Novel Agents for the Prevention and Management of Hyperkalemia
Peter A. McCullough, MD, MPH, FACC, FACP, FAHA, FCCP, FNKF, FNLA,1 Maria Rosa Costanzo, MD,2 Marc Silver, MD,3 Bruce Spinowitz, MD,4 Jun Zhang, MD, MS,1 Norman E. Lepor, MD, FACC, FAHA, FSCAI5
1 Baylor University Medical Center, Baylor Heart and Vascular Institute, Baylor Jack and Jane Hamilton Heart and Vascular Hospital, Dallas, TX, and The Heart Hospital, Plano, TX; 2Advocate Heart Institute, and Edward Heart Hospital, Naperville, IL; 3Advocate Christ Medical Center, Oak Lawn, IL; 4Division of Nephrology, New York Hospital Queens, and Nephrology Associates, Flushing, NY; 5David Geffen School of Medicine at UCLA and Cedars-Sinai Medical Center, Los Angeles, CA, and Westside Medical Associates of Los Angeles, Beverly Hills, CA
Hyperkalemia is defined as serum potassium concentrations elevated above the upper limit of normal (> 5.0 mEq/L). It has become more common in cardiovascular practice due to the growing population of patients with chronic kidney disease and the broad application of drugs that modulate renal elimination of potassium by reducing production of angiotensin II (angiotensin-converting enzyme inhibitors, direct renin inhibitors, β-adrenergic receptor antagonists), blocking angiotensin II receptors (angiotensin receptor blockers), or antagonizing the action of aldosterone on mineralocorticoid receptors (mineralocorticoid receptor antagonists). The risk of hyperkalemia is a major limiting factor for the use of these disease-modifying drugs in both acute and chronic cardiorenal syndromes. Thus, agents to control the plasma concentration of potassium are needed in the multidrug treatment of cardiorenal disease, including chronic kidney disease, heart failure, and acute kidney injury. Novel oral therapies in development for both acute and extended use in the management of hyperkalemia include patiromer sorbitex calcium and sodium zirconium cyclosilicate. Important biochemical differences between these compounds result in unique product profiles and electrolyte outcomes in patients treated for hyperkalemia. This review highlights the major mechanisms of hyperkalemia and key results from randomized trials in a range of clinical scenarios in patients with, and at risk for, hyperkalemia.
[Rev Cardiovasc Med. 2015;16(2):140-155 doi: 10.3909/ricm0782]
© 2015 MedReviews®, LLC
Novel Agents for the Prevention and Management of Hyperkalemia
Peter A. McCullough, MD, MPH, FACC, FACP, FAHA, FCCP, FNKF, FNLA,1 Maria Rosa Costanzo, MD,2 Marc Silver, MD,3 Bruce Spinowitz, MD,4 Jun Zhang, MD, MS,1 Norman E. Lepor, MD, FACC, FAHA, FSCAI5
1 Baylor University Medical Center, Baylor Heart and Vascular Institute, Baylor Jack and Jane Hamilton Heart and Vascular Hospital, Dallas, TX, and The Heart Hospital, Plano, TX; 2Advocate Heart Institute, and Edward Heart Hospital, Naperville, IL; 3Advocate Christ Medical Center, Oak Lawn, IL; 4Division of Nephrology, New York Hospital Queens, and Nephrology Associates, Flushing, NY; 5David Geffen School of Medicine at UCLA and Cedars-Sinai Medical Center, Los Angeles, CA, and Westside Medical Associates of Los Angeles, Beverly Hills, CA
Hyperkalemia is defined as serum potassium concentrations elevated above the upper limit of normal (> 5.0 mEq/L). It has become more common in cardiovascular practice due to the growing population of patients with chronic kidney disease and the broad application of drugs that modulate renal elimination of potassium by reducing production of angiotensin II (angiotensin-converting enzyme inhibitors, direct renin inhibitors, β-adrenergic receptor antagonists), blocking angiotensin II receptors (angiotensin receptor blockers), or antagonizing the action of aldosterone on mineralocorticoid receptors (mineralocorticoid receptor antagonists). The risk of hyperkalemia is a major limiting factor for the use of these disease-modifying drugs in both acute and chronic cardiorenal syndromes. Thus, agents to control the plasma concentration of potassium are needed in the multidrug treatment of cardiorenal disease, including chronic kidney disease, heart failure, and acute kidney injury. Novel oral therapies in development for both acute and extended use in the management of hyperkalemia include patiromer sorbitex calcium and sodium zirconium cyclosilicate. Important biochemical differences between these compounds result in unique product profiles and electrolyte outcomes in patients treated for hyperkalemia. This review highlights the major mechanisms of hyperkalemia and key results from randomized trials in a range of clinical scenarios in patients with, and at risk for, hyperkalemia.
[Rev Cardiovasc Med. 2015;16(2):140-155 doi: 10.3909/ricm0782]
© 2015 MedReviews®, LLC
Novel Agents for the Prevention and Management of Hyperkalemia
Peter A. McCullough, MD, MPH, FACC, FACP, FAHA, FCCP, FNKF, FNLA,1 Maria Rosa Costanzo, MD,2 Marc Silver, MD,3 Bruce Spinowitz, MD,4 Jun Zhang, MD, MS,1 Norman E. Lepor, MD, FACC, FAHA, FSCAI5
1 Baylor University Medical Center, Baylor Heart and Vascular Institute, Baylor Jack and Jane Hamilton Heart and Vascular Hospital, Dallas, TX, and The Heart Hospital, Plano, TX; 2Advocate Heart Institute, and Edward Heart Hospital, Naperville, IL; 3Advocate Christ Medical Center, Oak Lawn, IL; 4Division of Nephrology, New York Hospital Queens, and Nephrology Associates, Flushing, NY; 5David Geffen School of Medicine at UCLA and Cedars-Sinai Medical Center, Los Angeles, CA, and Westside Medical Associates of Los Angeles, Beverly Hills, CA
Hyperkalemia is defined as serum potassium concentrations elevated above the upper limit of normal (> 5.0 mEq/L). It has become more common in cardiovascular practice due to the growing population of patients with chronic kidney disease and the broad application of drugs that modulate renal elimination of potassium by reducing production of angiotensin II (angiotensin-converting enzyme inhibitors, direct renin inhibitors, β-adrenergic receptor antagonists), blocking angiotensin II receptors (angiotensin receptor blockers), or antagonizing the action of aldosterone on mineralocorticoid receptors (mineralocorticoid receptor antagonists). The risk of hyperkalemia is a major limiting factor for the use of these disease-modifying drugs in both acute and chronic cardiorenal syndromes. Thus, agents to control the plasma concentration of potassium are needed in the multidrug treatment of cardiorenal disease, including chronic kidney disease, heart failure, and acute kidney injury. Novel oral therapies in development for both acute and extended use in the management of hyperkalemia include patiromer sorbitex calcium and sodium zirconium cyclosilicate. Important biochemical differences between these compounds result in unique product profiles and electrolyte outcomes in patients treated for hyperkalemia. This review highlights the major mechanisms of hyperkalemia and key results from randomized trials in a range of clinical scenarios in patients with, and at risk for, hyperkalemia.
[Rev Cardiovasc Med. 2015;16(2):140-155 doi: 10.3909/ricm0782]
© 2015 MedReviews®, LLC
KEY WORDS
Potassium • Hyperkalemia • Patiromer sorbitex calcium • Sodium zirconium cyclosilicate • Polymer Ion trap • Chronic kidney disease • Heart failure • Renin-angiotensin system • Aldosterone
KEY WORDS
Potassium • Hyperkalemia • Patiromer sorbitex calcium • Sodium zirconium cyclosilicate • Polymer Ion trap • Chronic kidney disease • Heart failure • Renin-angiotensin system • Aldosterone
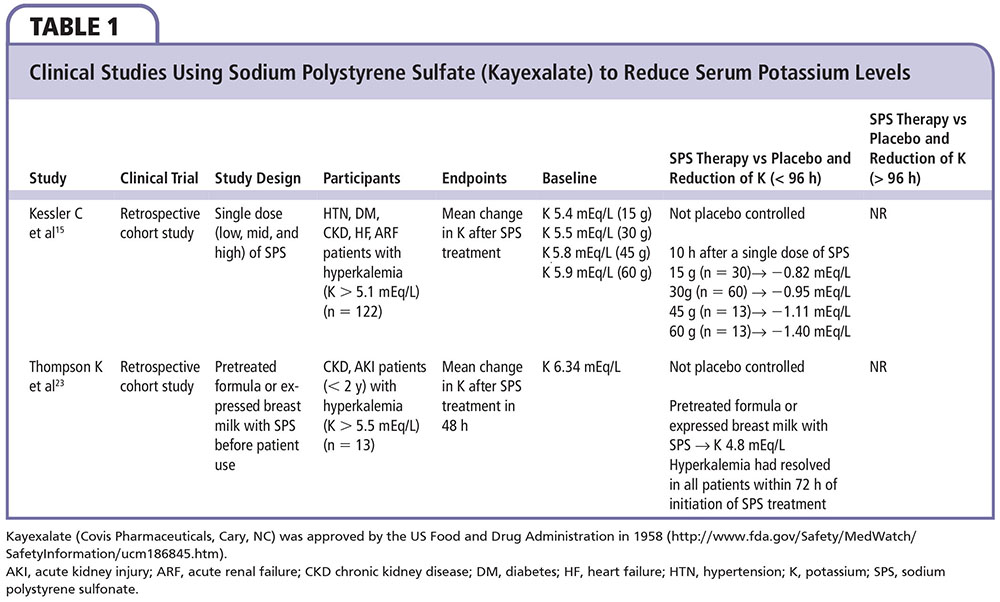
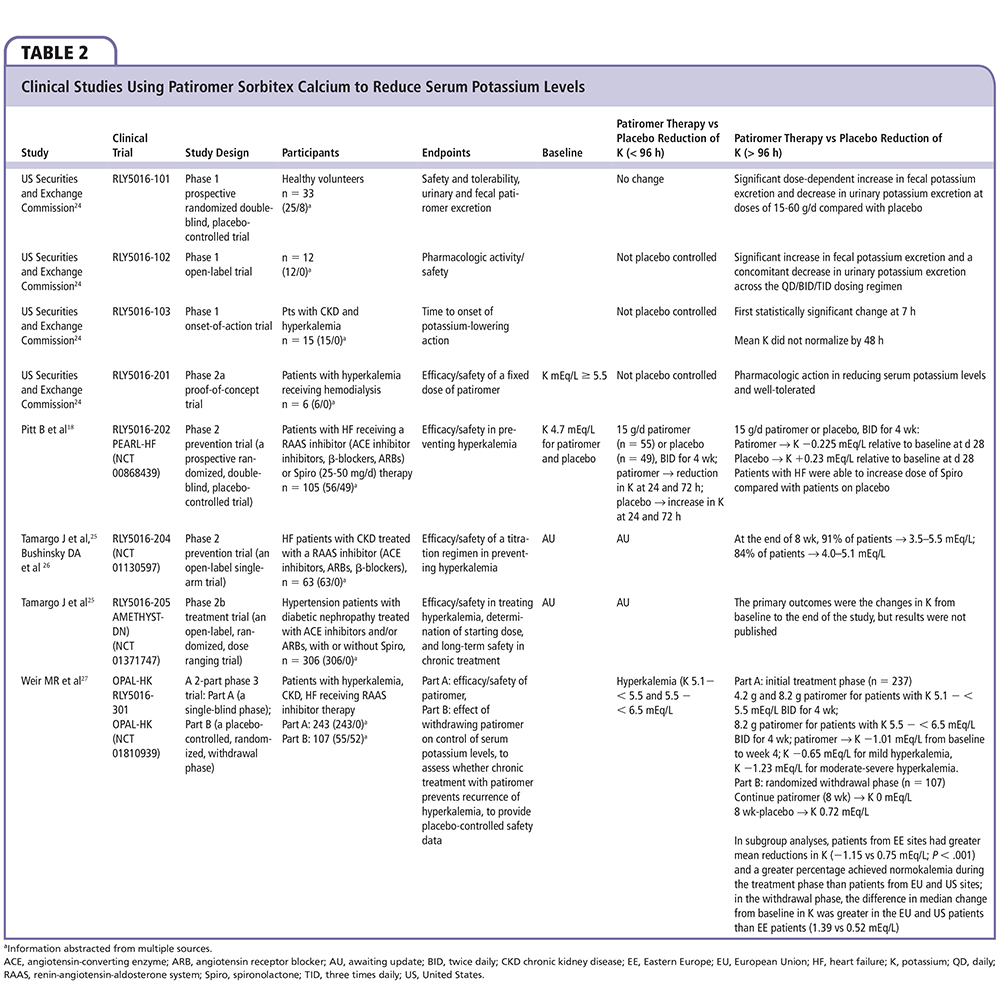
Figure 1. Mean reductions in serum potassium in stable hyperkalemic patients initially randomized to patiromer calcium, 4.2 g by mouth twice daily or 8.4 g by mouth twice daily depending on the initial potassium value. bid, twice daily; po, by mouth. Adapted from Weir MR et al.27
Figure 2. Time to the first occurrence of hyperkalemia in those subjects who achieved normokalemia with patiromer sorbitex calcium and then were randomized to either continue the agent or be assigned placebo. Adapted from Weir MR et al.27
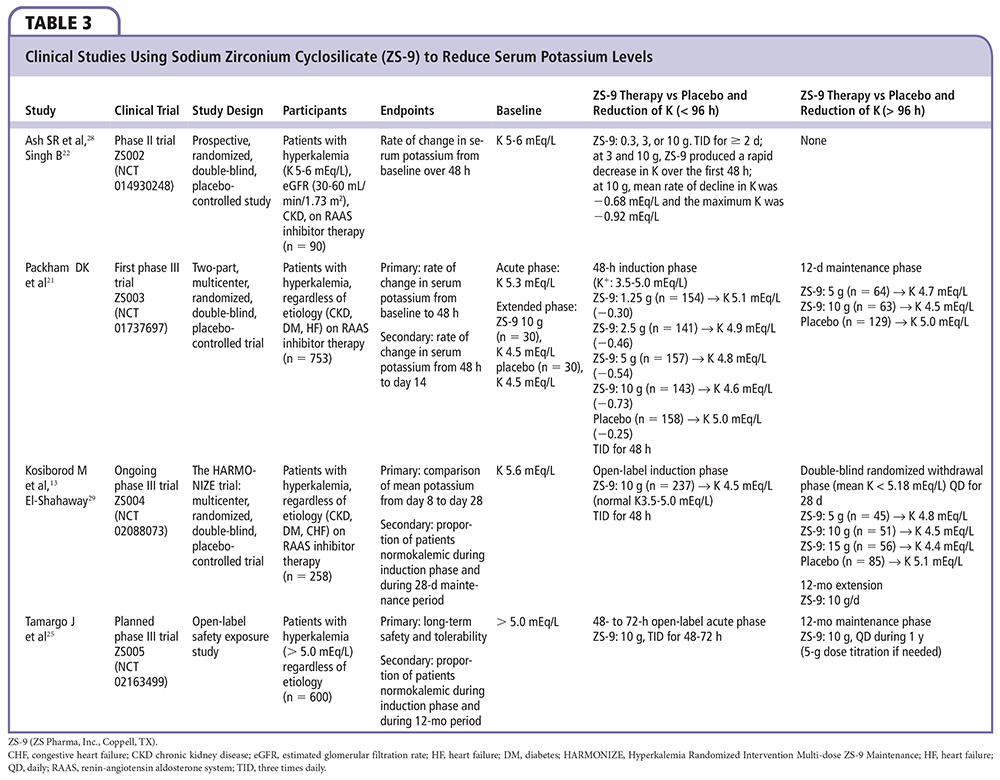
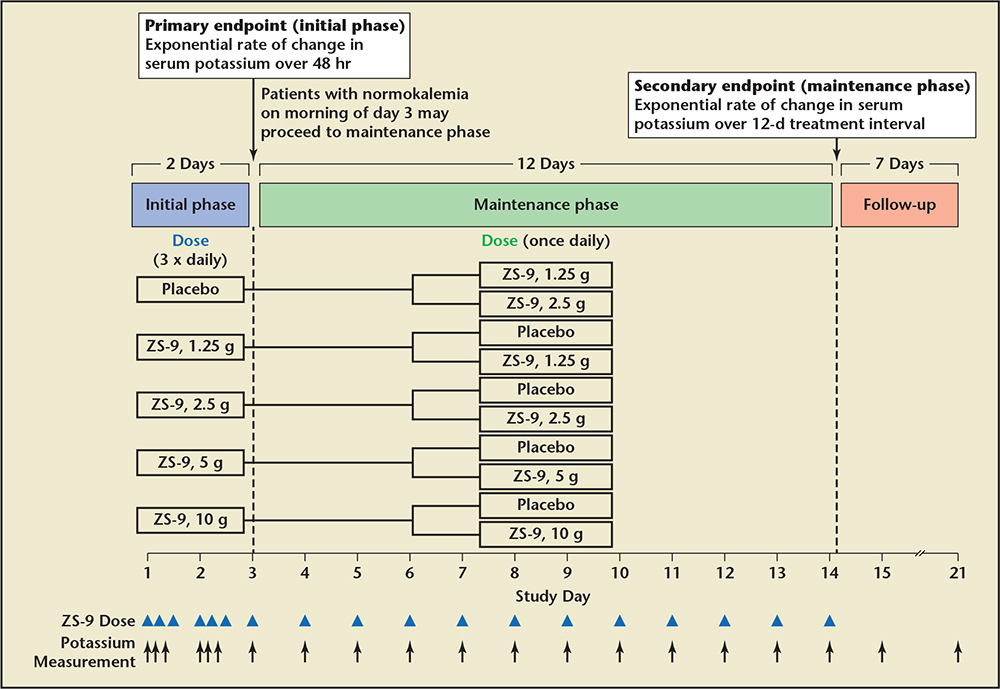
Figure 3. Study design for Packham DK et al.21 Patients whose serum potassium level decreased to 3.5 to 4.9 mEq/L at 48 hours during the initial phase of the study were randomly assigned to receive either their original sodium zirconium cyclosilicate dose or placebo once daily before breakfast on days 3 to 15 (maintenance phase). Patients assigned to the placebo group in the initial phase were randomly assigned to receive either 1.25 g or 2.5 g of sodium zirconium cyclosilicate in the maintenance phase. ZS-9 (sodium zirconium cyclosilicate; ZS Pharma, Inc., Coppell, TX).
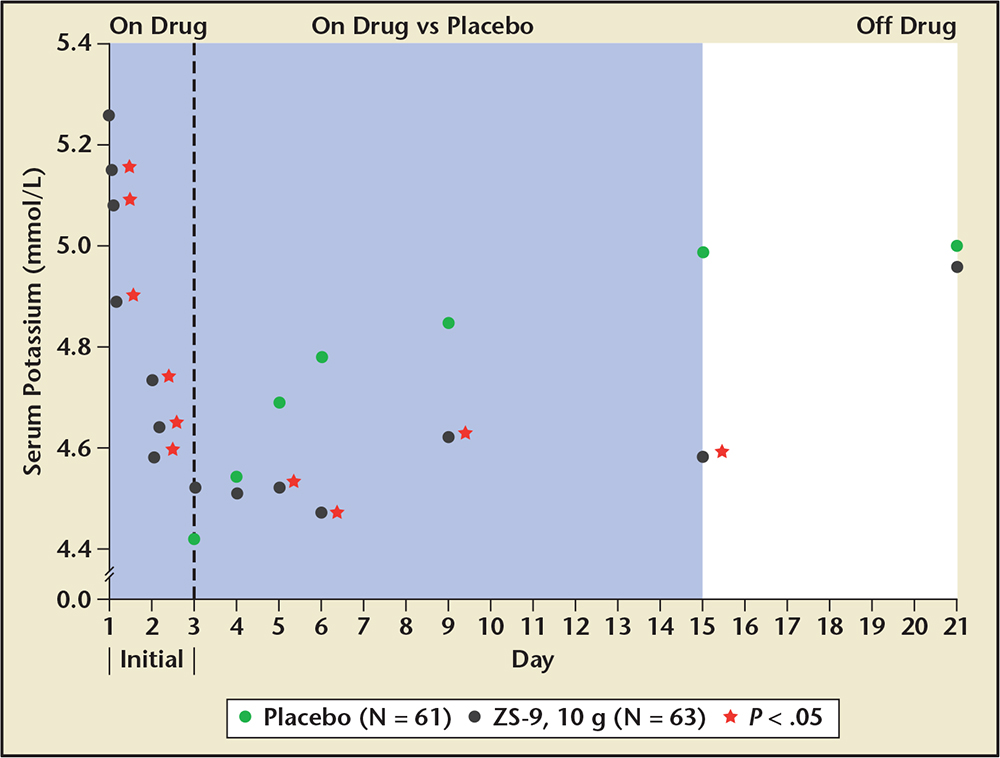
Figure 4. Extended use sodium zirconium cyclosilicate (ZS-9), 10 g three times daily by mouth versus placebo in those who initially achieved normokalemia and were followed for 21 days. ZS-9 (ZS Pharma, Inc., Coppell, TX). Adapted from Packham DK et al.21
Figure 5. Acute use of sodium zirconium cyclosilicate (ZS-9) and placebo with resultant potassium concentrations. ZS-9 (ZS Pharma, Inc., Coppell, TX). Adapted from Packham DK et al.21
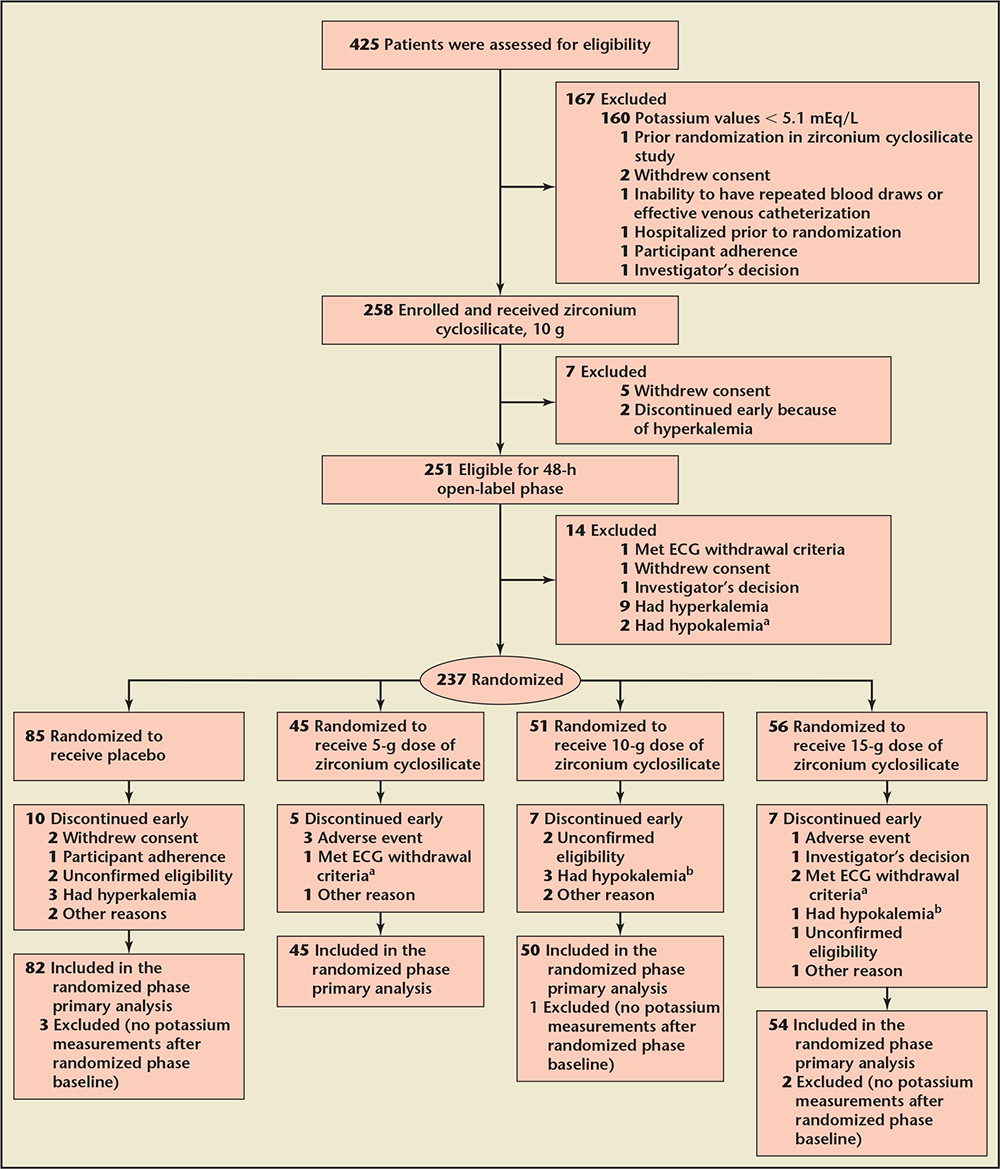
Figure 6. Design of the HARMONIZE trial, which was a phase 3, randomized, double-blind, placebo-controlled trial that enrolled ambulatory patients with a potassium level of 5.1 mEq/L or greater in an ambulatory setting. aElectrocardiogram (ECG) withdrawal criteria included significant increase in PR interval (. 250 ms in the absence of preexisting atrioventricular block), or widening of the QRS complex (. 140 ms in the absence of preexisting bundle branch block) or peaked T-wave or an increase in QTc interval . 25 ms to more than 500 ms or . 25 ms in somebody with a baseline QTc of . 500 ms. bAccording to the study protocol, all clinical decisions to withdraw patients because of a potassium level , 3.0 mEq/L were based on i-STAT values; however, in all of these cases, subsequent central laboratory values for serum potassium were $ 3.0 mEq/L. ECG, electrocardiographic; HARMONIZE, Hyperkalemia Randomized Intervention Multidose ZS-9 Maintenance. Reproduced with permission from Kosiborod M et al.13
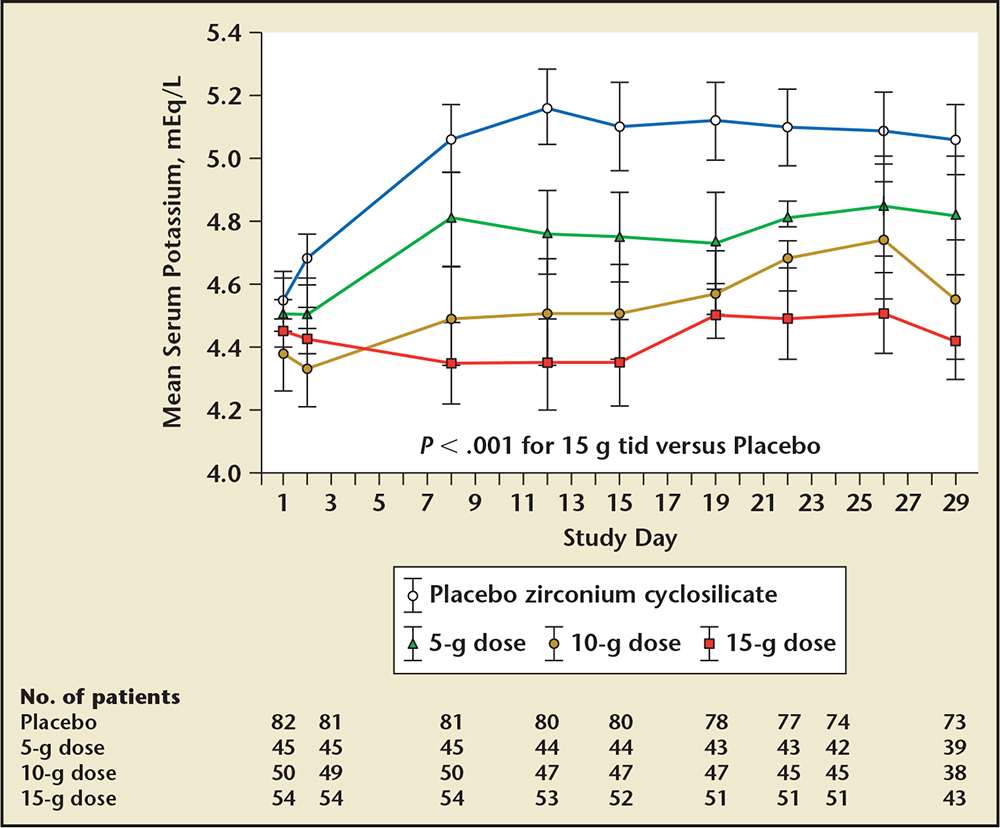
Figure 7. Results from the HARMONIZE trial with serial potassium concentrations over time. HARMONIZE, Hyperkalemia Randomized Intervention Multidose ZS 9 Maintenance; tid, three times daily. Adapted from Kosiborod M et al.13
Main Points
• Hyperkalemia, defined as serum potassium concentrations elevated above the upper limit of normal (> 5.0 mEq/L), has become more common in cardiovascular practice due to the growing population of patients with chronic kidney disease and the broad application of drugs that modulate renal elimination of potassium.
• The risk of hyperkalemia is a major limiting factor for the use of these disease-modifying drugs in both acute and chronic cardiorenal syndromes; therefore, agents to control the plasma concentration of potassium are needed in the multidrug treatment of cardiorenal disease, including chronic kidney disease, heart failure, and acute kidney injury.
• Patiromer sorbitex calcium (RLY5016; Relypsa Inc., Redwood City, CA) is currently under development as a novel potassium exchange resin formulated as a dry, odorless powder for suspension in water. Patiromer is insoluble in typical solvents, passes through the gastrointestinal tract without degradation, and has its principal site of action in the colon approximately 7 hours after ingestion. It is being developed as a chronic therapy to treat hyperkalemia.
• Sodium zirconium cyclosilicate (ZS-9; ZS Pharma, Inc., Coppell, TX), is currently under development as a treatment for acute and long-term chronic hyperkalemia. ZS-9 is a selective potassium ion trap that is engineered to have a highly selective, high-capacity crystalline lattice structure to preferentially entrap monovalent cations (specifically excess potassium ions) over divalent cations. ZS-9 also appears to bind ammonium, resulting in net acid loss, systemic reduction in blood urea nitrogen, and elevation in plasma bicarbonate.
Main Points
• Hyperkalemia, defined as serum potassium concentrations elevated above the upper limit of normal (> 5.0 mEq/L), has become more common in cardiovascular practice due to the growing population of patients with chronic kidney disease and the broad application of drugs that modulate renal elimination of potassium.
• The risk of hyperkalemia is a major limiting factor for the use of these disease-modifying drugs in both acute and chronic cardiorenal syndromes; therefore, agents to control the plasma concentration of potassium are needed in the multidrug treatment of cardiorenal disease, including chronic kidney disease, heart failure, and acute kidney injury.
• Patiromer sorbitex calcium (RLY5016; Relypsa Inc., Redwood City, CA) is currently under development as a novel potassium exchange resin formulated as a dry, odorless powder for suspension in water. Patiromer is insoluble in typical solvents, passes through the gastrointestinal tract without degradation, and has its principal site of action in the colon approximately 7 hours after ingestion. It is being developed as a chronic therapy to treat hyperkalemia.
• Sodium zirconium cyclosilicate (ZS-9; ZS Pharma, Inc., Coppell, TX), is currently under development as a treatment for acute and long-term chronic hyperkalemia. ZS-9 is a selective potassium ion trap that is engineered to have a highly selective, high-capacity crystalline lattice structure to preferentially entrap monovalent cations (specifically excess potassium ions) over divalent cations. ZS-9 also appears to bind ammonium, resulting in net acid loss, systemic reduction in blood urea nitrogen, and elevation in plasma bicarbonate.
Potassium is the most abundant cation in the human body; 98% is intracellular (140 mEq/L) and 2% is extracellular (3.8-5.0 mEq/L).1 The pathophysiology of the hyperkalemic states is multifaceted and involves dietary and supplemental intake, neurohumoral systems, acid-base balance, and— most importantly—function of the principal cells in the collecting duct of the kidney2 This review focuses on patients with hyperkalemia, particularly predialysis chronic kidney disease (CKD) patients with either acute or chronic cardiorenal disease (most commonly chronic heart failure [HF] combined with CKD), in whom disease-modifying drugs result in elevations of potassium concentration.3
There are several key mechanisms underlying the development of hyperkalemia. Any known cause of a reduction in the secretion of renin can begin a cascade of biochemical events worsened by direct renin inhibitors, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin II receptor blockers (ARBs), leading to less angiotensin II stimulation of the zona glomerulosa cells within the adrenal glands, and reduced production and circulation of aldosterone.2Mineralocorticoid receptor antagonists (MRAs) are powerful inhibitors of aldosterone-mediated potassium excretion in the distal nephron. The principal cell in the collecting duct is the major regulator of urinary potassium excretion; the epithelial sodium channel (ENaC) located at its luminal surface recovers sodium from the urine and, under normal conditions, leads to the lumen-negative potential essential for potassium and proton secretion.4 Distal sodium delivery is a critical determinant of potassium elimination; thus, decreases in sodium delivery as a result of a reduced nephron mass limits potassium secretion and creates the potential for hyperkalemia. As a major regulator of potassium balance, aldosterone stimulates the intracellular mineralocorticoid receptor (MR), resulting in ENaC-mediated signal transduction for greater sodium reabsorption and more potassium excretion into the urine via renal outer medullary potassium channels.5 Therefore, in patients with CKD, reducing aldosterone activity on the MR by any means can result in a failure to excrete potassium, leading to hyperkalemia. Patients with diabetes and type 4 distal renal tubular acidosis are a notable high-risk subgroup, because aldosterone deficiency or resistance impairing the secretion of hydrogen and potassium ions results in systemic hyperchloremic metabolic acidosis with hyperkalemia.6 The principal cells in the collecting duct sense low sodium delivery and are at high risk for the development of severe hyperkalemia after initiation of ACE inhibitors, ARBs, or MRAs.1 As a general heuristic, starting at an estimated glomerular filtration rate (eGFR) < 45 mL/min/1.73 m2 and potassium > 4.5 mEq/L, hyperkalemia increases in frequency and severity after initiation of renin-angiotensin-aldosterone system (RAAS) blockade with lower levels of renal filtration and higher baseline serum potassium concentrations.7
Clinical Consequences
The outcomes of patients who have periodic or persistent hyperkalemia are consistently poor across the care continuum. In a study from An and colleagues,8 1803 of 282,832 (0.6%) hospitalizations were associated with hyperkalemia. A total of 923 nonhospice cases with complete data were analyzed (mean age 61 years, 41% with diabetes, 70% with CKD, 17% on dialysis). In those patients hospitalized for or with acute severe hyperkalemia (potassium ≥ 6.5 mEq/L), 40% had hyperkalemia upon presentation and 60% developed it sometime during the hospitalization, with 70% displaying typical electrocardiographic changes, including peaked T-waves, QRS widening, and loss of atrial P-waves. The most common presenting symptom was cardiac arrest with asystole or sinus arrest, followed by other arrhythmias, and skeletal muscle weakness. A total of 22% of patients had new-onset acute kidney injury (AKI), whereas 52% had AKI plus CKD. As a part of resuscitation, 24% had hyperkalemia-causing drugs discontinuation and 27% underwent some form of renal replacement therapy. Severe hyperkalemia improved in 715 patients (77.5%), and a total of 283 patients (30.7%) died. Infection, volume depletion, and bleeding were significantly associated with a higher case fatality rate. Furthermore, the development of AKI in patients with normal baseline renal function was a predictor of increased mortality (odds ratio [OR] 5.23; 95% confidence interval [CI], 3.75-7.30; P < .001). In contrast, the mortality rate was lower in patients with AKI plus CKD (OR 0.53; 95% CI, 0.40-0.70; P < .001), suggesting CKD-related adaptation to chronically higher levels of potassium may have been protective. In contrast, Jain and colleagues7 found that individually, CKD stage and hyperkalemia were independently associated with mortality among a large cohort (N = 15,803) treated with cardiovascular drugs.7
Einhorn and colleagues9 analyzed 66,259 hyperkalemic (potassium ≥ 5.5 mEq/L) events (3.2% of a representative sample of hospitalization events) from a Veterans Administration cohort and found that 47% were detected on outpatient encounters. As expected, ACE inhibitors, ARBs, and MRAs were associated with hyperkalemia. The 1-day risk of a hyperkalemic event was higher in patients with CKD than in those without; however, the risk of death was higher in those without pre-existing CKD as compared with those with CKD, which was consistent with the findings of An and colleagues.8
McMahon and coworkers10 studied 39,705 adult patients (mean age 63 years, 16% with AKI) who were hospitalized in the intensive care unit over a 10-year period. Higher admission potassium values were associated with AKI and end-stage renal disease, but otherwise occurred in a broad spectrum of patients. The highest potassium concentration on the day of critical care initiation was an independent predictor of 30-day death in a graded manner compared with the referent group with potassium levels between 4.0 and 4.5 mEq/L: potassium = 4.5 to 5.0 mEq/L (OR 1.25; 95% CI, 1.16-1.35; P < .0001); potassium = 5.0 to 5.5 mEq/L (OR 1.42; 95% CI, 1.29-1.56; P < .0001); potassium = 5.5 to 6.0 mEq/L (OR 1.67; 95% CI, 1.47-1.89; P < .0001); potassium = 6.0 to 6.5 mEq/L (OR 1.63; 95% CI, 1.36-1.95; P < .0001); and potassium > 6.5 mEq/L (OR 1.72; 95% CI, 1.49-1.99; P < .0001). Interestingly, in patients whose potassium concentration declined ≥ 1 mEq/L after 48 hours in the intensive care unit, the association between hyperkalemia and mortality was no longer statistically significant, suggesting either treatment of hyperkalemia or natural resolution was favorable with regard to mortality. This was likely due to a combination of effects, including discontinuing drugs that may have played a role, treatment for hyperkalemia, recovery of renal function, or overall concomitant improvement of multiorgan system function.
Sodium Polystyrene Sulfonate
Potassium monitoring is the cornerstone of management and is associated with lower rates of hyperkalemia among patients who are at risk.1,11 For both acute and chronic therapy, sodium polystyrene sulfonate and calcium polystyrene sulphonate (available in Europe) are adjunctive therapies for severe hyperkalemia. Sodium polystyrene sulfonate, an organic enteral potassium-sodium exchange resin, nonselectively binds potassium and other cations (especially divalent cations such as calcium and magnesium). Sodium polystyrene sulfonate was approved by the US Food and Drug administration (FDA) in 1958 with very little clinical data, 4 years before the Kefauver-Harris amendment, which required new therapies to have proven efficacy.1,12 Sodium polystyrene sulfonate is commonly given with sorbitol, which often results in diarrhea.12 Diarrhea lowers potassium via colonic epithelial cells that upregulate their ability to facilitate luminal losses of potassium in CKD; however, it is an uncomfortable and potentially dangerous side effect for hospitalized patients who are at risk for volume shifts. In 2006, the FDA advised against the use of a 70% sorbitol solution, given concerns regarding bowel injury, primarily with retention enemas; in 2009 the agency further recommended that sorbitol not be added to sodium polystyrene sulfonate, thus making a pharmacy-mixed solution the de facto hospital formulary item.14 Today, approximately 5 million doses are given per year, most commonly in a formulation of 15 g of sodium polystyrene sulfonate, which is either given in 20 g of sorbitol (33% sorbitol) despite the FDA warning mentioned above, or mixed with water or syrup, and usually administered in 15- to 30-g doses. In a single-center observational study, Kessler and coworkers15 found a range of sodium polystyrene sulfonate doses were associated with a graded decrease in the potassium concentration (Table 1).15 As noted by Watson and colleagues,16 sodium polystyrene sulfonate is associated with frequent adverse effects and carries the risk of acute bowel necrosis as both an oral solution and as a retention enema, particularly in critically ill and postsurgical patients.16 In addition, hypernatremia has been reported as a response to excessive (∼ 240 g) short-term use.17 Thus, sodium polystyrene sulfonate is infrequently prescribed as an outpatient, chronic oral therapy by internists and cardiologists because of diarrhea and concerns about its unknown efficacy and poor tolerability
Patiromer Sorbitex Calcium
Patiromer sorbitex calcium (patiromer; RLY5016; Relypsa Inc., Redwood City, CA) is a novel potassium exchange polymer formulated as a dry, odorless powder for suspension in water. The powder consists of spherical beads with an average diameter of 100 μm, which results in a lower viscosity than polymeric drugs made in bulk and ground into a powder (eg, sodium polystyrene sulfonate). The beads contain sorbitol, as well as calcium; sorbitol accounts for approximately 29% of the weight of patiromer, whereas calcium accounts for approximately 11% (2 g of sorbitol + 0.8 g of calcium for every 4.2 g of patiromer). Patiromer is insoluble in typical solvents, passes through the gastrointestinal tract without degradation, and its principal site of action is in the colon approximately 7 hours after ingestion. It is being developed as a chronic therapy to treat hyperkalemia.
Clinical trials with patiromer sorbitex calcium are detailed in Table 2. The Polymeric Potassium Binder, in a Double-blind, Placebo-controlled Study in Patients with Chronic Heart Failure (PEARL-HF) included 105 patients with HF and a history of hyperkalemia resulting in discontinuation of ACE inhibitors, ARBs, or β-adrenergic receptor antagonists, or CKD confirmed by an eGFR of < 60 mL/min/1.73 m2, who were randomized to double-blind treatment with 30 g/d RLY5016 or placebo for 4 weeks.18 Eligible patients were required to have a serum potassium concentration between 4.3 and 5.1 mEq/L at screening. Spironolactone, initiated at 25 mg/d, was increased to 50 mg/d on day 15 if potassium was ≤ 5.1 mEq/L. Compared with placebo, RLY5016 had significantly lower serum potassium levels (−0.45 mEq/L; P < .001), lower rates of hyperkalemia, defined as potassium > 5.5 mEq/L (7.3% RLY5016 vs 24.5% placebo; P = .015), and a higher proportion of patients successfully received spironolactone, 50 mg/d (91% RLY5016 vs 74% placebo; P = .019) at the end of treatment.19 The most common adverse events were gastrointestinal disorders (flatulence, diarrhea, constipation, and vomiting), which were reported with higher frequency in the RLY5016 group (21% vs 6%, respectively). Magnesium also binds to patiromer, resulting in greater rates of hypomagnesemia, defined as magnesium < 1.8 mg/dL during the treatment period (24% RLY5016 vs 2.1% placebo). Patients who developed hypomagnesemia did not appear to have higher rates of muscle cramping, paresthesias, or other related symptoms.
In a Two-Part, Single-Blind, Phase 3 Study Evaluating the Efficacy and Safety of Patiromer for the Treatment of Hyperkalemia (OPAL-HK), patients with stage 3 or 4 CKD (eGFR 15-59 mL/min/1.73 m2) who had serum potassium concentrations of 5.1 to 6.4 mEq/L at two screenings were eligible for the trial.20 Patients were required to have received stable doses of RAAS blockers for the prior 28 days, and had to be stable clinic patients without acute cardiac or renal events requiring hospitalization within the previous 3 months. In the first part of the trial, 92 subjects with potassium concentrations 5.1 to 5.4 mEq/L received oral patiromer polymer 4.2 g twice daily titrated up to an average daily dose of 12.8 g, and 151 patients with baseline potassium concentrations 5.5 to 6.4 mEq/L received double the dose, or 8.4 g oral patiromer polymer (contains sorbitol, 4 g/dose) twice daily titrated up to an average daily dose of 21.4 g for 4 weeks. The maximum dose of patiromer polymer allowed was 50.4 g/d (12 4.2-g packets). Each 4.2 g of patiromer polymer is complexed with an additional 0.8 g of calcium and 2 g of sorbitol, for approximately 7 g of material per 4.2 g packet; thus, the average daily maintenance dose of 21.4 g of patiromer polymer equates to approximately 35 g of material. Patiromer was given to all subjects with breakfast and dinner in a 40- to 120-mL water suspension per dosage. Both groups had a reduction in serum potassium concentrations (Figure 1) with the overall mean change from baseline to week 4 (primary outcome) of −1.01 ± 0.03 mEq/L (95% CI, −1.07 to −0.95; P < .001), and 76% of patients achieved the target potassium range (3.8-5.0 mEq/L). Only patients with higher baseline potassium (5.5-6.4 mEq/L; n = 151) were eligible to continue into the randomized maintenance phase. Of patients who completed the 4-week initial phase and achieved a serum potassium level of 3.8 to 5.0 mEq/L, 107 were randomized to either continuing their stable dose of patiromer or to placebo. Early withdrawal from the randomized phase occurred in 10 (18%) and 22 (42%) patients in the patiromer and placebo groups, respectively. The most common reason for discontinuation was hyperkalemia. Follow-up potassium concentrations were assessed at 3 and 7 days, and, if needed, at 14 days; the time to the first hyperkalemic event (potassium > 5.5 mEq/L) is shown in Figure 2. The median change in the potassium concentration from the start of the randomized withdrawal phase-out to 4 weeks was + 0.72 mEq/L in the placebo group and 0 mEq/L in the patiromer group (average daily dose, 21.1 g), for a between-group difference of 0.72 mEq/L (95% CI, 0.46-0.99; P < .001). Mild to moderate constipation (4%), diarrhea (4%), and nausea (4%) were the most common gastrointestinal side effects in the patiromer-treated group, and occurred in none of the patients in the placebo group. Over the course of treatment with patiromer, hypokalemia (potassium < 3.5 mEq/L) and hypomagnesemia (magnesium < 1.4 mg/dL) each developed in 3% of subjects. It appears that the majority of patients in practice will require an oral dose of 8.4 g twice daily, or higher, and thus will receive approximately 8 to 12 g of sorbitol per day. The constipating effects of the polymer and sorbitol likely account for the noted differences in gastrointestinal side effects.
Sodium Zirconium Cyclosilicate
A novel agent, sodium zirconium cyclosilicate (ZS-9; ZS Pharma, Inc., Coppell, TX), is under development as a treatment for acute and long-term chronic hyperkalemia. ZS-9 (ZS-9 was the 9th out of 12 candidates that entered drug optimization for 3-dimensional structure, affinity to potassium, and balanced ratio of exchangeions) is a selective potassium ion trap that is engineered to have a high-capacity, highly selective crystalline lattice that preferentially entraps potassium cations over other cations (specifically excess potassium ions) over divalent cations (eg, calcium and magnesium). ZS-9 also appears to bind ammonium, resulting in net acid loss, systemic reduction in blood urea nitrogen, and elevation in plasma bicarbonate. ZS-9 will be available as a tasteless, odorless, insoluble, and nonabsorbed powder (given with 40-120 mL of water per dose), and potentially a tablet; it requires no special handling, refrigeration, or special preparation, and does not have to be given in solution or with cathartics such as sorbitol. The clinical trials that have tested ZS-9 are shown in Table 3.
In the Multicenter, Two-phase, Multi-dose, Prospective, Randomized, Double-blind, Placebo-Controlled Study of Safety and Efficacy of Microporous, Fractionated, Protonated Zirconium Silicate in Mild to Moderate Hyperkalemia trial (ZS-003), a total of 753 patients with potassium levels 5.0 to 6.5 mEq/L (including patients with CKD, HF, and diabetes, and those on ACE inhibitors, ARBs, or MRAs) were randomized to receive 1 of 4 doses of ZS-9 (1.25 g, 2.5 g, 5 g, or 10 g) or placebo, administered 3 times daily for the initial 48-hour acute phase (Figure 3).21 Patients with normokalemia (serum potassium 3.5-4.9 mEq/L) at 48 hours were then randomly assigned to receive either ZS-9 or placebo once daily on days 3 to 14. The primary endpoint was the rate of change in serum potassium from baseline throughout the 48-hour acute phase. ZS-9 (10 g, three times daily by mouth) rapidly reversed hyperkalemia compared with placebo (Figure 4). At 48 hours, there were absolute mean reductions of 0.46 mEq/L (95% CI, −0.53 to −0.39) in the 2.5-g group, 0.54 mEq/L (95% CI, −0.62 to −0.47) in the 5-g group, and 0.73 mEq/L (95% CI.−0.82 to −0.65) in the 10-g group, as compared with a mean reduction of 0.25 mEq/L (95% CI, −0.32 to −0.19) in the placebo group (P < .001 for all comparisons; Figure 5). The mean reduction from baseline to 1 hour after the first 10-g dose of ZS-9 was 0.11 mEq/L (95% CI, −0.17 to −0.05; P = .009) suggesting a potassium binding effect in the gastrointestinal lumen at the level of the stomach and proximal small intestine. A total of 99% of patients in the group receiving 10 g three times daily achieved normokalemia within 48 hours.22 Across the entire study group, a total of 543 of 753 (72.1%) patients were randomized in the maintenance phase (Figure 4). Figure 4 shows the rapid and significant reversal of hyperkalemia with oral ZS-9,10 g three times daily versus placebo acutely, as well as with once-daily extended use over 14 days; ZS-9 suppressed potassium concentrations to < 4.6 mEq/L whereas potassium concentrations steadily rose to > 5.0 mEq/L in the placebo group. The normalization of serum potassium with 5 g and 10 g ZS-9 doses were achieved regardless of the baseline potassium level, eGFR, concomitant RAASi usage, and history of HF, CKD, or diabetes. In the ZS-9 group, two cases of hypokalemia (serum potassium 3.1 mEq/L on the 2.5-g dose in the maintenance phase and 3.4 mEq/L on the 10-g dose in the initial phase) were reported. Both cases were transient and resolved with dose adjustment. Overall, adverse events were reported in 12.9% and 10.8% during the acute phase and 25.1% and 24.5% during the maintenance phase of ZS-9-treated and placebo patients, respectively. Adverse events did not appear to be dose dependent. There were no differences in overall or gastrointestinal adverse events between ZS-9 and placebo.
In a second multicenter trial (Hyperkalemia Randomized Intervention Multidose ZS-9 Maintenance [HARMONIZE]), 258 stable clinic patients with a potassium concentration ≥ 5.1 mEq/L at baseline received 10 g of oral ZS-9 three times daily during an initial 48-hour open-label phase. Patients achieving normokalemia (3.5-5.0 mEq/L) were then randomized to receive ZS-9, 5 g (n = 45), 10 g (n = 51), 15 g (n = 56), or placebo (n = 85) daily for 28 days (Figure 6).13Potassium was significantly reduced (−0.2 mEq/L; 95% CI, −0.3 to −0.2) 1 hour after the first 10-g dose compared with baseline (P < .001). At 2 and 4 hours after the first dose, mean change in potassium was −0.4 mEq/L (95% CI, −0.5 to −0.4) and −0.5 mEq/L (95% CI, −0.6 to −0.5), respectively (P < .001 for both time points). The absolute change in serum potassium was −0.7 mEq/L (95% CI, −0.7 to −0.6; −12%) at 24 hours and -1.1 mEq/L (95% CI, −1.1 to −1.0; −19%) at 48 hours (P < .001 for both time points). Median time to normalization was 2.2 hours. Normokalemia was achieved by 84% and 98% of patients at 24 and 48 hours, respectively. Efficacy of ZS-9 was consistent across all subgroups and all ranges of hyperkalemia, including those with ≥ 6.0 mEq/L at baseline. The primary endpoint was a comparison of mean serum potassium levels among placebo and each treatment group during days 8 through 29 of the randomized withdrawal phase. As shown in Figure 7, patients receiving 5 g, 10 g, or 15 g of ZS-9 were maintained at 4.8, 4.5, and 4.4 mEq/L versus 5.1 mEq/L among placebo patients (P < .001 all comparisons). In addition, hyperkalemia was controlled in 80%, 90%, and 94% of 5-, 10-, 15-g ZS-9-treated patients versus only 46% of placebo-treated patients (P < .001 all doses). Importantly, the potassium-lowering effects and maintenance of normokalemia occurred across all disease subtypes (CKD, HF, and diabetes) without patients being removed from RAASi therapies. Consistent with previous studies, there was no significant difference in gastrointestinal adverse events with ZS-9 as compared with placebo. Edema occurred in 2% (2/85 patients), 2% (1/45), 6% (3/51), and 14% (8/56) in the placebo, 5-, 10-, and 15-g groups, respectively; however, edema resolved or did not require treatment in all patients on 5 g and 10 g, and 3 of the 15 g patients. No increase in body weight, blood pressure, or urinary sodium excretion was observed in the ZS-9-treated patients. In long-term, 12-month studies, as well as in the larger 753 patient study, ZS-9-treated patients have not experienced edema at rates greater than placebo.
Comparative Efficacy
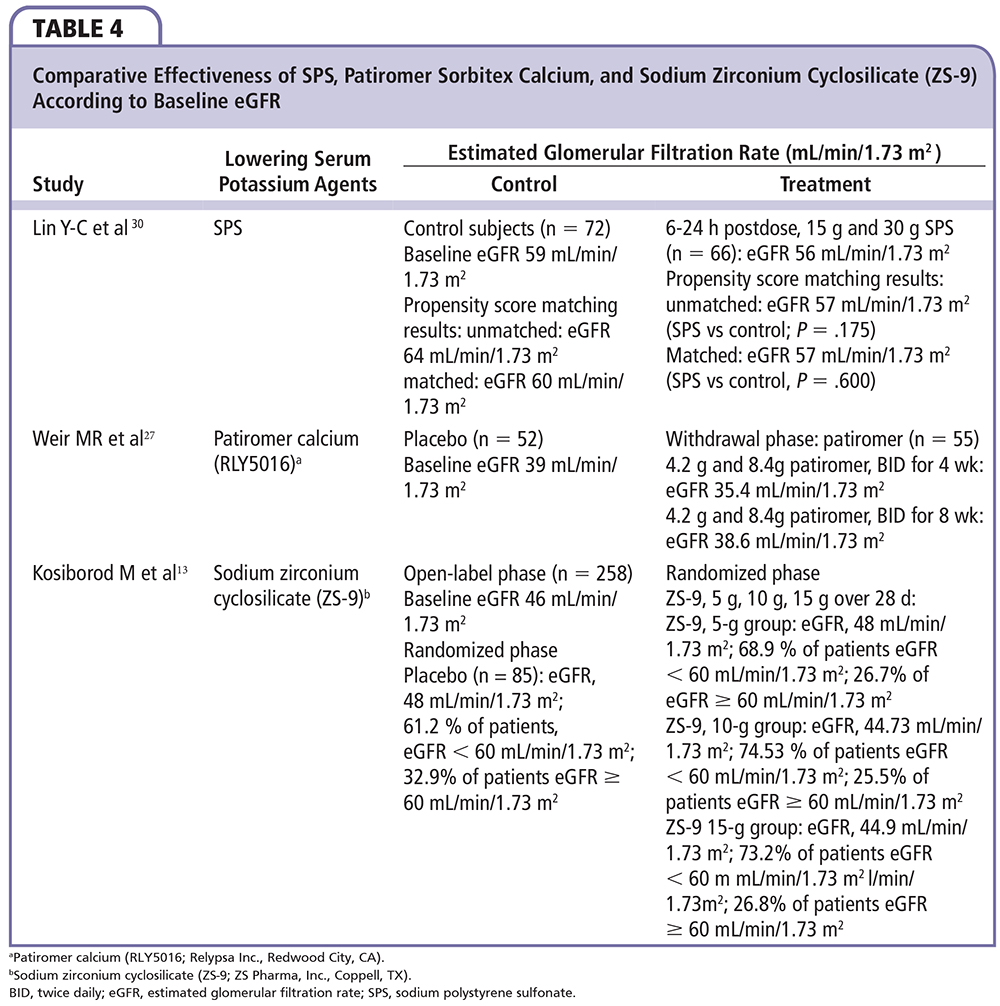
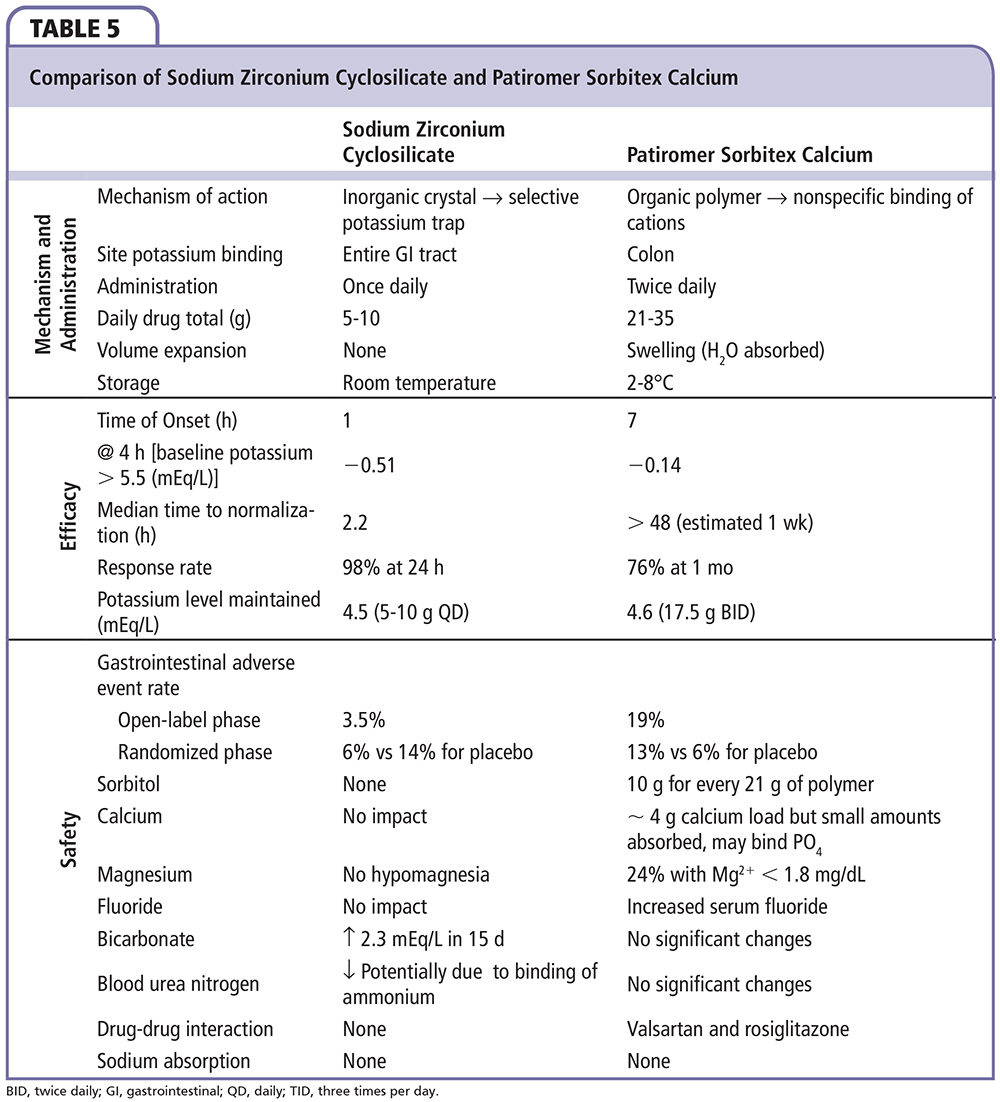
A summary of comparative efficacy according to eGFR for sodium polystyrene sulfate, patiromer sorbitex calcium, and ZS-9 is shown in Table 4. Among the two novel agents, patiromer and ZS-9, there are several notable differences, including the biochemical structures and effects in clinical testing (Table 5). Patiromer is a polymer containing sorbitol that is administered with small volumes of water (40 mL), and is associated with a higher incidence of gastrointestinal adverse effects. The amount of sorbitol is less than that typically used with sodium polystyrene sulfonate; thus, patiromer represents a safer and easier to use agent than the standard currently employed. ZS-9 appears to have an advantage of not being a polymer but an inorganic, selective cation trap (given in as little as 40 mL of water in ongoing trials). As a result, ZS-9 has not been associated with increases in gastrointestinal side effects in clinical trials, but potential association with edema is being evaluated in ongoing long-term studies.
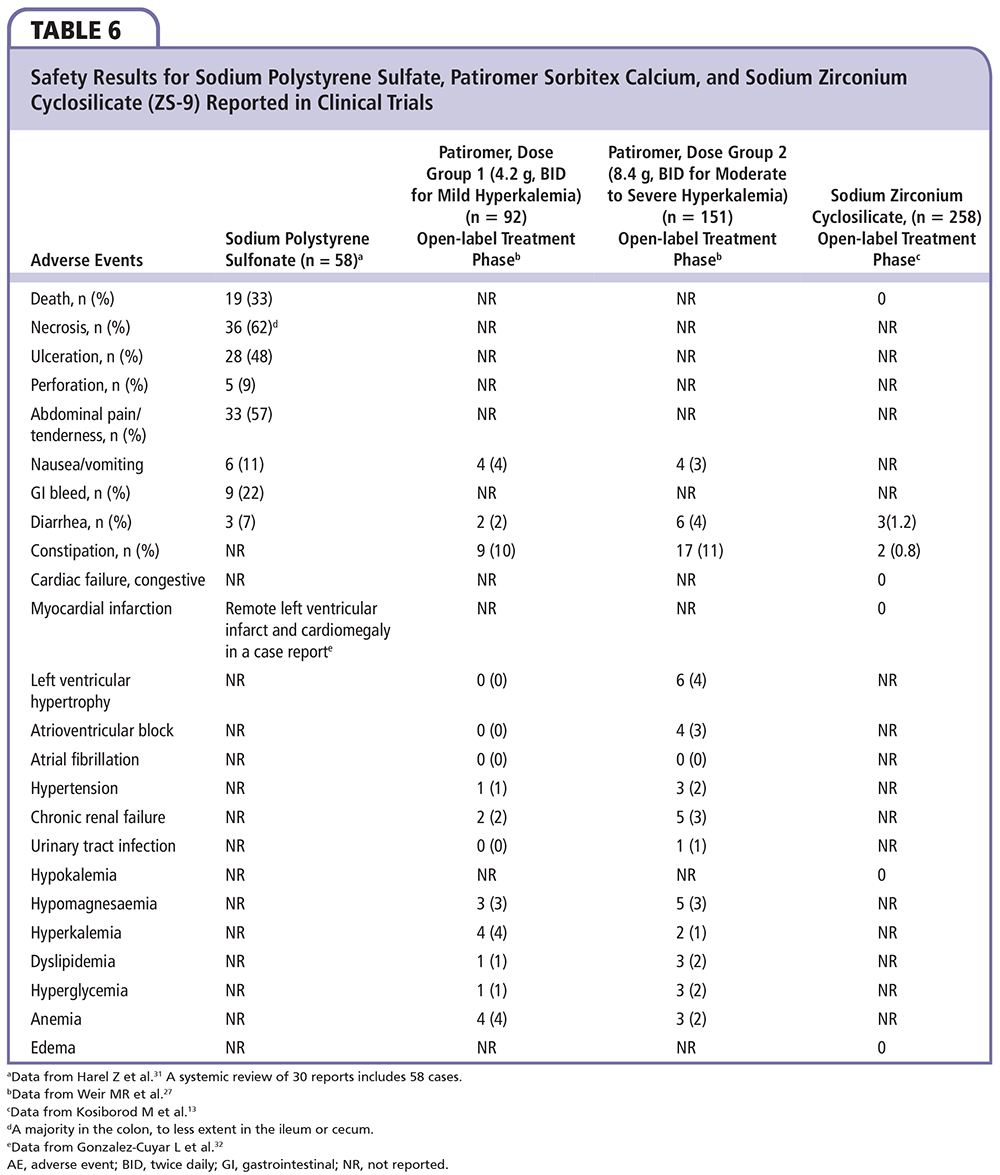
Control of hyperkalemia was good with both agents. The onset of action is much quicker (∼ 2 h) with ZS-9 as it appears to work in the proximal and distal gastrointestinal tract in contrast to patiromer (∼ 7 h), which probably has its first site of action in the colon. The median time to normalization was 2.2 hours for ZS-9 versus > 48 hours for patiromer. In addition to the rapid onset, ZS-9 appears to maintain normokalemia with a lower, more convenient dose of 5 g to 15 g once daily. Patiromer required dose titration in 62% of patients in the clinical testing, ranging from 2 to 12 packets per day given twice daily. Lastly, ZS-9 has no impact on serum magnesium or calcium, but results in a reduction in blood urea nitrogen and a small rise in bicarbonate due to the binding of ammonium in the gastrointestinal lumen. Thus, the overall electrolyte picture is favorable for patients with cardiorenal disease who have chronic azotemia and metabolic acidosis. In contrast, patiromer releases calcium and binds both potassium and magnesium, resulting in hypomagnesemia in some patients. Changes in magnesium will require correction in some individuals and monitoring in all. A summary of these and other safety events from trials of sodium polystyrene sulfate, patiromer sorbitex calcium, and ZS-9 are shown in Table 6. Both novel agents represent major advances over sodium polystyrene sulfonate and may allow the greater use of RAAS/MRA-blocking agents in patients with cardiorenal disease, representing a therapeutic breakthrough in an area in which concern for or actual hyperkalemia was previously a dose-limiting toxicity. Based on published trials, it appears that a majority of patients most likely to benefit from RAAS/MRA blockade may be able to receive these life-saving therapies with the utilization of the new potassium-controlling agents described herein.
Limitations
There are considerable limitations to the cumulative data presented thus far for agents that prevent and treat hyperkalemia. First, there are no head-to-head randomized, controlled trials of two or more agents (novel agent vs novel agent or versus sodium polystyrene sulfate). Second, the most common expected clinical scenarios, including acute emergency department treatment of hyperkalemia and use of these novel agents on hospital wards or the intensive care unit, have not been tested. Importantly, in the setting of AKI, in which a progressive rise in serum potassium is present, the effects of patiromer sorbitex calcium and ZS-9 are unknown. Third, various routes of administration, such as nasogastric tube or rectal administration for patiromer sorbitex calcium and ZS-9, have not been tested to date. Thus, if approved and marketed, the most common clinical scenarios in which hyperkalemia requires treatment have not been evaluated in clinical trials, and important inferences on safety and efficacy cannot be made at this time.
Conclusions
Both acute and chronic hyperkalemia complicate the management of CKD, HF, and AKI. Novel therapies, including the polymer patiromer sorbitex calcium and ZS-9 ion trap, are promising both as acute remedies and as adjunctive therapies for hyperkalemia, and may allow greater use of ACE inhibitors, ARBs, and MRAs in the vulnerable populations who have an especially great need for neurohormonal blockade. ![]()
References
- McCullough PA, Beaver TM, Bennett-Guerrero E, et al. Acute and chronic cardiovascular effects of hyperkalemia: new insights into prevention and clinical management. Rev Cardiovasc Med. 2014;15:11-23.
- Palmer BF. Managing hyperkalemia caused by inhibitors of the renin-angiotensin-aldosterone system. N Engl J Med. 2004;351:585-592.
- Canaud B, Tetta C, McCullough P, Chawla LS. An integrated approach for cardiorenal management. Blood Purif. 2014;37(suppl 2):1.
- Palmer BF. Regulation of potassium homeostasis [published online ahead of print May 1, 2014]. Clin J Am Soc Nephrol. doi: 10.2215/ CJN.08580813
- Bhargava A, Pearce D. Mechanisms of mineralocorticoid action: determinants of receptor specificity and actions of regulated gene products. Trends Endocrinol Metab. 2004;15:147-153.
- Karet FE. Mechanisms in hyperkalemic renal tubular acidosis. J Am Soc Nephrol. 2009;20:251-254.
- Jain N, Kotla S, Little BB, et al. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2012;109:1510-1513.
- An JN, Lee JP, Jeon HJ, et al. Severe hyperkalemia requiring hospitalization: predictors of mortality. Crit Care. 2012;16:R225.
- Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156-1162.
- McMahon GM, Mendu ML, Gibbons FK, Christopher KB. Association between hyperkalemia at critical care initiation and mortality. Intensive Care Med. 2012;38:1834-1842.
- Raebel MA, Ross C, Xu S, et al. Diabetes and drugassociated hyperkalemia: effect of potassium monitoring. J Gen Intern Med. 2010;25:326-333.
- Flinn RB, Merrill JP, Welzant WR. Treatment of the oliguric patient with a new sodium-exchange resin and sorbitol; a preliminary report. N Engl J Med. 1961;264:111-115.
- Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: the HARMONIZE randomized clinical trial. JAMA. 2014;312:2223-2233.
- Sterns RH, Rojas M, Bernstein P, Chennupati S. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol. 2010;21: 733-735.
- Kessler C, Ng J, Valdez K, et al. The use of sodium polystyrene sulfonate in the inpatient management of hyperkalemia. J Hosp Med. 2011;6:136-140.
- Watson M, Abbott KC, Yuan CM. Damned if you do, damned if you don’t: potassium binding resins in hyperkalemia. Clin J Am Soc Nephrol. 2010;5: 1723-1726.
- Nepal M, Bucaloiu ID, Norfolk ER. Hypernatremia in a patient treated with sodium polystyrene sulfonate. Int J Nephrol Renovasc Dis. 2010;3:141-143.
- Pitt B, Anker SD, Bushinsky DA, et al; PEARL-HF Investigators. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32:820-828.
- Buysse JM, Huang IZ, Pitt B. PEARL-HF: prevention of hyperkalemia in patients with heart failure using a novel polymeric potassium binder, RLY5016. Future Cardiol. 2012;8:17-28.
- Weir MR, Bakris GL, Bushinsky DA, et al; OPAL-HK Investigators. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211-221.
- Packham DK, Rasmussen HS, Lavin PT, et al. Sodium zirconium cyclosilicate in hyperkalemia. N Engl J Med. 2015;372:222-231.
- Singh B. ZS-9 for serum potassium reduction in hyperkalemic patients from a Phase 3 multicenter, randomized, double-blind, placebo-controlled trial. Presented at: National Kidney Foundation 2014 Spring Clinical Meetings; April 22-26, 2014; Las Vegas, NV.
- Thompson K, Flynn J, Okamura D, Zhou L. Pretreatment of formula or expressed breast milk with sodium polystyrene sulfonate (Kayexalate®) as a treatment for hyperkalemia in infants with acute or chronic renal insufficiency. J Renal Nutrition. 2013;23: 333-339.
- US Securities and Exchange Commission. Commission File Number 001-36184. Washington, DC; 2014. http://www.sec.gov/Archives/
edgar/data/1416792/000119312515075163/
d884519d10qa.htm. Accessed April 29, 2015. - Tamargo J, Caballero R, Delpón E. New drugs for the treatment of hyperkalemia in patients treated with renin-angiotensin-aldosterone system inhibitors - hype or hope? Discov Med. 2014;18:249-254.
- Bushinsky DA, Bakris GL, Williams G, et al. Patiromer induced a rapid onset of action and sustained K1 lowering throughout the dosing period in CKD patients with hyperkalemia. Presented at: Kidney Week 2014: American Society of Nephrology Annual Meeting; November 11-16, 2014; Philadelphia, PA. SA-PO153.
- Weir MR, Bakris GL, Bushinsky DA, et al; OPAL-HK investigators. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med. 2015;372:211-221.
- Ash SR, Singh B, Lavin P, et al. Safety and efficacy of ZS-9, a novel selective potassium trap: a phase 2 study of the treatment of hyperkalemia in patients with chronic kidney diseases [published online ahead of print 4 February 2015]. Kidney Int. doi: 10.1038/ ki.2014.382.
- El-Shahaway MA. Subgroup analysis of a phase e multicenter, randomized, double-blind, placebo control trial of patients with hyperkalemia. Late-Breaking Clinical Trials. Presented at: 18th Annual Scientific Meeting for the Heart Failure Society of America; September 14-17, 2014; Las Vegas, NV.
- Lin Y-C, Batterink J, Legal GM, et al. Efficacy of sodium polystyrene sulfonate for the treatment of hyperkalemia. http://www.vhpharmsci.com/
residency/resources/projects_2012_files/
LIN%20-%20SPS.pdf. - Harel Z, Harel S, Shan PS, et al. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systemic review. Am J Med. 2013;126:264.e9-264.e24.
- Gonzalez-Cuyar LF, Cresswell NB, Burke AP. Sodium polystyrene sulfonate (Kayexalate) aspiration. Diagn Pathol. 2008;3:27.