Coronary Plaque Characteristics Affect No-Reflow During Primary Percutaneous Coronary Intervention: A Pooled Analysis of 14 Observational Studies Using Intravascular Ultrasound
Bu-Chun Zhang, MD, PhD,1 Cheng Wang, MD,1 Zhi-Wen Zhou, MD, PhD,2 Yan-Feng Ma, MD,1 Wen-Hua Li, MD,1 Dong-Ye Li, MD1
1Department of Cardiology, The Affiliated Hospital of Xuzhou Medical College, Jiangsu 221002, China;
2Department of Cardiology, Shanghai Xuhui Central Hospital, Shanghai, China
The association between coronary plaque composition and no-reflow during percutaneous coronary intervention (PCI) is still debated. We performed a systematic literature search using MEDLINE, Embase, Cochrane, and Ovid databases for intravascular ultrasound (IVUS) studies evaluating the relationship between coronary plaque characteristics and no-reflow after PCI. Fourteen observational trials were included in the meta-analysis, including 1457 patients (237 in the no-reflow group, 1220 in the normal reflow group). Pooled analysis indicated that the no-reflow group had a significantly higher absolute volume of fibrofatty plaque (weighted mean differences [WMD], 4.94 mm3; 95% confidence interval [CI], 1.83-8.06; P = .002), external elastic membrane cross-sectional area (EEM-CSA) (WMD, 3.40 mm2; 95% CI, 2.22-4.58; P < .00001), plaque area (WMD, 4.06 mm2; 95% CI, 2.24-5.89; P < .0001), and artery remodeling index (WMD, 0.09; 95% CI, 0.06-0.13; P < .00001), and a smaller percentage of fibrous plaque (WMD, −5.89 %; 95% CI, −0.66 to −11.12; P = .03) than in the normal reflow group. There were no significant differences in the other plaque components between the two groups. This meta-analysis confirmed that high absolute volume of fibrofatty plaque, EEM-CSA, plaque area, and coronary artery remodeling index, and a decreased percentage of fibrous plaque as detected by IVUS in culprit lesions, are linked with the development of the no-reflow phenomenon after PCI.
[Rev Cardiovasc Med. 2015;16(3):200-213 doi: W.3909/ricm0780]
© 2015 MedReviews®, LLC
Coronary Plaque Characteristics Affect No-Reflow During Primary Percutaneous Coronary Intervention: A Pooled Analysis of 14 Observational Studies Using Intravascular Ultrasound
Bu-Chun Zhang, MD, PhD,1 Cheng Wang, MD,1 Zhi-Wen Zhou, MD, PhD,2 Yan-Feng Ma, MD,1 Wen-Hua Li, MD,1 Dong-Ye Li, MD1
1Department of Cardiology, The Affiliated Hospital of Xuzhou Medical College, Jiangsu 221002, China;
2Department of Cardiology, Shanghai Xuhui Central Hospital, Shanghai, China
The association between coronary plaque composition and no-reflow during percutaneous coronary intervention (PCI) is still debated. We performed a systematic literature search using MEDLINE, Embase, Cochrane, and Ovid databases for intravascular ultrasound (IVUS) studies evaluating the relationship between coronary plaque characteristics and no-reflow after PCI. Fourteen observational trials were included in the meta-analysis, including 1457 patients (237 in the no-reflow group, 1220 in the normal reflow group). Pooled analysis indicated that the no-reflow group had a significantly higher absolute volume of fibrofatty plaque (weighted mean differences [WMD], 4.94 mm3; 95% confidence interval [CI], 1.83-8.06; P = .002), external elastic membrane cross-sectional area (EEM-CSA) (WMD, 3.40 mm2; 95% CI, 2.22-4.58; P < .00001), plaque area (WMD, 4.06 mm2; 95% CI, 2.24-5.89; P < .0001), and artery remodeling index (WMD, 0.09; 95% CI, 0.06-0.13; P < .00001), and a smaller percentage of fibrous plaque (WMD, −5.89 %; 95% CI, −0.66 to −11.12; P = .03) than in the normal reflow group. There were no significant differences in the other plaque components between the two groups. This meta-analysis confirmed that high absolute volume of fibrofatty plaque, EEM-CSA, plaque area, and coronary artery remodeling index, and a decreased percentage of fibrous plaque as detected by IVUS in culprit lesions, are linked with the development of the no-reflow phenomenon after PCI.
[Rev Cardiovasc Med. 2015;16(3):200-213 doi: W.3909/ricm0780]
© 2015 MedReviews®, LLC
Coronary Plaque Characteristics Affect No-Reflow During Primary Percutaneous Coronary Intervention: A Pooled Analysis of 14 Observational Studies Using Intravascular Ultrasound
Bu-Chun Zhang, MD, PhD,1 Cheng Wang, MD,1 Zhi-Wen Zhou, MD, PhD,2 Yan-Feng Ma, MD,1 Wen-Hua Li, MD,1 Dong-Ye Li, MD1
1Department of Cardiology, The Affiliated Hospital of Xuzhou Medical College, Jiangsu 221002, China;
2Department of Cardiology, Shanghai Xuhui Central Hospital, Shanghai, China
The association between coronary plaque composition and no-reflow during percutaneous coronary intervention (PCI) is still debated. We performed a systematic literature search using MEDLINE, Embase, Cochrane, and Ovid databases for intravascular ultrasound (IVUS) studies evaluating the relationship between coronary plaque characteristics and no-reflow after PCI. Fourteen observational trials were included in the meta-analysis, including 1457 patients (237 in the no-reflow group, 1220 in the normal reflow group). Pooled analysis indicated that the no-reflow group had a significantly higher absolute volume of fibrofatty plaque (weighted mean differences [WMD], 4.94 mm3; 95% confidence interval [CI], 1.83-8.06; P = .002), external elastic membrane cross-sectional area (EEM-CSA) (WMD, 3.40 mm2; 95% CI, 2.22-4.58; P < .00001), plaque area (WMD, 4.06 mm2; 95% CI, 2.24-5.89; P < .0001), and artery remodeling index (WMD, 0.09; 95% CI, 0.06-0.13; P < .00001), and a smaller percentage of fibrous plaque (WMD, −5.89 %; 95% CI, −0.66 to −11.12; P = .03) than in the normal reflow group. There were no significant differences in the other plaque components between the two groups. This meta-analysis confirmed that high absolute volume of fibrofatty plaque, EEM-CSA, plaque area, and coronary artery remodeling index, and a decreased percentage of fibrous plaque as detected by IVUS in culprit lesions, are linked with the development of the no-reflow phenomenon after PCI.
[Rev Cardiovasc Med. 2015;16(3):200-213 doi: W.3909/ricm0780]
© 2015 MedReviews®, LLC
KEY WORDS
No-reflow phenomenon • Plaque characteristics • Intravascular ultrasound • Coronary disease • Meta-analysis
KEY WORDS
No-reflow phenomenon • Plaque characteristics • Intravascular ultrasound • Coronary disease • Meta-analysis
The pathophysiology of no-reflow is likely multifactorial; the type of plaque may be an important predictor for no-reflow after PCI.
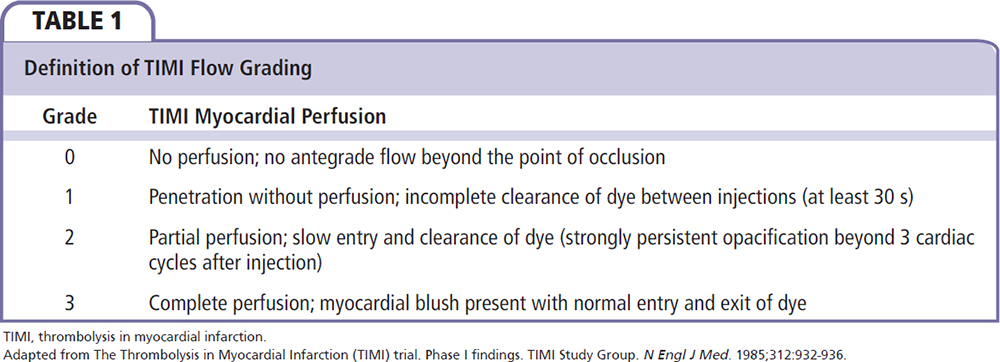
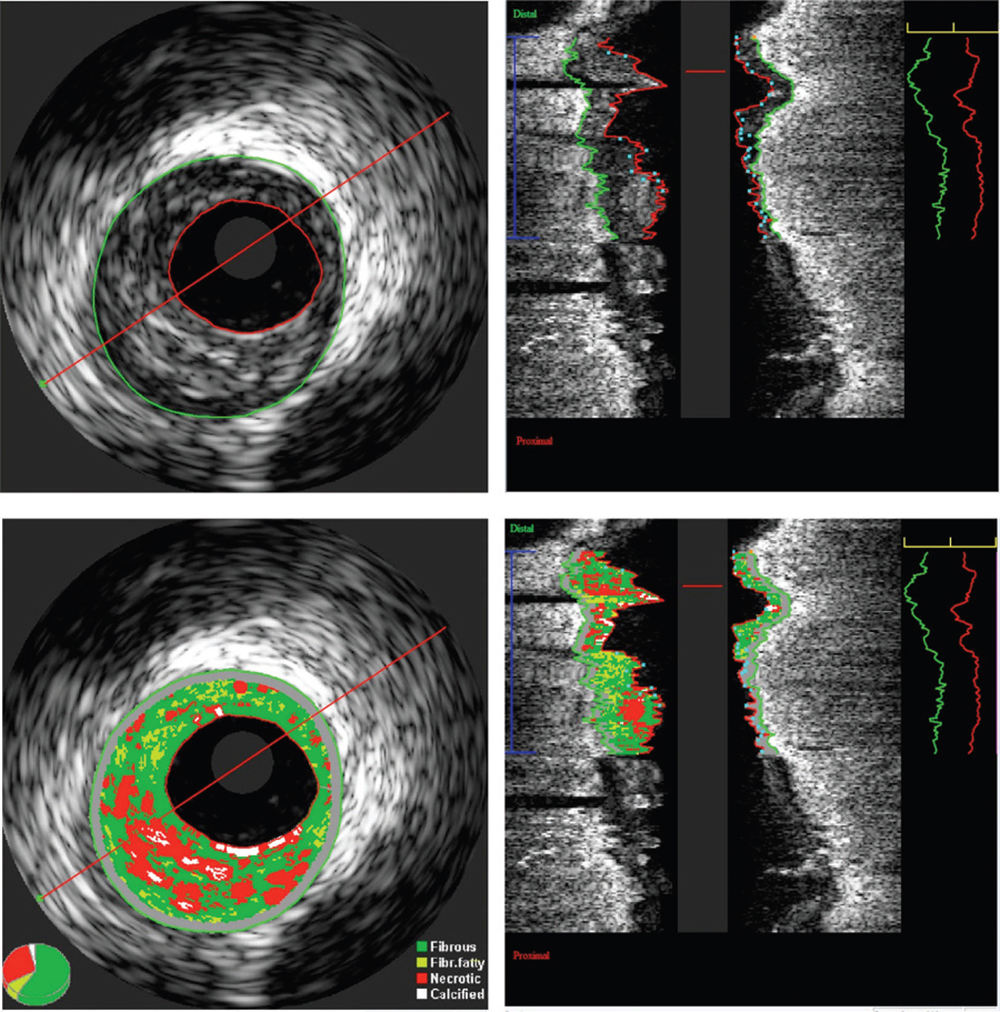
Figure 1. Information theoretic quantification of the absolute plaque volume. Grayscale intravascular ultrasound (IVUS) image (top). Virtual histology IVUS image (bottom). A region of interest is defined and analyzed by indicating the outlines of the lumen and the external elastic membrane in all frames. Out of this analysis, lumen, vessel, and plaque volumes are calculated together with virtual histology data such as fibrofatty and fibrous plaque volumes. Image courtesy of Jurgen M.R. Ligthart, BSc.
The meta-analysis showed the no-reflow group had a significantly higher absolute volume of fibrofatty plaque … compared with the normal reflow group.

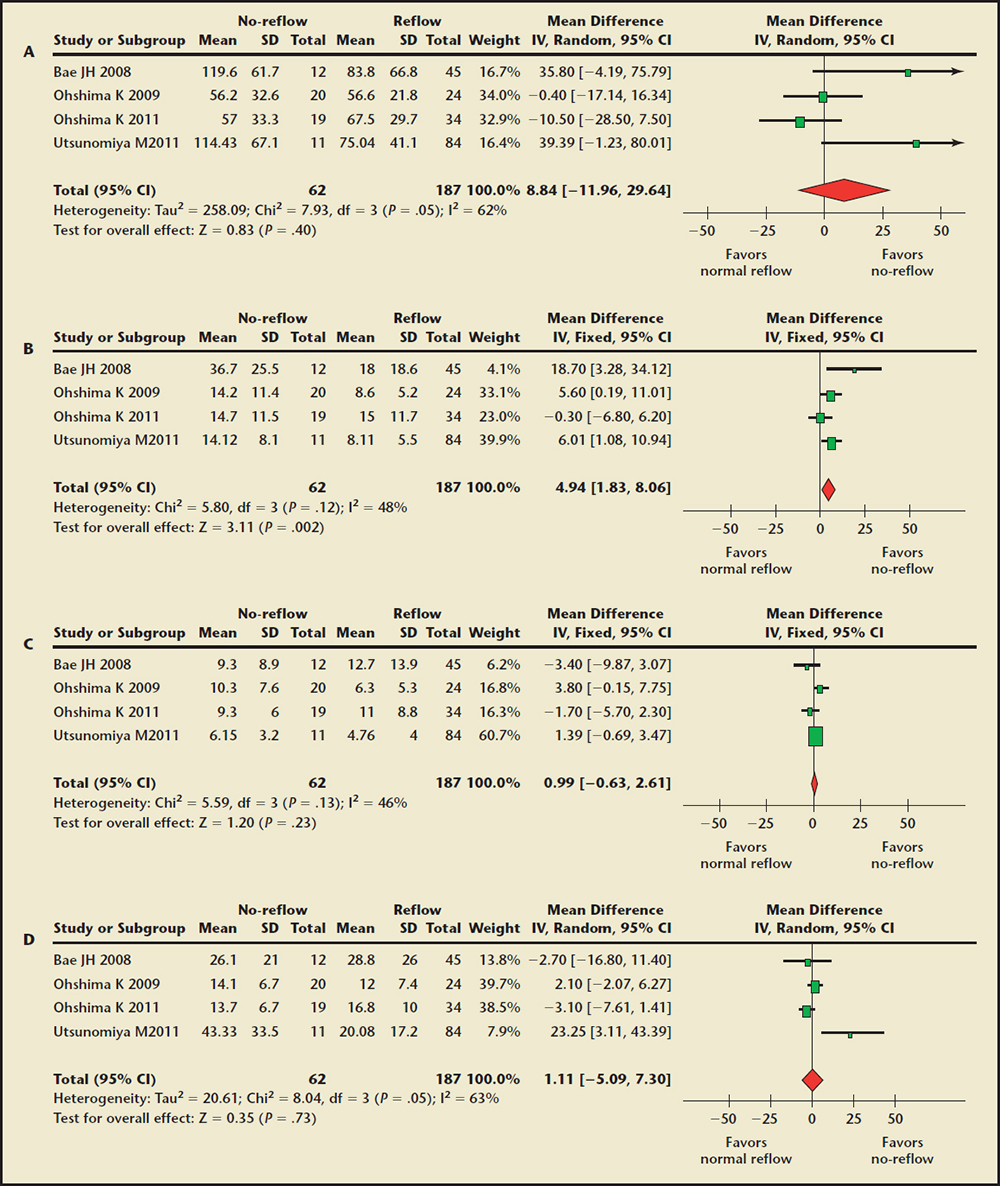
Figure 2. Forest plot of weighted mean difference for absolute plaque component volume in the no-reflow and normal reflow groups. (A) Absolute fibrous volume comparison. (B) Absolute fibrofatty volume comparison. (C) Absolute dense calcium volume comparison. (D) Absolute necrotic core volume comparison. CI, confidence interval; IV, instrumental variable; SD, standard deviation.
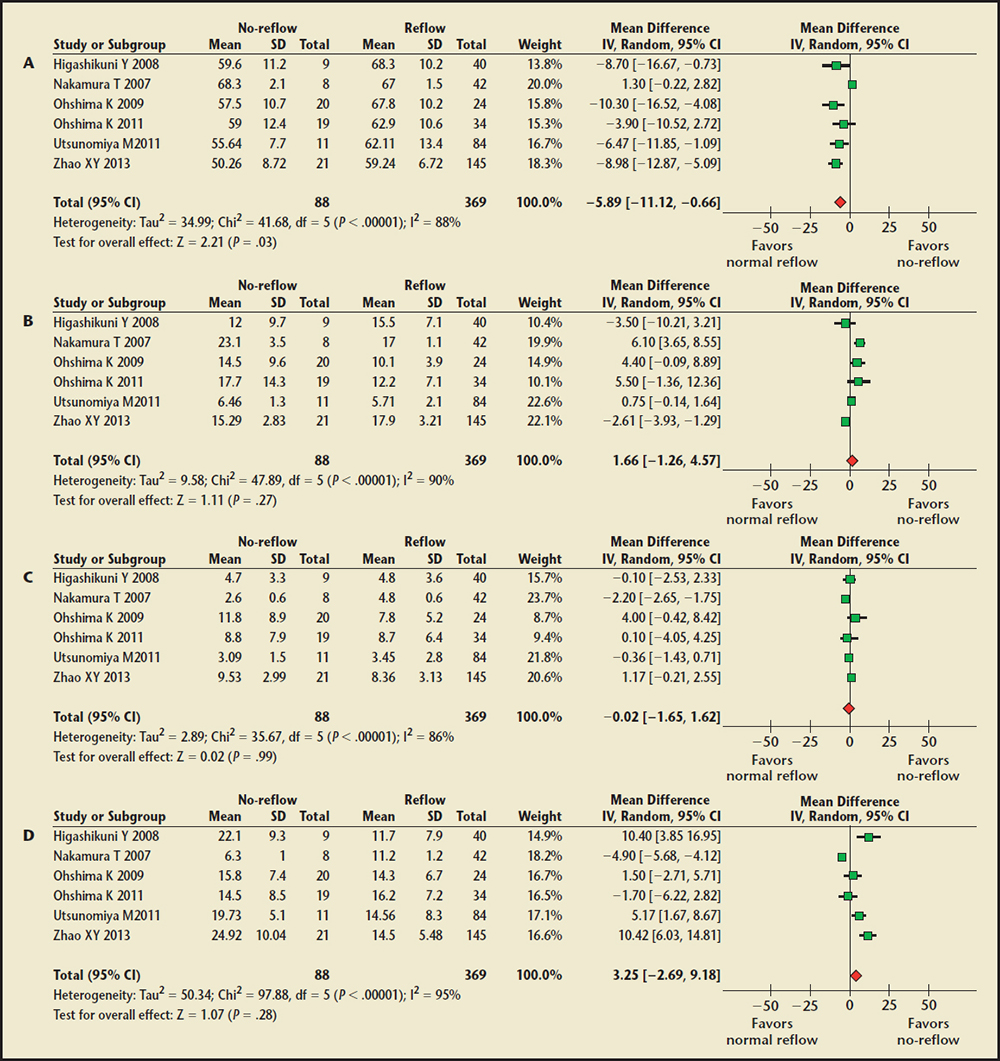
Figure 3. Forest plot of weighted mean difference for percentage of plaque composition in the no-reflow and normal reflow groups. (A) Fibrous percentage comparison. (B) Fibrofatty percentage comparison. (C) Dense calcium percentage comparison. (D) Necrotic core percentage comparison. CI, confidence interval; IV, instrumental variable; SD, standard deviation.
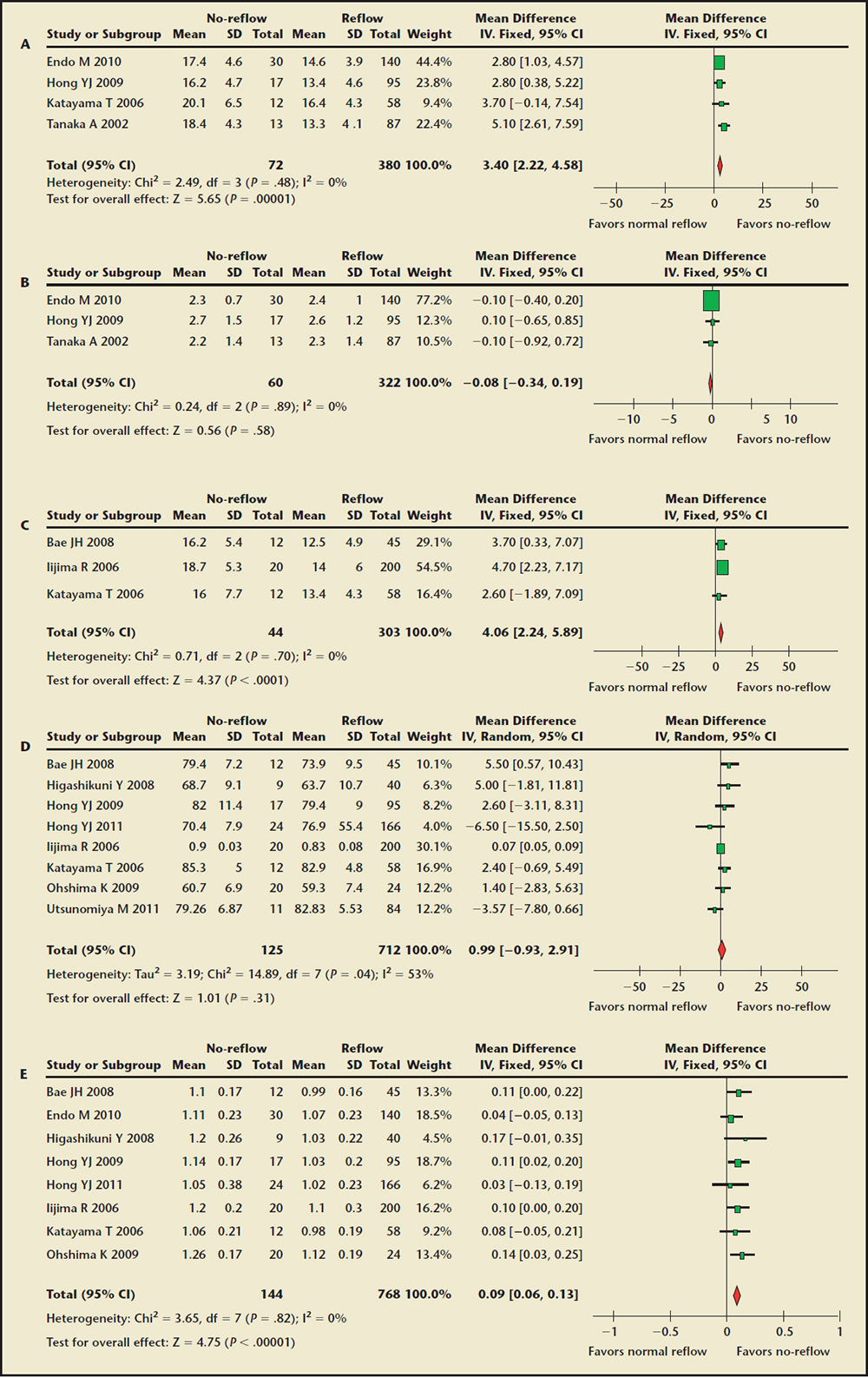
Figure 4. Forest plot of weighted mean difference for the entire culprit lesion analysis in the no-reflow and normal reflow groups. (A) External elastic membrane crosssectional area comparison. (B) Lumen cross-sectional area comparison. (C) Plaque area comparison. (D) Plaque burden comparison. (E) Coronary artery remodeling index comparison. CI, confidence interval; IV, instrumental variable; SD, standard deviation.
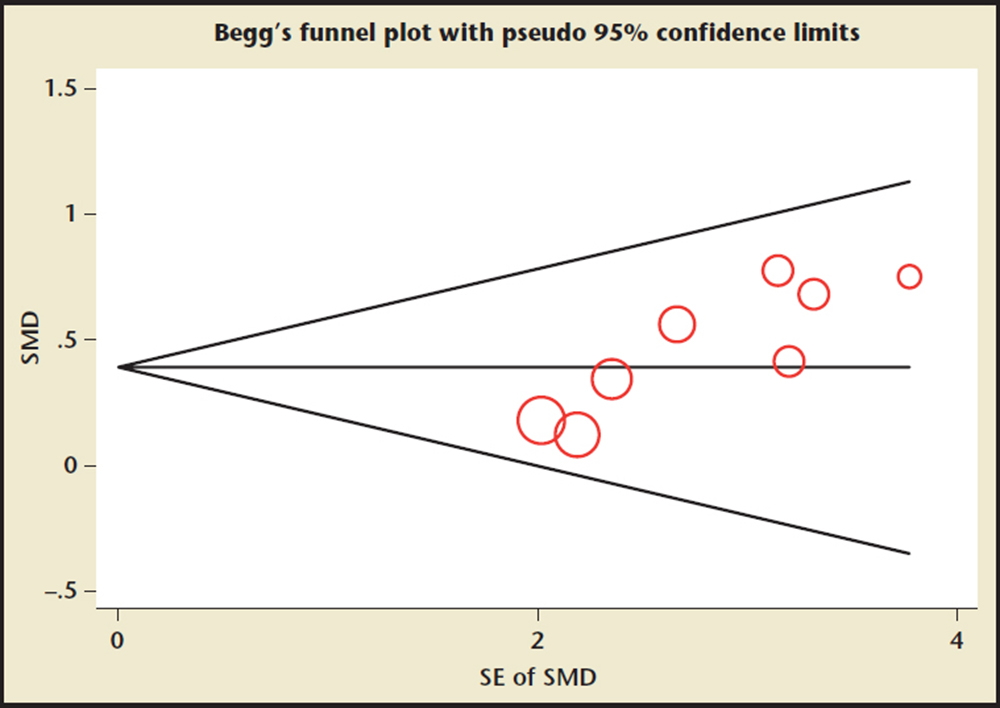
Figure 5. Funnel plots of the remodeling index for assessment of publication bias. SE, standard error; SMD, standardized mean difference.
[The no-reflow phenomenon] is a complex phenomenon and is caused by the variable combination of four pathogenetic components: distal atherothrombotic embolization, ischemic injury, reperfusion injury, and susceptibility of coronary microcirculation to injury.
With regard to the coronary artery remodeling index detected by IVUS in patients with ACS, previous clinical studies have shown that preintervention findings, including remodeling index, are predictable risk factors for the angiographic no-reflow phenomenon.
Main Points
• The “no-reflow” phenomenon refers to the inability to reperfuse myocardial tissue despite the reopening of the infarct-related artery. No-reflow often happens in acute myocardial infarction patients during primary percutaneous coronary intervention (PCI), which has a strong negative impact on clinical outcome, negating the potential benefit of primary PCI. No-reflow is associated with a higher rate of mortality and early postinfarction complications.
• Meta-analysis based on currently available published observational studies demonstrates that, among intravascular ultrasound (IVUS) measurement parameters, absolute volume of fibrofatty plaque, external elastic membrane cross-sectional area, plaque area, and coronary artery remodeling index in culprit lesions are significantly greater in patients with no-reflow after PCI compared with patients with normal reflow; however, the percentage of the fibrous plaque was significantly smaller in the patients with no-reflow.
• Coronary plaque composition of culprit/target lesions based on IVUS analysis is closely related to the development of impaired myocardial perfusion following primary angioplasty, which suggests the importance of evaluation of plaque volume and composition by IVUS prior to mechanical therapy.
Main Points
• The “no-reflow” phenomenon refers to the inability to reperfuse myocardial tissue despite the reopening of the infarct-related artery. No-reflow often happens in acute myocardial infarction patients during primary percutaneous coronary intervention (PCI), which has a strong negative impact on clinical outcome, negating the potential benefit of primary PCI. No-reflow is associated with a higher rate of mortality and early postinfarction complications.
• Meta-analysis based on currently available published observational studies demonstrates that, among intravascular ultrasound (IVUS) measurement parameters, absolute volume of fibrofatty plaque, external elastic membrane cross-sectional area, plaque area, and coronary artery remodeling index in culprit lesions are significantly greater in patients with no-reflow after PCI compared with patients with normal reflow; however, the percentage of the fibrous plaque was significantly smaller in the patients with no-reflow.
• Coronary plaque composition of culprit/target lesions based on IVUS analysis is closely related to the development of impaired myocardial perfusion following primary angioplasty, which suggests the importance of evaluation of plaque volume and composition by IVUS prior to mechanical therapy.
The “no-reflow” phenomenon refers to the inability to reperfuse myocardial tissue despite the reopening of the infarct-related artery.1 No-reflow often happens in acute myocardial infarction (MI) patients during primary percutaneous coronary intervention (PCI), which has a strong negative impact on clinical outcome, negating the potential benefit of primary PCI. No-reflow is associated with a higher rate of mortality and early postinfarction complications.2 The occurrence of no-reflow can be evaluated first in the catheterization laboratory by the use of angiographic indexes, Doppler wire, and electrocardiographic assessment.3 On angiograms, the no-reflow phenomenon is defined as substantial coronary antegrade flow reduction (less than thrombolysis in MI flow grade 3) without mechanical obstruction (Table 1).4,5
The pathophysiology of no-reflow is likely multifactorial; the type of plaque may be an important predictor for no-reflow after PCI. A few observational studies have reported that the angiographic no-reflow phenomenon is associated with atherosclerotic plaque characteristics detected by intravascular ultrasound (IVUS) before PCI in acute MI patients,6,7 however, with inconsistent results.8-16 Because of the small sample size, the power achieved in those studies was not sufficient to detect whether coronary plaque composition increased risk of no-reflow after PCI (Figure 1). Using all available published data to increase statistical power, meta-analysis is an efficient way of analytically combining the results of individual studies together to detect and quantify an effect with more precision.
The purpose of this pooled analysis is to combine primary data from all relevant studies to produce reliable estimates of the associations of coronary plaque composition assessed by IVUS with the incidence of no-reflow after PCI among patients with acute coronary syndrome (ACS).
Methods
Search Strategy
We performed a computerized literature search in PubMed, Embase, Cochrane, and Ovid databases (up to November, 2014), using the key words no-reflow, slow reflow, intravascular ultrasound, virtual histology, plaque characteristic, plaque composition, and percutaneous coronary intervention along with a filter for studies in human beings. Citations were screened and evaluated using the established inclusion/exclusion criteria at the abstract level by two operators (Drs. Zhang and Wang), and relevant studies were retrieved as full manuscripts. There were no language restrictions. Our systematic review was conducted according to the Meta-analysis of Observational Studies in Epidemiology guidelines.12
Selection
Inclusion criteria were (1) grayscale and/or virtual histology (VH)-IVUS examination before PCI; and (2) direct comparison of lesion characteristics and/or plaque composition in patients with or without no reflow/slow reflow phenomenon after PCI. Studies were excluded from the meta-analyses if enrolled subjects without control group and IVUS measurements cannot provide appropriate quantitative results.
Data Extraction
Two independent investigators (Drs. Li and Zhou) reviewed each report to determine its eligibility and then extracted and tabulated all of the relevant data. Disagreement was resolved by consensus between the two authors. The following basic information was obtained from each article: first author, year of publication, country of origin, sample size, mean age, sex distribution, hypertension, diabetes, clinical setting, definition of no-reflow, and IVUS type. In addition, we retrieved conventional IVUS data including the volume and percentage of each tissue component of plaque, plaque area, plaque burden, culprit lesions, external elastic membrane cross-sectional areas (EEM-CSA) and lumen CSA, and coronary artery remodeling index from included trials.
Statistical Analysis
To ensure adequate statistical power, we only conducted pooled analysis for IVUS quantitative measurements with available data from at least three independent studies. All analyses were performed using Review Manager 5.0 software (available from The Cochrane Collaboration at http://www.cochrane.org) and software STATA version 11.2 (Stata Corporation, College Station, TX). The Mantel-Haenszel method for fixed effects and the DerSimonian-Laird method for random effects were used to estimate pooled weighted mean differences (WMD). We tested heterogeneity of the included studies with Q statistics and the extent of inconsistency between results with I2 statistics.13 In the absence of heterogeneity between studies, the methods produced very similar results. We report fixed-effects estimates because fixed effects are more robust in meta-analysis calculations when there are small numbers of events. Possibility of publication bias was assessed by funnel plot analysis. Sensitivity analysis was also done by omitting one study at a time to examine influence of one study on the overall summary estimate. Data are presented as WMD with 95% confidence intervals (CIs). P < .05 was considered statistically significant.
Results
Description of Studies
Of 253 potentially relevant articles initially screened, 207 publications were excluded because they did not meet the inclusion criteria. Of the remaining 46 complete reports, 32 studies without appropriate IVUS measurements or a comparison of normal reflow were excluded. Finally, 14 observational trials8-11,14-23 met inclusion criteria and were included in the final meta-analysis, consisting of a total of 1457 patients (237 in the no-reflow group, 1220 in the normal reflow group). Five studies had a prospective design,9,10,14,15,18 and nine studies had a retrospective design.8,11,16,17,19-23
Of the 14 studies, 9 studies involved acute MI patients,8-10,14,15,17-20 4 studies enrolled ACS (including both acute MI and unstable angina) patients,11,16,21,22 and the remaining study involved unstable angina patients.23 In this pooled analysis, six used grayscale IVUS,8,14-17,19 two used both grayscale and VH-IVUS,10,20 five used VH-IVUS,9,11,18,21,23 and one study used grayscale and iMap-IVUS only.22 The baseline characteristics of patients and coronary plaque characteristics included in the meta-analysis are reported in Tables 2 through 4. All studies were published in English. The subject population was derived from three countries.
Relationship Between Coronary Plaque Characteristics and No-Reflow After Primary PCI
The Absolute Plaque Component Volume Findings. Four trials10,18,20,22 of 249 patients were included. The meta-analysis showed the no-reflow group had a significantly higher absolute volume of fibrofatty plaque (WMD, 4.94 mm3; 95% CI, 1.83-8.06; P = .002) compared with the normal reflow group (Figure 2). There were no significant differences in absolute volume of fibrous, dense calcified, and necrotic core at the culprit lesions between the two groups. On sensitive analysis, the results remained unchanged by excluding any individual trial.
The Percentage of Plaque Composition Findings. Six trials9,11,18,20,22,23 of 457 patients were included. Pooled analysis showed the percentage of the fibrous plaque was significantly smaller in the no-reflow group (WMD, −5.89%; 95% CI, −0.66 to −11.12; P = .03) compared with the normal reflow group (Figure 3). No significant differences were noted in the percentage of fibrofatty, dense calcified, and necrotic core at the culprit lesions between the two groups. Substantial statistical heterogeneity was detected in all of the comparisons among the trials.
Sensitivity analysis for the percentage of the fibrous plaque, excluding the studies by Higashikuni and colleagues,16 Ohshima and colleagues,18 Utsunomiya and associates,22 and Zhao and colleagues23 from the pooled analysis, resulted in a borderline statistical significance between the two groups (P = .06 for excluding either, and P = .07 for excluding either). With regard to the percentage of necrotic core, sensitivity analysis showed that omitting one study by Nakamura and associates,14 it was significantly greater in the no-reflow group compared with the normal reflow group (WMD, 4.95%; 95% CI, 0.54-9.36; P = .03).
The Culprit Lesions EEM-CSA and Lumen CSA Findings. Four studies8,14,17,19 were used for the analysis of the EEM-CSA, including 452 patients. As shown in Figure 4A, culprit lesions EEM-CSA of the no-reflow group were larger than in the normal reflow group (WMD, 3.40 mm2; 95% CI, 2.22-4.58; P < .00001). However, lumen CSA was not different between the two groups (Figure 4B). No evidence of statistical heterogeneity was identified (I2 = 0%). Influence analysis demonstrated that no single study significantly altered the summary estimates.
The Plaque Area and Plaque Burden Findings. Data for plaque area were available from three studies8,10,16 including 347 patients, and are shown in Figure 4C. Overall, plaque area was significantly greater in the no-reflow group compared with the normal reflow group (WMD, 4.06 mm2; 95% CI, 2.24-5.89; P < .0001). A total of 837 patients were included in eight studies8,10,11,16-18,21,22 reporting plaque burden. There was no significant difference between the two groups (WMD, 0.99%; 95% CI, -0.93-2.91; P = .31) (Figure 4D). On sensitivity analyses, the results remained unchanged by omitting one study at a time.
Coronary Artery Remodeling Index Findings. A total of 912 patients were included in eight studies8,10,11,16-19,21 reporting coronary artery remodeling index. It was significantly greater in the no-reflow group compared with the normal reflow group (WMD, 0.09; 95% CI, 0.06-0.13; P < .00001) (Figure 4E). There was no evidence of heterogeneity (P = .82, I2 = 0%). Sensitivity analysis indicated that the results of the meta-analysis were reliable and stable.
Publication Bias Diagnostics
Because the coronary artery remodeling index comes from eight studies, funnel plots were performed for remodeling index data. The funnel plot did not show an asymmetric pattern (Figure 5).
Discussion
The present meta-analysis based on currently available published observational studies demonstrates that, among IVUS measurement parameters, absolute volume of fibrofatty plaque, EEM-CSA, plaque area, and coronary artery remodeling index in culprit lesions are significantly greater in patients with no-reflow after PCI compared with patients with normal reflow However, the percentage of the fibrous plaque was significantly smaller in the patients with no-reflow. The no-reflow phenomenon has been recognized as an uncommon complication after reperfusion therapy (mechanical or thrombolytic) for acute MI and after PCI. It is a complex phenomenon and is caused by the variable combination of four pathogenetic components: distal atherothrombotic embolization, ischemic injury, reperfusion injury, and susceptibility of coronary microcirculation to injury.24 As a consequence, early identification of a potent mechanism may prevent the occurrence of no-reflow.
The present study adds to the current literature confirming that coronary plaque composition of culprit/target lesions based on IVUS analysis is closely related to the development of impaired myocardial perfusion following primary angioplasty, which suggests the importance of evaluation of plaque volume and composition by IVUS prior to mechanical therapy.25 Our data support most previous observations of an association between the culprit plaque composition and subsequent no-reflow phenomenon after PCI. Tanaka and associates10 demonstrated that lesion EEM-CSA, not lumen CSA, are independent predictive factors of no-reflow after reperfusion in patients with acute MI. In addition, our study is in accordance with previous studies,9 showing larger plaque volume (high fibrofatty plaque and plaque area) in the no-reflow group. The fibrofatty plaque is also known as lipid-rich plaque, which is associated with positive vascular remodeling via matrix metalloproteinase production.26 With regard to the coronary artery remodeling index detected by IVUS in patients with ACS, previous clinical studies have shown that preintervention findings, including remodeling index, are predictable risk factors for the angiographic no-reflow phenomenon.27 In our analysis, we noted patients with the no-reflow phenomenon had a smaller percentage of fibrous component in culprit plaques. Our study is in line with previously published data.11
The present meta-analysis has several features that distinguish it from a similar meta-analysis.28,29 First, to limit bias in the selection of included studies, we used only patients with no-reflow or slow-reflow phenomenon. Second, compared with meta-analysis by Jang and associates,28 we included two additional observational studies.22,23 Third, compared with meta-analysis based on 10 studies by Ding and colleagues,29 14 studies were included in our pooled analysis. In our meta-analysis, we also analyzed IVUS measurement parameters such as EEM-CSA, lumen CSA, plaque area, plaque burden, and remodeling index, except for the absolute volume and percentage of four different plaque compositions.
Study Limitations
There are limitations to the present study. First, 14 studies included in our meta-analysis were observational studies. The potential effects of selection bias and confounding must be considered when interpreting their results. Second, some heterogeneity was observed among the included studies, which was due primarily to the design of the included trials (most were not randomized controlled trials), IVUS measurements were not usually reported uniformly in the individual studies, and the patient characteristics. Third, not all studies included in our review reported complete IVUS data concerning the no-reflow phenomenon.
Conclusions
Our pooled analysis showed that high absolute volume of fibrofatty plaque, EEM-CSA, plaque area, coronary artery remodeling index, and decreased percentage of fibrous plaque in culprit lesions are linked with the patients with no-reflow after PCI. ![]()
The authors declare no real or apparent conflicts of interest. This work was supported by China Postdoctoral Science Foundation Research Funds (Grant No: 2013M540468), The Natural Science Foundation of Jiangsu Province (Grant No: BK20141137), and Jiangsu Planned Projects for Postdoctoral Research Funds (Grant No: 1302169C). We also thank Mr. Jurgen M.R. Ligthart (senior technician, Department of Interventional Cardiology, Thoraxcenter, Erasmus University Medical Center, The Netherlands) for providing the IVUS image.
References
- Kloner RA, Ganote CE, Jennings RB. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. J Clin Invest. 1974;54:1496-1508.
- Harrison RW, Aggarwal A, Ou FS, et al; American College of Cardiology National Cardiovascular Data Registry. Incidence and outcomes of noreflow phenomenon during percutaneous coronary intervention among patients with acute myocardial infarction. Am J Cardiol. 2013;111:178-184.
- Rezkalla SH, Kloner RA. Coronary no-reflow phenomenon: from the experimental laboratory to the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2008;72:950-957.
- Ding S, Pu J, Qiao ZQ, et al. TIMI myocardial perfusion frame count: a new method to assess myocardial perfusion and its predictive value for short-term prognosis. Catheter Cardiovasc Interv. 2010;75:722-732.
- Pu J, Shan P, Ding S, et al. Gender differences in epicardial and tissue-level reperfusion in patients undergoing primary angioplasty for acute myocardial infarction. Atherosclerosis. 2011;215:203-208.
- Sato H, Iida H, Tanaka A, et al. The decrease of plaque volume during percutaneous coronary intervention has a negative impact on coronary flow in acute myocardial infarction: a major role of percutaneous coronary intervention-induced embolization. J Am Coll Cardiol. 2004;44:300-304.
- Pu J, Mintz GS, Brilakis ES, et al. In vivo characterization of coronary plaques: novel findings from comparing greyscale and virtual histology intravascular ultrasound and near-infrared spectroscopy. Eur Heart J. 2012;33:372-383.
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012.
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18:2693-2708.
- Tanaka A, Kawarabayashi T, Nishibori Y, et al. Noreflow phenomenon and lesion morphology in patients with acute myocardial infarction. Circulation. 2002;105:2148-2152.
- Watanabe T, Nanto S, Uematsu M, et al. Prediction of no-reflow phenomenon after successful percutaneous coronary intervention in patients with acute myocardial infarction: intravascular ultrasound findings. Circ J. 2003;67:667-671.
- Iijima R, Shinji H, Ikeda N, et al. Comparison of coronary arterial finding by intravascular ultrasound in patients with “transient no-reflow” versus “reflow” during percutaneous coronary intervention in acute coronary syndrome. Am J Cardiol. 2006;97:29-33.
- Katayama T, Kubo N, Takagi Y, et al. Relation of atherothrombosis burden and volume detected by intravascular ultrasound to angiographic no-reflow phenomenon during stent implantation in patients with acute myocardial infarction. Am J Cardiol. 2006;97:301-304.
- Nakamura T, Kubo N, Ako J, Momomura S. Angiographic no-reflow phenomenon and plaque characteristics by virtual histology intravascular ultrasound in patients with acute myocardial infarction. J Interv Cardiol. 2007;20:335-339.
- Bae JH, Kwon TG, Hyun DW, et al. Predictors of slow flow during primary percutaneous coronary intervention: an intravascular ultrasound-virtual histology study. Heart. 2008;94:1559-1564.
- Higashikuni Y, Tanabe K, Tanimoto S, et al. Impact of culprit plaque composition on the no-reflow phenomenon in patients with acute coronary syndrome: an intravascular ultrasound radiofrequency analysis. Circ J. 2008;72:1235-1241.
- Hong YJ, Jeong MH, Choi YH, et al. Predictors of noreflow after percutaneous coronary intervention for culprit lesion with plaque rupture in infarct-related artery in patients with acute myocardial infarction. J Cardiol. 2009;54:36-44.
- Ohshima K, Ikeda S, Watanabe K, et al. Relationship between plaque composition and no-reflow phenomenon following primary angioplasty in patients with ST-segment elevation myocardial infarction—analysis with virtual histology intravascular ultrasound. J Cardiol. 2009;54:205-213.
- Endo M, Hibi K, Shimizu T, et al. Impact of ultrasound attenuation and plaque rupture as detected by intravascular ultrasound on the incidence of noreflow phenomenon after percutaneous coronary intervention in ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2010;3:540-549.
- Ohshima K, Ikeda S, Kadota H, et al. Cavity volume of ruptured plaque is an independent predictor for angiographic no-reflow phenomenon during primary angioplasty in patients with ST-segment elevation myocardial infarction. J Cardiol. 2011;57: 36-43.
- Hong YJ, Jeong MH, Choi YH, et al. Impact of plaque components on no-reflow phenomenon after stent deployment in patients with acute coronary syndrome: a virtual histology-intravascular ultrasound analysis. Eur Heart J. 2011;32:2059-2066.
- Utsunomiya M, Hara H, Sugi K, Nakamura M. Relationship between tissue characterisations with 40 MHz intravascular ultrasound imaging and slow flow during coronary intervention. EuroIntervention. 2011;7:340-346.
- Zhao XY, Wang XF, Li L, et al. Plaque characteristics and serum pregnancy-associated plasma protein A levels predict the no-reflow phenomenon after percutaneous coronary intervention. J Int Med Res. 2013;41:307-316.
- Niccoli G, Cosentino N, Spaziani C, et al. New strategies for the management of no-reflow after primary percutaneous coronary intervention. Expert Rev Cardiovasc Ther. 2011;9:615-630.
- Pu J, Mintz GS, Biro S, et al. Insights into echoattenuated plaques, echolucent plaques, and plaques with spotty calcification: novel findings from comparisons among intravascular ultrasound, nearinfrared spectroscopy, and pathological histology in 2,294 human coronary artery segments. J Am Coll Cardiol. 2014;63:2220-2233.
- Pasterkamp G, Schoneveld AH, Hijnen DJ, et al. Atherosclerotic arterial remodeling and the localization of macrophages and matrix metalloproteases 1, 2 and 9 in the human coronary artery. Atherosclerosis. 2000;150:245-253.
- Lee SY, Mintz GS, Kim SY, et al. Attenuated plaque detected by intravascular ultrasound: clinical, angiographic, and morphologic features and postpercutaneous coronary intervention complications in patients with acute coronary syndromes. JACC Cardiovasc Interv. 2009;2:65-72.
- Jang JS, Jin HY, Seo JS, et al. Meta-analysis of plaque composition by intravascular ultrasound and its relation to distal embolization after percutaneous coronary intervention. Am J Cardiol. 2013;111: 968-972.
- Ding S, Xu L, Yang F, et al. Association between tissue characteristics of coronary plaque and distal embolization after coronary intervention in acute coronary syndrome patients: insights from a metaanalysis of virtual histology-intravascular ultrasound studies. PLoS One. 2014;9: e106583.