of average weight in heparin dosing led to rapid and efficient anticoagulation in the majority of patients.17 It should be noted that these guidelines don't completely address dosing in overweight and obese patients, making the correct application of this weight-based heparin therapy important with obesity's prevalence in the United States. Heparin has a small volume of distribution, and adipose tissue is less vascular than lean tissue, making the volume of distribution difficult to assess in obese patients.17
Lower doses of heparin may be a viable strategy to reduce the risk of bleeding complications. Higher ACT has been associated with more bleeding complications, but statistically similar rates of ischemic events across increasing ACT quartiles (quartile 1: < 256 s; quartile 2: 257-296 s; quartile 3: 297-347 s; quartile 4: > 348 s).18 One analysis of randomized trials revealed that higher doses of heparin during PCI yielded fewer ischemic complications, but more bleeding complications.19 In the Aspirin Plus Dipyridamole Versus Aspirin Alone After Cerebral Ischaemia of Arterial Origin (ESPRIT) trial, lower ACT levels reduced bleeding complications but did not increase ischemic complications.20 A single-center prospective study from the UCLA Medical Center demonstrated that low-dose heparin (40 IU/kg) provided excellent protection from bleeding complications without compromising ischemic events in 300 patients who underwent elective transfemoral PCI with pretreatment with aspirin, 325 mg, and clopidogrel, 600 mg.21 It is possible that the preloading of clopidogrel offsets the potential risk of ischemic events with low-dose heparin. Another prospective registry included 418 patients who underwent PCI with 30 IU/kg of heparin.22 The average dose of heparin was 2253 IU and the final ACT was 174 seconds. The composite rate of repeat revascularization, myocardial infarction (MI), or death at 1 month was 2.9%, whereas the rate of serious vascular complication requiring a blood transfusion and surgical repair was 0.24%. Most of the patients were not pretreated with dual antiplatelet therapy. An analysis from the Safety and Efficacy of Intravenous Enoxaparin in Elective Percutaneous Coronary Intervention: An International Randomized Evaluation (STEEPLE) trial comparing intravenous unfractionated heparin with intravenous enoxaparin demonstrated that bleeding significantly increased with ACT values > 325 seconds, whereas ischemic events increased with ACT values < 325 seconds. Together, these studies suggest that heparin has a relatively narrow therapeutic window and that a general relationship exists between heparin dosing and outcomes, such that lower heparin doses are equally effective as higher doses and potentially safer.23
The Coronary Interventions Antiplatelet-based Only (CIAO) trial suggested that it might even be feasible to perform PCI without heparin if patients are pretreated with dual antiplatelet therapy.24 However, PCI was only performed in simple uncomplicated lesions. Given the paucity of current data, PCI with ultralow heparin doses (or no heparin) cannot be recommended.
Proper dosing of heparin must be carefully considered in patients with chronic kidney disease (CKD). Patients with CKD have an increased risk of hemorrhagic and adverse ischemic events after undergoing primary PCI for ST segment elevation MI (STEMI).25 Analyses show that the bleeding risk of patients with CKD (creatinine clearance [CrCl] < 30 mL/min) is greater than in patients with CrCl > 30 mL/min regardless of the anticoagulant used. Instead, baseline risk factors such as uremia, increased age, and concurrent treatment and morbidities determine the higher risk of bleeding in CKD.8 Such morbidities include smoking and diabetes. Thus, conservative dosing of heparin is recommended in patients with severe renal impairment when treating thromboembolism.8 No adjusted heparin dosing guidelines for CKD patients undergoing PCI currently exist, but lower doses should also be used in this setting. Patients with CKD undergoing PCI should be initially treated conservatively with unfractionated heparin and monitored closely to avoid adverse bleeding risks. Lower doses will primarily be cleared by the reticuloendothelial system and require less secondary clearance of heparin via renal excretion.8
Physicians must also consider patient factors that may alter the response to heparin. For example, shorter aPTTs are associated with smoking and diabetes in the setting of ACS, whereas longer aPTTs are associated with older age, low body weight, African ancestry, and female sex.26 Careful review of patient medical charts may identify potential drug interactions or comorbidities. In addition, the indication for anticoagulation is critical in determining the correct heparin dosage, as different heparin dosing guidelines exist for various settings. The AHA and the ACCP recommend protocols using weight-based heparin dosing, which provides efficacious anticoagulation with favorable aPTT responses.17 Using lower doses of heparin with these protocols may provide additional benefit by lowering bleeding risks without sacrificing effective anticoagulation.23 This approach is especially important in patients with CKD undergoing PCI who are already at a higher risk of bleeding with any anticoagulant. It is hoped that future studies will establish specific heparin dosing guidelines for this specific group of patients in the setting of PCI.
Heparin With GP IIb/IIIa Inhibitors
The combination of heparin and GP IIb/IIIa inhibitors has a potent anti-ischemic effect, but an increased risk of serious bleeding complications that must be balanced carefully. Although randomized trials have demonstrated the reduction of ischemic events with GP IIb/IIIa inhibitors in patients who undergo PCI for non-ST elevation ACS and STEMI, bleeding complications are also increased.27,28 However, the Intracoronary Stenting and Antithrombotic: Regimen Rapid Early Action for Coronary Treatment (ISAR-REACT) 2 trial reported a lack of demonstrated benefit to the use of GP IIb/IIIa inhibitor in troponin-negative patients.29 In elective PCI, routine use of GP IIb/IIIa is not a universally accepted treatment strategy due to the lack of efficacy data and the increased risk of bleeding complications. Its use has decreased over 50% in the United Kingdom since 2005, and a similar trend is taking place throughout the rest of Europe.30 Heparin combined with a GP IIb/IIIa inhibitor may be best used in specific cases, such as ACS in the presence of large thrombus burden or inadequate antiplatelet loading during primary PCI.31,32 The introduction of more potent and rapidly acting oral antiplatelet agents for ACS like ticagrelor and prasugrel, as well as preloading with clopidogrel and aspirin at least 6 hours prior to elective PCI, may obviate the need for incremental anti-ischemic protection provided by a GP IIb/IIIa inhibitor.33
If a GP IIb/IIIa inhibitor is used, the dose of heparin should be adjusted to target an ACT of 200 to 250 seconds.34 Lower heparin doses and targeted ACT levels in patients who received GP IIb/IIIa inhibitors were associated with a 90% reduction in vascular bleeding rates (20.2% to 2.2%).35 Further studies are still needed to compare the efficacy of various heparin doses with and without GP IIb/ IIIa inhibitors against other drugs, including bivalirudin.
Heparin Versus Bivalirudin for PCI
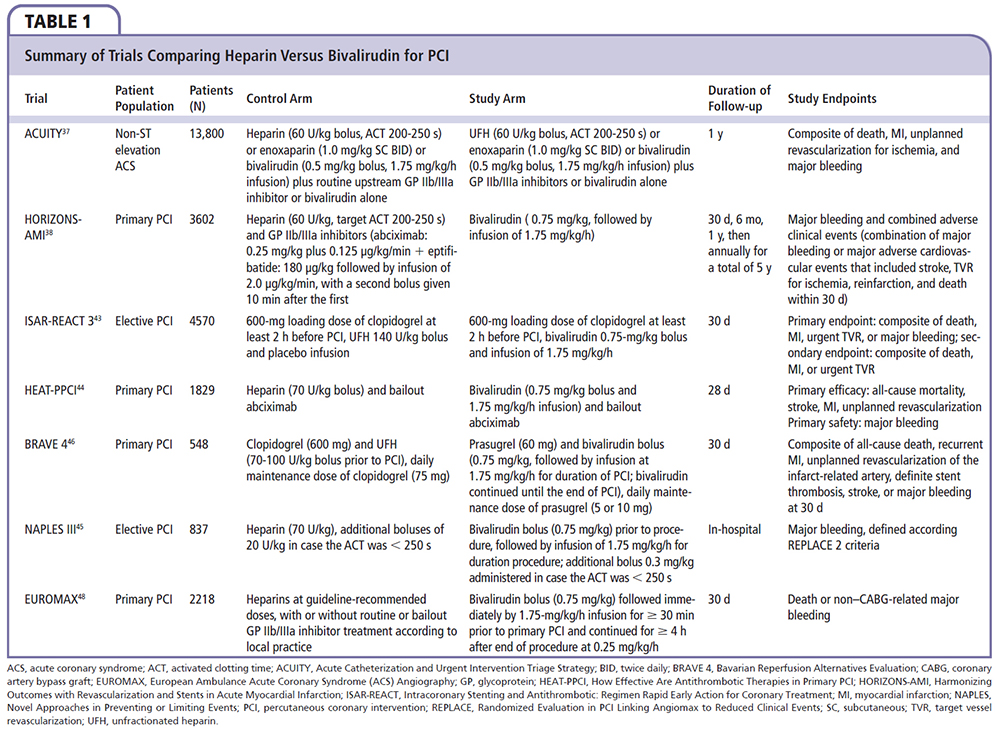
Bivalirudin is a direct thrombin inhibitor that is associated with fewer bleeding complications compared with heparin plus a GP IIb/IIIa inhibitor when used for the full spectrum of coronary artery disease treated with PCI.36-38 It is currently the most widely used AT agent in the United States for patients undergoing PCI.39 In patients with moderate- or high-risk ACS undergoing invasive treatment in the Acute Catheterization and Urgent Intervention Triage Strategy (ACUITY) trial, bivalirudin, as compared with heparin plus a GP IIb/IIIa inhibitor, was associated with a noninferior rate of the composite ischemia endpoint (7.8% and 7.3%, respectively; relative risk [RR] 1.08; 95% confidence interval [CI], 0.93-1.24; P = .32) and significantly reduced rates of major bleeding (3.0% vs 5.7%; RR, 0.53; 95% CI, 0.43-0.65; P < .001) and the net clinical outcome endpoint (10.1% vs 11.7%; RR, 0.86; 95% CI, 0.77-0.97; P = .02).37 However, patients who were not treated with a thienopyridine prior to PCI had a significantly higher rate of composite ischemia with bivalirudin when compared with heparin plus a GP IIb/IIIa inhibitor (10.3% vs 7.5%; P ≤ .05).
The Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial reported a 34% relative and 1.0% absolute reduction in 30-day mortality in STEMI patients who underwent primary PCI with bivalirudin compared with heparin plus a GP IIb/IIIa inhibitor.38 Furthermore, major bleeding was reduced by 40% in patients treated with bivalirudin.38 The use of a GP lib/ Ilia inhibitor might explain the increased bleeding in the heparin groups studied. Stent thrombosis was higher within 24 hours with bivalirudin, but no significant increase was present by 30 days.
Although several studies support the use of bivalirudin over heparin and a GP IIb/IIIa inhibitor, study limitations and other data findings question their superiority. Bivalirudin possesses several pharmacologic advantages over heparin, but no clear evidence suggests bivalirudin has any advantage with regard to ischemic protection.30 The Randomized Evaluation in PCI Linking Angiomax to Reduced Clinical Events (REPLACE)-l trial reported no differences in bleeding or ischemic complications in a head-to-head comparison with bivalirudin and moderate-dose heparin (70 U/kg) in elective PCI.40 When the investigators in the REPLACE-2 trial added mandatory GP IIb/IIIa inhibitor administration to the heparin arm, more bleeding complications predictably occurred in this study arm when compared with bivalirudin monotherapy.40
The ISAR-REACT 3 trial was another study that had its shortcomings; it used a significantly higher dose of heparin (150 U/ kg) than suggested by current international guidelines.41,42 The results showed no difference in ischemic outcomes compared with bivalirudin, but showed more bleeding complications with heparin. When patients in a third arm were treated with a lower dose of heparin (100 U/kg) in the ISAR-REACT 3A trial, there was no longer a significant difference in bleeding outcomes compared with patients who received bivalirudin.43
In the How Effective Are Antithrombotic Therapies in Primary PCI (HEAT-PPCI) trial, 1829 patients from a single center with STEMI referred for primary PCI were randomized to bivalirudin or heparin (70 U/ kg bolus). Use of a GP IIb/IIIa inhibitor as a bailout was similar (13.5% vs 15.5%; P = NS).44 Nearly all patients (99.6%) were treated with dual antiplatelet therapy prior to PCI, with 60% receiving ticagrelor and 27% receiving prasugrel. The primary efficacy endpoint of major adverse cardiac events (MACE) at 4 weeks was lower in the heparin group (5.7% vs 8.7%; RR 1.52; 95% CI, 1.1-2.1, P = .01), which was driven by less target lesion revascularization and reinfarction without any increase in bleeding. In addition, definite or probable stent thrombosis was lower (0.9% vs 3.4%; RR 3.91; 95% CI, 1.6-9.5; P = .001) compared with the bivalirudin group, whereas mortality, as well as the primary safety outcome of major bleeding, was similar (3.1% vs 3.5%; P = NS).
In the Novel Approaches in Preventing or Limiting Events (NAPLES) III trial, 837 patients deemed to be at high risk for bleeding complications during their elective transfemoral PCI were randomized to bivalirudin or heparin administration (70 U/kg bolus followed by 20 U/kg to maintain ACT above 250 s).45 Bailout use of a GP IIb/IIIa inhibitor was uncommon in both arms of the study (0.5% vs 1.3%; P = .22). The primary endpoint of in-hospital major bleeding was similar in both the heparin and bivalirudin arms (3.3% vs 2.6%, odds ratio [OR], 1.28; 95% CI, 0.58-2.86; P = .54), as well as the clinical end-points at 30 days: stent thrombosis (0.5% vs 0.5%; P = .99), MI (0.2% vs 0%; P = .50), MACE (6.5% vs 4.3%; P = .17), death (2.4% vs 1.4%; P = .31), and major bleeding (3.3% vs 2.6%; P = .58).
The Bavarian Reperfusion Alternatives Evaluation (BRAVE) 4 trial was a randomized, open-label, multicenter trial that included 548 STEMI patients comparing a planned primary PCI strategy of prasugrel 60 mg plus bivalirudin (0.75 mg/kg bolus followed by 1.75 mg/kg/h infusion) against the administration of clopidogrel, 600 mg plus heparin (70-100 U/kg bolus with additional heparin administered during PCI based on ACT).46 All patients received 500 mg of intravenous aspirin. The primary endpoint of unplanned revascularization of the infarct-related artery, stent thrombosis, stroke, major bleeding, MI, or death at 30 days was similar in both groups (15.6% prasugrel plus bivalirudin vs 14.5% clopidogrel plus heparin; P = .68).47 The secondary ischemic endpoint of revascularization of the infarct-related artery, stent thrombosis, stroke, MI, or death at 30 days was also similar (4.8% vs 5.5%; P = .89).
The European Ambulance ACS Angiography (EUROMAX) trial was conducted at 65 European centers and randomized 2218 patients with STEMI transported for primary PCI to bivalirudin (0.75 mg/kg bolus followed by 1.75 mg/kg/h infusion and continuation up to 4 hours post-PCI at a rate of 0.25 mg/kg/h) or unfractionated heparin or enoxaparin with an optional GP IIb/IIIa inhibitor.48 Bivalirudin reduced the risk of the primary outcome of the composite of death or major non-coronary artery bypass grafting bleeding (5.1% vs 8.5%; RR, 0.60; 95% CI, 0.43-0.82; P = .001), driven by a reduction in major bleeding (2.6% vs 6.0%; RR, 0.43; 95% CI, 0.28-0.66; P < .001). The use of a GP IIb/IIIa inhibitor was lower in the bivalirudin group (11.5% vs 69.1%). The risk of acute stent thrombosis was higher with bivalirudin (1.1% vs 0.2%; RR, 6.11; 95% CI, 1.37-27.24; P = .007). There was no significant difference in rates of death (2.9% vs 3.1%) or reinfarction (1.7% vs 0.9%).
In a meta-analysis of 16 randomized trials that included 33,958 patients, comparing bivalirudin-based regimens versus heparin-based regimens in those treated with PCI, the risk of MACE at 30 days was higher with bivalirudin-based regimens compared with heparin-based regimens (RR 1.09; 95% CI, 1.01-1.17; P = .0204), which was largely driven by increases in MI (RR 1.12; 95% CI, 1.03-1.23) and by ischemia-driven revascularization (RR 1.16; 95% CI 0.997-1.34), with no effect on mortality (RR 0.99; 95% CI, 0.82-1.18). Bivalirudin was associated with an increased risk of stent thrombosis (RR 1.38; 95% CI, 1.09-1.74; P = .0074). Overall, bivalirudin-based regimens lowered the risk of major bleeding (RR 0.62; 95% CI, 0.49-0.78; P < .0001), but the magnitude of this effect varied greatly (P < .0001) depending on whether GP IIb/IIIa inhibitors were used predominantly in the heparin arm only (RR 0.53; 95% CI, 0.47-0.61; P < .0001), provisionally in both arms (RR 0.78; 95% CI, 0.51-1.19; P = .25), or planned in both arms (RR 1.07; 95% CI, 0.87-1.31; P = .53).
One clear advantage to using heparin over bivalirudin during PCI is that it is substantially less expensive. A cost prediction model utilizing the results of the ISAR-REACT 3 trial demonstrated that there were increased costs associated with the use of bivalirudin in all but a small group of patients at high risk for bleeding.49 Bivalirudin costs approximately $400 to $500 per PCI compared with less than $10 for heparin.21 Thus, the use of weight-based guideline-recommended heparin regimens might be the more cost-effective method to protect against ischemic events and reduce hemorrhagic complications. In addition, bivalirudin must be reconstituted at the time of administration, making its use a little more labor intensive. Another advantage that heparin has over bivalirudin is that it possesses a specific antidote to reverse its anticoagulant effects: protamine sulfate.9,21 However, the lack of a specific bivalirudin antidote is less problematic than for longer-acting anticoagulants because bivalirudin has a half-life of 25 minutes after intravenous injection.50,51 However, the half-life extends to 57 minutes for CrCl between 10 to 29 mL/ min and 3.5 hours in patients on hemodialysis. Therefore, the infusion of bivalirudin in patients with CrCl 10 to 29 mL/min should be reduced from 2 mg/kg/h to 1 mg/kg/h.52
Conclusions
Heparin plays a critical role in preventing acute thrombotic events in patients undergoing PCI. Despite the pharmacologic disadvantages, heparin has its advantages in reversibility and cost, but discrepancies concerning its effectiveness in PCI compared with bivalirudin need to be addressed. The optimal heparin dosing regimen, which minimizes the risk of bleeding complications while maintaining protection from ischemic com plications, has yet to be elucidated. The addition of a GP IIb/IIIa inhibitor increases the risk of bleeding and therefore should be used judiciously A large-scale multicenter randomized trial comparing heparin monotherapy and bivalirudin with pretreatment with clopidogrel is still needed to clarify the ideal AT for elective PCI. ![]()
References
- Linhardt RJ. Heparin: an important drug enters its seventh decade. Chemistry Industry. 1991;2:45-50.
- Francis CW, Crowther M. Principles of antithrombotic therapy. In: Lichtman MA, Kipps TJ, Seligsohn U, et al, eds. Williams Hematology. 8th ed. New York, NY: McGraw-Hill; 2010:353-368 : chapter 23.
- Hirsh J, Anand SS, Halperin JL, Fuster V. Mechanism of action and pharmacology of unfractionated heparin. Arterioscler Thromb Vasc Biol. 2001;21: 1094-1096.
- Nelson DL, Cox M. Lehninger Principles of Biochemistry. 4th ed. New York, NY: W. H. Freeman; 2004.
- Gray E, Hogwood J, Mulloy B. The anticoagulant and antithrombotic mechanisms of heparin. Handb Exp Pharmacol. 2012;207:43-61.
- Linhardt RJ, Gunay NS. Production and chemical processing of low molecular weight heparins. Semin Thromb Hemost. 1999;25(suppl 3):5-16.
- Eikelboom JW, Hankey GJ. Low molecular weight heparins and heparinoids. Med J Aust. 2002;177: 379-383.
- Hughes S, Szeki I, Nash MJ, Thachil J. Anticoagulation in chronic kidney disease patients—the practical aspects. Clinical Kidney Journal. 2014;7:442-449.
- Kearney TE. Protamine. In: Olson KR, ed. Poisoning & Drug Overdose. 6th ed. New York, NY: McGraw-Hill; 2012: chapter 228.
- Marmur JD, Bullock-Palmer RP, Poludasu S, Cavusoglu E. Avoiding intelligence failures in the cardiac catheterization laboratory: strategies for the safe and rational use of dalteparin or enoxaparin during percutaneous coronary intervention. J Invasive Cardiol. 2009;21:653-664.
- Partial thromboplastin time (PTT). Medline Plus. U.S. National Library of Medicine website. http://www.nlm.nih.gov/medlineplus/ency/article/003653.htm. Accessed July 28, 2015.
- Bowers J, Ferguson JJ 3rd. The use of activated clotting times to monitor heparin therapy during and after interventional procedures. Clin Cardiol. 1994; 17:357-361.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58: E44-E122.
- Ndrepepa G, Berger PB, Mehilli J, et al. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end point. J Am Coll Cardiol. 2008;51:690-697.
- Feit F, Voeltz MD, Attubato MJ, et al. Predictors and impact of major hemorrhage on mortality following percutaneous coronary intervention from the REPLACE-2 trial. Am J Cardiol. 2007;100: 1364-1369.
- Hirsch J, Anand SS, Halperin JL, Fuster V; American Heart Association. Guide to anticoagulant therapy: heparin. Circulation. 2001;103:2994-3018.
- Safani M, Hill SE, Winters R, et al. The use of average body weight in dosing unfractionated heparin. Chest. 2013;143:1840-1841.
- Brener SJ, Moliterno DJ, Lincoff AM, et al. Relationship between activated clotting time and ischemic or hemorrhagic complications: analysis of 4 recent randomized clinical trials of percutaneous coronary intervention. Circulation. 2004;110: 994-998.
- Chew DP, Bhatt DL, Lincoff AM, et al. Defining the optimal activated clotting time during percutaneous coronary intervention: aggregate results from 6 randomized, controlled trials. Circulation. 2001;103:961-966.
- Tolleson TR, O’Shea JC, Bittl JA, et al. Relationship between heparin anticoagulation and clinical outcomes in coronary stent intervention: observations from the ESPRIT trial. J Am Coll Cardiol. 2003;41: 386-393.
- Lee MS, Oyama J, Iqbal Z, Tarantini G. Lowdose heparin for elective percutaneous coronary intervention. J Interv Cardiol. 2014;27:58-62.
- Godon P, Rioufol G, Finet G, et al. Efficacy and safety of low-dose heparin (30 IU/kg) during coronary angioplasty [Article in French]. Arch Mal Coeur Vaiss. 2001;94;984-988.
- Rao SV, Ohman EM. Anticoagulant therapy for percutaneous coronary intervention. Circ Cardiovasc Interv. 2010;3:80-88.
- Stabile E, Nammas W, Salemme L, et al. The CIAO (Coronary Interventions Antiplatelet-based Only) study: a randomized study comparing standard anticoagulation regimen to absence of anticoagulation for elective percutaneous coronary intervention. J Am Coll Cardiol. 2008;52:1293-1298.
- Saltzman AJ, Stone GW, Claessen BE, et al. Long-term impact of chronic kidney disease in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention: the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) Trial. JACC Cardiovascular Interventions. 2011;4:1011-1019.
- Menon V, Berkowitz SD, Antman EM, et al. New heparin dosing recommendations for patients with acute coronary syndromes. Am J Med. 2001;110: 641-650.
- Inhibition of platelet glycoprotein IIb/IIIa with eptifibatide in patients with acute coronary syndromes. The PURSUIT Trial Investigators. Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy. N Engl J Med. 1998;339:436-443.
- Montalescot G, Barragan P, Wittenberg O, et al; ADMIRAL Investigators. Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction. N Engl J Med. 2001;344:1895-1903.
- Kastrati A, Mehilli J, Neumann FJ, et al; Intracoronary Stenting and Antithrombotic: Regimen Rapid Early Action for Coronary Treatment 2 (ISAR-REACT 2) Trial Investigators. Abciximab in patients with acute coronary syndromes undergoing percutaneous coronary intervention after clopidogrel pretreatment: the ISAR-REACT 2 randomized trial. JAMA. 2006;295:1531-1538.
- Shahzad A, Cooper RM, Stables RH. Antithrombic therapy in PCI: why not heparin? EuroIntervention. 2013;9:423-426.
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78-e140.
- Steg PG, James SK, Atar D, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619.
- Steinhubl SR, Berger PB, Mann JT 3rd, et al; CREDO Investigators. Clopidogrel for the Reduction of Events During Observation. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411-2420.
- O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of STelevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:485-510.
- Blankenship JC, Balog C, Sapp SK, et al. Reduction in vascular access site bleeding in sequential abciximab coronary intervention trials. Catheter Cardiovasc Interv. 2002;57:476-483.
- Lincoff AM, Bittl JA, Harrington RA, et al; REPLACE-2 Investigators. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA. 2003;289:853-863.
- Stone GW, McLaurin BT, Cox DA, et al; ACUITY Investigators. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355:2203-2216.
- Stone GW, Witzennbichler B, Guagliumi G, et al; HORIZONS-AMI Trial Investigators. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218-2230.
- Dehmer GJ, Weaver D, Roe MT, et al. A contemporary view of diagnostic cardiac catheterization and percutaneous coronary intervention in the United States: a report from the CathPCI Registry of the National Cardiovascular Data Registry, 2010 through June 20 11. J Am Coll Cardiol. 2012;60: 2017-2031.
- Lincoff AM, Bittl JA, Kleiman NS, et al; REPLACE-1 Investigators. Comparison of bivalirudin versus heparin during percutaneous coronary intervention (the Randomized Evaluation of PCI Linking Angiomax to Reduced Clinical Events [REPLACE]-1 trial). Am J Cardiol. 2004;93:1092-1096.
- Kastrati A, Neumann FJ, Mehilli J, et al; ISAR-REACT 3 Trial Investigators. Bivalirudin versus unfractionated heparin during percutaneous coronary intervention. N Engl J Med. 2008;359:688-696.
- Wijns W, Kolh P, Danchin N, et al. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501-2555.
- Schulz S, Mehilli J, Neumann FJ, et al; Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISARREACT) 3A Trial Investigators. ISAR-REACT 3A: a study of reduced dose of unfractionated heparin in biomarker negative patients undergoing percutaneous coronary intervention. Eur Heart J. 2010;31:2482-2491.
- Shahzad A, Kemp I, Mars C, et al. Unfractionated heparin versus bivalirudin in primary percutaneous coronary intervention (HEAT-PPCI): an open-label, single center, randomized controlled trial. Lancet. 2014;384:1849-1858.
- Brigouri C, Bisconti G, Focaccio A, et al. Novel approaches in preventing or limiting events (NAPLES) III trial: randomized comparison of bivalirudin versus unfractionated heparin at increased risk of bleeding undergoing transfemoral elective coronary stenting. JACC Cardiovasc Interv. 2015;8: 414-423.
- Schulz S, Richardt G, Laugwitz KL, et al. Comparison of prasugrel and bivalirudin vs. clopidogrel and heparin in patients with STsegment elevation myocardial infarction: design and rationale of the Bavarian Reperfusion Alternatives Evaluation (BRAVE) 4 trial. Clin Cardiol. 2014;37: 270-276.
- Kumbhani DJ. Bavarian Reperfusion Alternatives Evaluation - BRAVE 4. http://www.cardiosource.org/Science-And-Quality/Clinical-Trials/B/BRAVE-4.aspx. Accessed July 28, 2015.
- Steg PG, van ‘t Hof A, Hamm CW, et al; EUROMAX Investigators. Bivalirudin started during emergency transport for primary PCI. N Engl J Med. 2013;369:2207-2217.
- Amin AP, Marso SP, Rao SV, et al. Cost-effectiveness of targeting patients undergoing percutaneous coronary intervention for therapy with bivalirudin versus heparin monotherapy according to predicted risk of bleeding. Circ Cardiovasc Qual Outcomes. 2010;3:358-365.
- Eikelboom J, Sobieraj-Teague M, Ginsberg JS. Anticoagulant therapy. In: McKean SC, Ross JJ, Dressler DD, et al, eds. Principles and Practice of Hospital Medicine. New York, NY: McGraw-Hill; 2012:2184-2190.
- Francis CW, Crowther M. Principles of antithrombotic therapy. In: Lichtman MA, Kipps TJ, Seligsohn U, et al, eds. Williams Hematology. 8th ed. New York, NY: McGraw-Hill; 2010:353-368.
- Bivalirudin. Medscape website. http://reference.medscape.com/drug/angiomax-angiox-bivalirudin-342137. Accessed July 28, 2015.
- Fauci AS, Braunwald E, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill Medical; 2008.