Management of Patients With Stable Angina and Type 2 Diabetes
Prakash C. Deedwania, MD, FACC, FAHA
University of California, San Francisco, Fresno, CA
Type 2 diabetes (T2D) is a well-established risk factor for patients with coronary artery disease (CAD). Patients with CAD and comorbid T2D also have a higher risk of cardiovascular complications, such as silent ischemia and stable angina. In treating the symptoms of stable angina in patients with CAD and comorbid T2D, it is vital to utilize therapies that reduce symptoms and improve outcomes. At the same time, there is significant concern about the preservation of glycometabolic parameters, such as glycosylated hemoglobin (HbA1c), particularly because some antianginal therapies, such as β-blockers and calcium channel blockers—although effective at improving the symptoms of stable angina and reducing ischemia—may also worsen glycemic control by increasing HbA1c levels. Available trial data on the efficacy of antianginal agents in patients with stable angina and comorbid T2D are limited. Therefore, in patients with stable angina and T2D, a tailored approach to treatment of stable angina by selecting therapies with a neutral or positive glycometabolic profile may improve outcomes and increase treatment compliance. Additionally, patients with a dual diagnosis may benefit from therapies that have beneficial effects on both stable angina and T2D, thereby reducing polypharmacy. Prospective studies in patients with stable angina and T2D are needed to guide therapy decisions.
[Rev Cardiovasc Med. 2015;16(2): 105-113 doi: 10.3909/ricm0742]
© 2015 MedReviews®, LLC
Management of Patients With Stable Angina and Type 2 Diabetes
Prakash C. Deedwania, MD, FACC, FAHA
University of California, San Francisco, Fresno, CA
Type 2 diabetes (T2D) is a well-established risk factor for patients with coronary artery disease (CAD). Patients with CAD and comorbid T2D also have a higher risk of cardiovascular complications, such as silent ischemia and stable angina. In treating the symptoms of stable angina in patients with CAD and comorbid T2D, it is vital to utilize therapies that reduce symptoms and improve outcomes. At the same time, there is significant concern about the preservation of glycometabolic parameters, such as glycosylated hemoglobin (HbA1c), particularly because some antianginal therapies, such as β-blockers and calcium channel blockers—although effective at improving the symptoms of stable angina and reducing ischemia—may also worsen glycemic control by increasing HbA1c levels. Available trial data on the efficacy of antianginal agents in patients with stable angina and comorbid T2D are limited. Therefore, in patients with stable angina and T2D, a tailored approach to treatment of stable angina by selecting therapies with a neutral or positive glycometabolic profile may improve outcomes and increase treatment compliance. Additionally, patients with a dual diagnosis may benefit from therapies that have beneficial effects on both stable angina and T2D, thereby reducing polypharmacy. Prospective studies in patients with stable angina and T2D are needed to guide therapy decisions.
[Rev Cardiovasc Med. 2015;16(2): 105-113 doi: 10.3909/ricm0742]
© 2015 MedReviews®, LLC
Management of Patients With Stable Angina and Type 2 Diabetes
Prakash C. Deedwania, MD, FACC, FAHA
University of California, San Francisco, Fresno, CA
Type 2 diabetes (T2D) is a well-established risk factor for patients with coronary artery disease (CAD). Patients with CAD and comorbid T2D also have a higher risk of cardiovascular complications, such as silent ischemia and stable angina. In treating the symptoms of stable angina in patients with CAD and comorbid T2D, it is vital to utilize therapies that reduce symptoms and improve outcomes. At the same time, there is significant concern about the preservation of glycometabolic parameters, such as glycosylated hemoglobin (HbA1c), particularly because some antianginal therapies, such as β-blockers and calcium channel blockers—although effective at improving the symptoms of stable angina and reducing ischemia—may also worsen glycemic control by increasing HbA1c levels. Available trial data on the efficacy of antianginal agents in patients with stable angina and comorbid T2D are limited. Therefore, in patients with stable angina and T2D, a tailored approach to treatment of stable angina by selecting therapies with a neutral or positive glycometabolic profile may improve outcomes and increase treatment compliance. Additionally, patients with a dual diagnosis may benefit from therapies that have beneficial effects on both stable angina and T2D, thereby reducing polypharmacy. Prospective studies in patients with stable angina and T2D are needed to guide therapy decisions.
[Rev Cardiovasc Med. 2015;16(2): 105-113 doi: 10.3909/ricm0742]
© 2015 MedReviews®, LLC
KEY WORDS
Coronary artery disease • Ranolazine • Stable angina • Type 2 diabetes
KEY WORDS
Coronary artery disease • Ranolazine • Stable angina • Type 2 diabetes
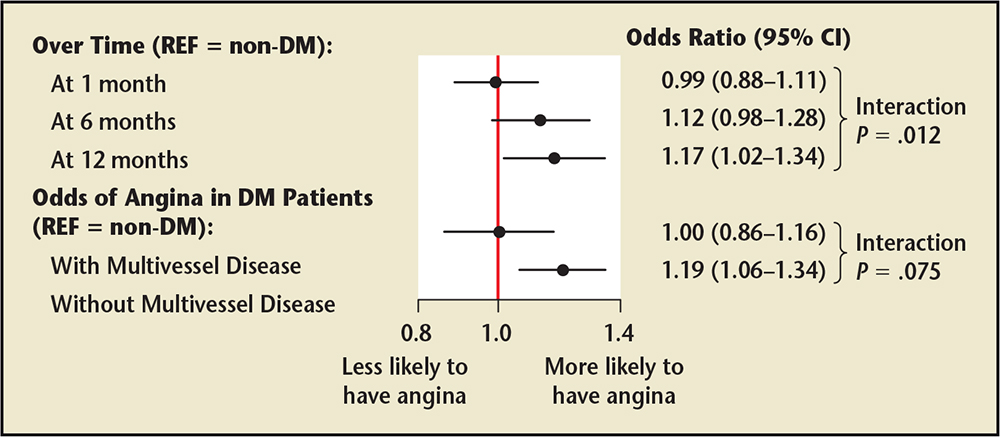
Figure 1. Odds ratio of experiencing angina after myocardial infarction in the Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status (TRIUMPH) registry. A hierarchical multivariable model examined the independent association of diabetes mellitus (DM) with post-myocardial infarction angina over 12 months of follow-up. DM was associated with greater odds for having angina at every time period during the 12-month follow-up period and was associated with greater odds of angina among patients without multivessel disease, but not among those with multivessel disease. CI, confidence interval; REF, reference group is patients without diabetes. Data from Arnold SV et al.21
… the ADA, ACCF, and AHA acknowledged that controlling nonglycemic risk factors (through BP control, lipid-lowering therapy, and lifestyle modification) should be the primary strategies for reducing the burden of CVD in patients with T2D.
With regard to medical therapy, β-blockers, calcium channel blockers, short- and long-acting nitrates, and ranolazine are all recommended for the relief of angina symptoms in patients with stable ischemic heart disease.
A post-hoc analysis of a long-term study comparing CABG and PCI in patients with CAD showed that 5-year survival was greater with CABG versus PCI in patients with diabetes.
Ranolazine, a late sodium current inhibitor approved for the treatment of stable angina. It is effective in reducing anginal frequency, time to onset of ST-segment depression, recurrent ischemia, and nitroglycerin use, and in increasing exercise tolerance without clinically meaningful effects on hemodynamic parameters, such as heart rate or BP.
Main Points
• Type 2 diabetes (T2D) is a well-established risk factor for coronary artery disease (CAD) and can result in increased mortality from myocardial infarction. CAD patients with T2D also have a higher risk of silent ischemia than those without T2D.
• In treating patients with CAD and comorbid T2D, a greater emphasis may need to be placed on antianginal pharmacotherapy. In addition to reducing symptoms and improving cardiovascular outcomes, it is also important to ensure that pharmacotherapies have no deleterious effects on a patient's glycometabolic parameters.
• The American Diabetes Association, the American College of Cardiology Foundation, and the American Heart Association acknowledge that controlling nonglycemic risk factors (through blood pressure control, lipid-lowering therapy, and lifestyle modification) should be the primary strategies for reducing the burden of cardiovascular disease in patients with T2D.
• A post-hoc analysis of a long-term study comparing coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI) in patients with CAD showed that 5-year survival was greater with CABG versus PCI in patients with diabetes.
• Antianginal therapy for patients with CAD and comorbid T2D is additionally complicated by the potential effects of antianginal agents on glucose control; studies have shown that therapy with antianginal agents, such as β-blockers and calcium channel blockers, may have a negative effect on glycemic control by increasing hemoglobin A1c levels in patients with T2D.
Main Points
• Type 2 diabetes (T2D) is a well-established risk factor for coronary artery disease (CAD) and can result in increased mortality from myocardial infarction. CAD patients with T2D also have a higher risk of silent ischemia than those without T2D.
• In treating patients with CAD and comorbid T2D, a greater emphasis may need to be placed on antianginal pharmacotherapy. In addition to reducing symptoms and improving cardiovascular outcomes, it is also important to ensure that pharmacotherapies have no deleterious effects on a patient's glycometabolic parameters.
• The American Diabetes Association, the American College of Cardiology Foundation, and the American Heart Association acknowledge that controlling nonglycemic risk factors (through blood pressure control, lipid-lowering therapy, and lifestyle modification) should be the primary strategies for reducing the burden of cardiovascular disease in patients with T2D.
• A post-hoc analysis of a long-term study comparing coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI) in patients with CAD showed that 5-year survival was greater with CABG versus PCI in patients with diabetes.
• Antianginal therapy for patients with CAD and comorbid T2D is additionally complicated by the potential effects of antianginal agents on glucose control; studies have shown that therapy with antianginal agents, such as β-blockers and calcium channel blockers, may have a negative effect on glycemic control by increasing hemoglobin A1c levels in patients with T2D.
Coronary artery disease (CAD) is the most common type of heart disease and a leading cause of death in the United States, responsible for approximately 385,000 deaths each year.1 Type 2 diabetes (T2D) is a well-established risk factor for CAD and can result in increased mortality from myocardial infarction (MI).2-5 CAD patients with T2D also have a higher risk of silent ischemia than those without T2D.6,7 Approximately 25% to 45% of patients with CAD and T2D have stable angina, a common clinical manifestation of ischemic heart disease, the symptoms of which can limit physical activity and impair quality of life (QoL).8-11 In general, patients with T2D and myocardial ischemia have more extensive CAD and more risk factors for cardiovascular disease (CVD), such as complex lipid abnormalities (dyslipidemia) and hypertension.9,12,13 Interestingly, the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) trial14 showed that patients with CAD and T2D have the same risk of cardiovascular events and mortality regardless of whether they have angina symptoms or not; thus, the management of CAD in patients with T2D should not be predicated solely on the presence or absence of angina symptoms.
The 2012 American College of Cardiology Foundation/American Heart Association (ACCF/AHA) stable ischemic heart disease treatment guideline defines the goals of successful treatment of stable ischemic heart disease as minimizing the likelihood of death while maximizing health and function.15 The ACCF/AHA guideline emphasizes maintenance or restoration of physical activity, functional capacity, and QoL that is satisfactory to the patient, with the complete or near complete elimination of ischemic symptoms, as primary objectives to achieving the goals of successful therapy.15
Strategies to achieve these objectives include modification of risk factors for ischemic heart disease and the use of evidence-based treatment to improve health and survival, with minimal medical therapy side effects.15 In concert with the ACCF/AHA guidelines, recommendations for patients with T2D from the American Diabetes Association (ADA) include lifestyle modifications, such as increased physical activity and weight control, in addition to pharmacologic therapy to achieve glycemic control.12 In treating patients with CAD and comorbid T2D, it is important to understand that, because angina restricts a patient's ability to exercise, a greater emphasis may need to be placed on antianginal pharmacotherapy. Another consideration is that, in addition to reducing symptoms and improving cardiovascular outcomes, it is also important to ensure that pharmacotherapies have no deleterious effects on a patient's glycometabolic parameters. This review discusses the effectiveness of common antianginal and anti-ischemic therapies and the potential implications of their effect on glucose control for treatment of patients with stable angina and comorbid T2D.
Pathophysiology of Myocardial Ischemia
Myocardial ischemia is a result of an imbalance in oxygen delivery to the myocardium and myocardial oxygen consumption.16 In patients with atherosclerotic CAD, increased oxygen demand (increased myocardial oxygen consumption) and decreased supply (decreased coronary blood flow), which can be the consequence of a variety of factors, can result in symptoms of stable angina during periods of increased stress.16,17
Stable angina is generally described as a discomfort or pain located in the chest or upper epigastrium, or as a pressure, heaviness, tightness, squeezing, burning, or choking sensation.18 Physicians generally see patients when their underlying ischemia results in anginal symptoms. Patients with typical angina have (1) substernal chest discomfort of a characteristic quality and duration that is (2) provoked by exertion or emotional stress and (3) relieved by rest or nitroglycerin. Patients with atypical angina have symptoms that meet only two of these three characteristics.15
Angina in T2D
Patients with T2D can often present with atypical symptoms of angina and silent ischemia.19 In a subgroup analysis of 2319 patients with CAD and T2D in the BARI 2D study, 971 patients (42%) with T2D and angiographically proven CAD had symptoms of angina, defined as any combination of typical angina and angina equivalents (defined as shortness of breath, dyspnea upon exertion, exertional fatigue, nausea, unexplained diaphoresis, or other).18
Analysis of data from the 24-center Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH) prospective registry,20 investigating the association between T2D and angina after an MI, showed that patients with diabetes were more likely to have angina pre-MI (P < .001) and post-MI (P < .001) than those without diabetes.21 The increased angina risk in patients with diabetes also increased significantly over time (Pinteraction = .012), and the association between diabetes and angina risk was stronger in patients without multivessel disease (Pinteraction = .075 vs patients with multivessel disease) (Figure 1).21
In a randomized long-term study (the Trial of Invasive versus Medical Therapy in the Elderly with Symptomatic CAD [TIME] study) in 301 elderly patients with symptomatic CAD stratified by diabetic status, although patients with diabetes (23% of the total cohort) had similar angina severity and antianginal drug use as those without diabetes, those with diabetes performed worse in daily activities and had diminished physical functioning.22
Coronary Risk Factors and Their Management in Patients With Stable Angina and T2D
Patients with CAD and comorbid T2D often have other CVD risk factors, such as hypertension, obesity, and dyslipidemia. The presence of these CVD risk factors often leads to an impaired QoL in patients with CAD and comorbid T2D, compared with those without T2D.23,24 The Investigation of Outcomes From Acute Coronary Syndromes (INFORM) study was a prospective analysis of health outcomes in a cohort of 1199 patients followed for 1 year after being admitted and treated for acute coronary syndrome (ACS).25 Approximately 326 patients (37%) in the INFORM study had T2D; these patients were more likely to have additional CVD risk factors such as hypertension and dyslipidemia at baseline than patients without T2D.25 At 1 year after-ACS, patients with T2D were also more likely to have angina, reported more cardiac-specific physical limitations, and reported greater impairment of QoL than patients without T2D.25 Findings from the TIME study in elderly patients with angina and diabetes also showed that presence of diabetes was associated with a higher incidence of at least two other risk factors.22
Several randomized controlled trials have evaluated the cardiovascular benefit of controlling risk factors such as elevated blood pressure (BP), lipid levels, and glucose levels on CVD outcomes in patients with T2D.26 In a joint statement on the effect of intensive glycemic control on the prevention of cardiovascular events, the ADA, ACCF, and AHA acknowledged that controlling nonglycemic risk factors (through BP control, lipid-lowering therapy, and lifestyle modification) should be the primary strategies for reducing the burden of CVD in patients with T2D.26 Patients with CAD and comorbid T2D need to have comprehensive reduction of CVD risk factors. The treatment guideline from the ACCF/AHA advocates reduction of CVD risk factors such as elevated BP, dyslipidemia, and elevated glucose levels in patients with stable ischemic heart disease.15 In addition to pharmacotherapy for the management of CVD risk factors and symptoms of stable angina, this guideline also recommends lifestyle modifications, including smoking cessation, weight management, depression screening, and a minimum of 150 minutes of moderate-intensity aerobic exercise weekly.15 This is in concert with recent recommendations from the ADA that patients with T2D attain a systolic and diastolic BP goal of < 140/80 mm Hg, a low-density lipoprotein cholesterol goal of < 100 mg/dL, a high-density lipoprotein cholesterol goal of > 40 mg/dL (> 50 mg/dL in women), and a target triglyceride level of < 150 mg/dL.12
Therapies for Myocardial Ischemia and Stable Angina in Patients With CAD and T2D
Guideline-directed Medical Therapy
Recommended management of CAD and stable angina consists of medical therapy as the initial strategy, followed by myocardial revascularization by percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) surgery in patients whose angina symptoms is not adequately controlled on medical therapy, depending on the individual patient's mortality risk.15,27 Although the ACCF/AHA treatment guidelines include patients with T2D in their overall recommendations,15 they have no specific guidelines for this population. This is due to the paucity of data. Only one primary study to date has evaluated the efficacy of antianginal therapy (ranolazine) in a large cohort of patients with stable angina and comorbid T2D,28 but this study was not completed when the guidelines were published. Other studies have investigated antianginal therapy in subgroup analyses of patients with and without diabetes29-31 and three pilot studies have assessed the efficacy of the nonantianginal agents sarpogrelate hydrochloride, thiazolidinedione, and metformin on anginal endpoints in patients with angina and T2D.32-34
With regard to medical therapy, β-blockers, calcium channel block-ers (CCBs), short- and long-acting nitrates, and ranolazine are all recommended for the relief of angina symptoms in patients with stable ischemic heart disease.15 β-blockers are recommended as initial therapy, whereas CCBs, long-acting nitrates, and ranolazine are recommended if β-blockers are not effective or well tolerated, or are contraindicated. Alternatively, if a β-blocker alone is not efficacious but is well tolerated, then dual therapy of a β-blocker combined with a CCB, long-acting nitrate, or ranolazine can be used.15 Revascularization is usually performed in CAD patients with unstable angina or MI, but can be used in selected patients with stable ischemic heart disease with persistent angina symptoms despite medical therapy.
Comparison of Revascularization Versus Medical Therapy
The comparative effectiveness of guideline-recommended medical therapy versus revascularization has been reviewed in one study and two secondary analyses in patients with stable angina and T2D,14,35,36 in one study in elderly patients with angina stratified by diabetic status,22 and in one study in a general CAD population.37
The BARI 2D study in patients with CAD and classic angina with comorbid T2D compared the efficacy of initial treatment with PCI or CABG and medical therapy with medical therapy alone.36 At 5-year follow-up, there was no statistically significant difference between the revascularization plus medical treatment group and the medical treatment alone group in the 5-year survival rate (88.3% vs 87.8%; P = .97) or in the rate of freedom from major cardiovascular events (77.2% vs 75.9%; P = .70).36 A secondary analysis of the BARI 2D study showed that patients undergoing revascularization (PCI or CABG) experienced lower rates of worsening angina, new angina, and subsequent revascularization compared with patients on medical therapy alone.35 The between-group difference was only statistically significant at year 1 (P < .001) and year 3 (P < .001) for worsening angina, whereas between-group differences in the outcomes of new angina and subsequent revascularization were significant through year 5 (P < .001).35 Another analysis of the BARI 2D study evaluated the effect of the presence of angina symptoms at baseline (typical angina symptoms; angina equivalent of dyspnea, fatigue, or diaphoresis on exertion; and asymptomatic ischemia) on primary outcomes.14 The three baseline groups included patients with typical angina symptoms, angina-equivalent symptoms (dyspnea, fatigue, or diaphoresis on exertion), and asymptomatic ischemia. After 5 years of follow-up, there were no differences between groups in the rate of all-cause death or in the rate of the composite outcome of all-cause death, nonfatal MI, or nonfatal stroke.14
Collectively, the data from the primary BARI 2D study and subsequent analyses suggest that guideline-directed medical therapy may be appropriate for most patients with CAD with stable angina and comorbid T2D, with revascularization reserved for high-risk patients with persistent, disabling symptoms despite guideline-directed medical therapy.27 Unlike the BARI 2D study, in which the mean age of the enrolled population was 62.4 years, the TIME study demonstrated the benefit of revascularization in an elderly population. TIME was a randomized long-term study designed to compare revascularization versus medical therapy in 301 elderly patients (aged ≥ 75 years) with symptomatic CAD and stratified by diabetic status (median follow-up, 4.1 years [range, 0.1-6.9]). The results showed that revascularization in addition to medical therapy significantly improved the overall and event-free survival rates (both P < .01 vs no revascularization), and the improvement was similar in patients with and without diabetes.22
The Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) study37 is a comparison of revascularization versus medical therapy in a general CAD population of which 33% of patients had a history of diabetes at baseline. The COURAGE trial compared the effect of PCI plus optimal medical therapy (β-blocker, diltiazem as needed, and aggressive management of CAD risk factors) versus optimal medical therapy alone in patients with stable CAD. Data showed that, during the median follow-up period of 55 months, patients in both treatment groups had similar rates of the primary end-point of death and nonfatal MI.37 A subgroup analysis comparing outcomes in patients with and without T2D was not performed.
For now, all patients with CAD and T2D, regardless of the presence or absence of angina symptoms, should be managed similarly with respect to risk stratification and preventive therapies to reduce the occurrence of cardiovascular events and death.14 Assiduous cardiovascular risk assessment and risk factor management is certainly indicated in patients with diabetes.7
PCI versus CABG
A post-hoc analysis of a long-term study comparing CABG and PCI in patients with CAD showed that 5-year survival was greater with CABG versus PCI (P = .003) in patients with diabetes.38 Findings from a retrospective data analysis also suggested that CABG might be associated with improved outcomes when compared with PCI in patients with diabetes.39 These findings were supported by the prospective Future Revascularization Evaluation in Patients with Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial,40 which evaluated the effectiveness of PCI using sirolimus or paclitaxel drug-eluting stents compared with CABG in patients with CAD and T2D. Data from the FREEDOM trial showed that there was a significantly lower rate of the composite outcome of all-cause death, nonfatal MI, or nonfatal stroke in patients with multivessel CAD and T2D treated with CABG compared with PCI (18.7% vs 26.6%; P = .005).40 These data suggest that patients with CAD and T2D who may have more extensive CAD and who are candidates for revascularization may benefit more from CABG compared with PCI, irrespective of the type of drug-eluting stent used.
A preliminary study in 77 patients with chronic angina and T2D showed that drug-eluting stent implantation and CABG had similar long-term outcomes in terms of major adverse cardiac events, but CABG was associated with more in-hospital complications and longer hospitalization.41
Overview of Medical Therapy
Traditional Antianginal Agents.
One study evaluated the relative efficacy of a β-blocker and a CCB in patients with chronic angina and diabetes (type 1 and type 2). This study of 809 patients with chronic angina treated with metoprolol or verapamil compared outcomes in three groups of patients: patients with diabetes (8.5%), patients with hyperglycemia in the absence of diabetes (8.3%), and normoglycemic patients (83.2%).29 The study found that diabetes and elevated blood glucose were independent risk factors for cardiovascular-related events; however, treatment was not shown to affect outcome or prognosis.29
A large meta-analysis of 90 studies attempted to compare the relative efficacy of β-blockers, CCBs, and nitrates in patients with stable angina in terms of reducing the rate of death and MI. There were no significant differences observed between β-blockers and CCBs in the rate of death or MI (P = .79), time until ≥ 1-mm ST-segment depression (P = .33), nitroglycerin use (P = .32), and the number of weekly angina episodes (P = .05). Significantly more patients taking CCBs were observed to have adverse events (AEs) resulting in discontinuation of medication compared with those taking β-blockers (P < .001).42 For the comparisons of β-blockers and CCBs with nitrates, there were no significant differences observed across the same endpoints, although the authors advised cautious interpretation of the results due to the low number of studies evaluated. Of the studies included in the meta-analysis, the mean proportion of patients at baseline with diabetes ranged from 2% to 24% across comparison groups and thus do not provide much guidance on treatment in patients with chronic angina and comorbid diabetes.42
In a longitudinal, observational study of 44,708 patients with known prior MI (n = 14,043), CAD but no history of MI (n = 12,012), or only risk factors for CAD (n = 18,653), patients with CAD risk factors only who received β-blocker therapy experienced a higher rate of cardiovascular death, nonfatal MI, or nonfatal stroke compared with those not receiving β-blocker therapy.43 In addition, among patients with CAD risk factors only receiving β-blocker therapy, there were more hospitalizations for atherothrombotic events or revascularization than for patients not receiving β-blocker therapy.43 Data from this study on patients with CAD challenged the conventional wisdom that β-blocker therapy has cardioprotective benefits in patients with stable heart disease.43
Ranolazine in Stable Angina.
Ranolazine, a late sodium current inhibitor approved for the treatment of stable angina. It is effective in reducing anginal frequency, time to onset of ST-segment depression, recurrent ischemia, and nitroglycerin use, and in increasing exercise tolerance without clinically meaningful effects on hemodynamic parameters, such as heart rate or BP.44,45 Ranolazine also has demonstrated efficacy with regard to angina-related endpoints when combined with other commonly used antianginal medications.46,47
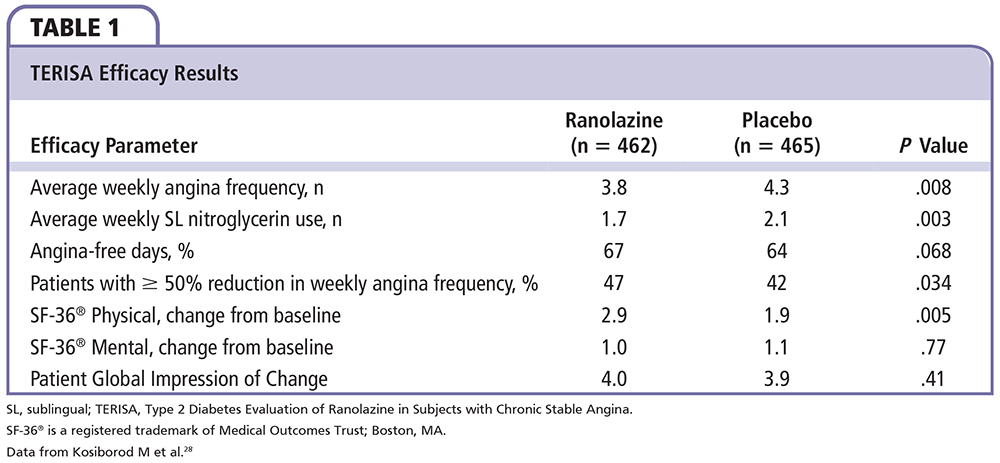
The recently completed Type 2 Diabetes Evaluation of Ranolazine in Subjects with Chronic Stable Angina (TERISA) study,28 an 8-week, phase 4, international, randomized, placebo-controlled study in 949 patients with T2D, CAD, and at least a 3-month history of chronic stable angina despite treatment with one or two antianginal agents, is the first large prospective study to evaluate the efficacy of ranolazine in a population of patients with T2D. The primary endpoint, the placebo-adjusted change from baseline in the average weekly angina frequency in the last 6 weeks of the double-blind treatment period, was significantly lower with ranolazine versus placebo, as was weekly nitroglycerin use during this time. Interestingly, the significantly greater efficacy of ranolazine versus placebo in terms of reduced weekly angina frequency (Table 1) was positively correlated with higher baseline hemoglobin A1c (HbA1c) (Pinteraction = .027). The number of angina-free days was greater with ranolazine treatment versus placebo, but not significantly so, and there were no differences between ranolazine and placebo for changes in the SF-36® Mental Component score (Medical Outcomes Trust; Boston, MA) and Patient's Global Impression of Change score (Table 1). The proportion of patients achieving ≥ 50% reduction in weekly angina frequency (P = .034) and increases in SF-36® Physical Component score were higher with ranolazine than with placebo (P = .005). Interestingly, in this study, an innovative electronic diary used for submission of patient-reported angina frequency and sublingual nitroglycerin use achieved a high rate of compliance (98% data capture in both groups). The overall incidence of AEs and the number of discontinuations due to AEs, serious AEs, and deaths were similar in the ranolazine and placebo groups.28 Nonserious AEs reported more frequently with ranolazine versus placebo were dizziness, nausea, constipation, and hypoglycemia.
Until the recent publication of TERISA, similar to studies of β-blockers, CCBs, and long-acting nitrates, other studies evaluating the use of ranolazine for the treatment of chronic angina evaluated 19% to 24% of patients with T2D at baseline.44,46,47 The data from TERISA confirmed exploratory and post-hoc findings from subgroup analyses of earlier studies of ranolazine in patients with T2D and angina—Monotherapy Assessment of Ranolazine in Stable Angina (MARISA)44 and Combination Assessment of Ranolazine in Stable Angina (CARISA).30 These subgroup analyses also had the benefit of being able to compare patients with and without diabetes directly. In the MARISA study, a subgroup analysis by diabetes status at baseline showed that patients with (24%) and without (76%) diabetes achieved similar total trough exercise durations with ranolazine (Pinteraction = .77), indicating no differential treatment effect by diabetes history.44 A secondary analysis of the CARISA trial also showed improvements with ranolazine in patients with (23%) and without (77%) diabetes, though no treatment interactions in exercise duration (Pinteraction = .89), time to onset of angina (Pinteraction = .54), time to onset of ≥ 1-mm ST-segment depression (Pinteraction = .44), weekly angina frequency (Pinteraction = .81), or nitroglycerin usage (Pinteraction = .063) were observed.30
Other Antianginal Agents.
Additional antianginal agents that are effective in treating stable angina and increasing exercise tolerance, but are not approved for use in the United States, include ivabradine, nicorandil, and trimeta-zidine.48 In a study comparing trimetazidine and metoprolol in patients with angina and T2D, both treatments provided similar, significant reductions in angina frequency, angina duration, nitroglycerin use, and improvements in time to angina and left ventricular ejection fraction from baseline; however, trimetazidine, but not metoprolol, did not affect heart rate, BP, blood glucose, and lipid levels. Ivabradine effectively increases exercise tolerance, time to onset of angina, time to limiting angina, and time to onset of ST-segment depression in patients with stable angina, but does not significantly reduce angina frequency or consumption of nitroglycerin.49,50
Effects on Glycemic Control
Antianginal therapy for patients with CAD and comorbid T2D is additionally complicated by the potential effects of antianginal agents on glucose control. Studies have shown that therapy with antianginal agents, such as β-blockers and CCBs, may have a negative effect on glycemic control by increasing HbA1c levels in patients with T2D.51,52 In a retrospective analysis evaluating the effect of β-blockers, CCBs, and angiotensin-converting enzyme inhibitors on HbA1c levels in patients with hypertension and T2D, β-blockers and nicardipine (a dihydropyridine CCB) were both found to cause deterioration in glucose metabolism (HbA1c was increased by 0.7% in 4 months with nicardipine and by ∼ 1.0% in 3 months by β-blocker therapy), whereas diltiazem and enalapril had no such effect.51 In a 20-week, randomized, controlled, double-blind study comparing the effect of combination therapy with extended-release verapamil plus trandolapril versus atenolol plus chlorthalidone on HbA1c levels in 463 patients with T2D and mild-to-moderate hypertension, HbA1c levels were unchanged with the former, whereas they increased significantly with the latter (P < .0001).52 In studies of the effect of ranolazine on glucose levels in patients with chronic angina or ACS and comorbid T2D, ranolazine treatment was shown to significantly improve glycemic control by decreasing mean HbA1c levels by 0.48% to 0.70% compared with placebo (P < .001).30,53,54 Six trials (four ongoing phase III studies with ranolazine as monotherapy,55-57 and ranolazine added to glimepiride,58 and two phase I trials assessing pharmacokinetics with met-formin59,60) will help to provide additional insight into the effects of ranolazine on glycometabolic parameters in patients with angina and T2D. One study evaluating ivabradine treatment showed it to have a neutral effect on HbA1c levels in patients with chronic angina and comorbid T2D.31
Tolerability of Antianginal Therapy
Antianginal agents are not without side effects. β-blocker therapy is associated with reductions in heart rate and BP, impaired sexual function and exercise tolerance, and fatigue.61-63 Adverse effects associated with dihydropyridine CCBs include exacerbation of angina, vasodilation, and systemic hypotension in some patients.15 Long-term therapy with short- and long-acting nitrates can lead to the development of nitrate tolerance.64 Specific adverse effects of ranolazine include constipation, dizziness, and nausea.46
Polypharmacy
Another concern with medical therapy in patients with chronic angina and comorbid T2D is polypharmacy. Patients with these chronic conditions are often on multiple medications to manage their disease states. Studies show that polypharmacy and an increased pill burden are associated with decreased compliance with therapy,65,66 and therapies that provide multiple benefits and a lower pill burden can improve treatment compliance.67,68 As such, therapies that provide a dual benefit in improving ischemia and glycemia may contribute to improved treatment compliance in patients with stable angina and comorbid T2D.
Conclusions
Many patients with chronic stable angina as part of CAD also have comorbid T2D. Antianginal therapy for these patients is complex and is complicated by the potential adverse effects of some antianginal agents on glucose control. Indeed, many of the traditionally prescribed antianginal agents are associated with increased HbA1c levels and adverse hemodynamic effects. Therefore, antianginal treatment options for patients with chronic angina and comorbid T2D who are at higher risk of cardiovascular complications require careful selection, and the metabolic profile of the agents must be considered. Patients with chronic angina and comorbid T2D will benefit from further research to evaluate the glycemic effects of antianginal therapies in patients with chronic angina and comorbid T2D, so that they can be offered evidence-based treatment with agents that provide both antianginal and glycemic benefits. ![]()
Luana Henderson, PhD, and Mary Mines of in Science Communications, Springer Healthcare (Philadelphia, PA), provided medical writing support funded by Gilead Sciences, Inc (Foster City, CA).
Dr. Deedwania has served as a consultant and on the advisory board for Gilead Sciences, Inc.
References
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6-e245.
- Haffner SM, Lehto S, Ronnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229-234.
- Juutilainen A, Lehto S, Ronnemaa T, et al. Type 2 diabetes as a “coronary heart disease equivalent”: an 18-year prospective population-based study in Finnish subjects. Diabetes Care. 2005;28:2901-2907.
- Donahoe SM, Stewart GC, McCabe CH, et al. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298:765-775.
- Fava S, Azzopardi J, Agius-Muscat H. Outcome of unstable angina in patients with diabetes mellitus. Diabet Med. 1997;14:209-213.
- Ditchburn CJ, Hall JA, de Belder M, et al. Silent myocardial ischaemia in patients with proved coronary artery disease: a comparison of diabetic and non-diabetic patients. Postgrad Med J. 2001;77:395-398.
- Stone PH. Ischemia dictates outcome, not symptoms. J Am Coll Cardiol. 2013;61:712-713.
- Tan MH. Diabetes and coronary heart disease. Diabetes Spectrum. 1999;12:80-83.
- Deedwania PC, Carbajal EV. Silent myocardial ischemia. A clinical perspective. Arch Intern Med. 1991;151:2373-2382.
- Deedwania PC, Carbajal EV. Prevalence and patterns of silent myocardial ischemia during daily life in stable angina patients receiving conventional antianginal drug therapy. Am J Cardiol. 1990;65: 1090-1096.
- Gardner AW, Montgomery PS, Ritti-Dias RM, Thadani U. Exercise performance, physical activity, and health-related quality of life in participants with stable angina. Angiology. 2011;62:461-466.
- American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(suppl 1):S11-S66.
- Franklin K, Goldberg RJ, Spencer F, et al; GRACE Investigators. Implications of diabetes in patients with acute coronary syndromes. The Global Registry of Acute Coronary Events. Arch Intern Med. 2004;164:1457-1463.
- Dagenais GR, Lu J, Faxon DP, et al; BARI 2D Study Group. Prognostic impact of the presence and absence of angina on mortality and cardiovascular outcomes in patients with type 2 diabetes and stable coronary artery disease: results from the BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) Trial. J Am Coll Cardiol. 2013;61:702-711.
- Fihn SD, Gardin JM, Abrams J, et al; American College of Cardiology Foundation/American Heart Association Task Force. 2012 ACCF/AHA/ACP/ AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126:e354-e471.
- Crossman DC. The pathophysiology of myocardial ischaemia. Heart. 2004;90:576-580.
- Ardehali A, Ports TA. Myocardial oxygen supply and demand. Chest. 1990;98:699-705.
- Krishnaswami A, Hardison R, Nesto RW, Sobel B; BARI 2D Investigators. Presentation in patients with angiographically documented coronary artery disease and type II diabetes mellitus (from the BARI 2D Clinical Trial). Am J Cardiol. 2012;109:36-41.
- Zellweger MJ, Hachamovitch R, Kang X, et al. Prognostic relevance of symptoms versus objective evidence of coronary artery disease in diabetic patients. Eur Heart J. 2004;25:543-550.
- Arnold SV, Chan PS, Jones PG, et al; Cardiovascular Outcomes Research Consortium. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status (TRIUMPH): design and rationale of a prospective multicenter registry. Circ Cardiovasc Qual Outcomes. 2011;4:467-476.
- Arnold SV, Spertus JA, Lipska KJ, et al. Association between diabetes mellitus and angina after acute myocardial infarction: analysis of the TRIUMPH prospective cohort study. Eur J Prev Cardiol. 2015;22:779-787.
- Jeger RV, Bonetti PO, Zellweger MJ, et al. Influence of revascularization on long-term outcome in patients > or = 75 years of age with diabetes mellitus and angina pectoris. Am J Cardiol. 2005;96:193-198.
- Cheung BM, Ong KL, Cherny SS, et al. Diabetes prevalence and therapeutic target achievement in the United States, 1999 to 2006. Am J Med. 2009;122:443- 453.
- Kahn R. Metabolic syndrome: is it a syndrome? Does it matter? Circulation. 2007;115:1806-1810.
- Peterson PN, Spertus JA, Magid DJ, et al. The impact of diabetes on one-year health status outcomes following acute coronary syndromes. BMC Cardiovasc Disord. 2006;6:41.
- Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. J Am Coll Cardiol. 2009;53:298-304.
- Deedwania PC, Carbajal EV. Medical therapy versus myocardial revascularization in chronic coronary syndrome and stable angina. Am J Med. 2011;124:681- 688.
- Kosiborod M, Arnold SV, Spertus JA, et al. Evaluation of ranolazine in patients with type 2 diabetes mellitus and chronic stable angina. Results from the TERISA randomized clinical trial. J Am Coll Cardiol. 2013;61:2038-2045.
- Held C, Björkander I, Forslund L, et al. The impact of diabetes or elevated fasting blood glucose on cardiovascular prognosis in patients with stable angina pectoris. Diabet Med. 2005;22:1326-1333.
- Timmis AD, Chaitman BR, Crager M. Effects of ranolazine on exercise tolerance and HbA1c in patients with chronic angina and diabetes. Eur Heart J. 2006;27:42-48.
- Borer JS, Tardif JC. Efficacy of ivabradine, a selective I(f) inhibitor, in patients with chronic stable angina pectoris and diabetes mellitus. Am J Cardiol. 2010;105:29-35.
- Watanabe H. Effects of sarpogrelate HCl, a selective 5-HT2A antagonist, on endothelial function and aortic stiffness in diabetic patients with stable angina [abstract]. J Am Coll Cardiol. 2004;43:197A.
- Murakami T, Mizuno S. Effects of thiazolodinedione on effort induced angina pectoris with type-2 diabetes mellitus [abstract]. Circulation. 2000;102(suppl II): 706-707.
- Watanabe J, Kakihana M. Effects of metformin on endothelial function, aortic stiffness, and exercise tolerance in diabetic patients with stable angina. Circ J. 2004;68:150.
- Dagenais GR, Lu J, Faxon DP, et al; Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Study Group. Effects of optimal medical treatment with or without coronary revascularization on angina and subsequent revascularizations in patients with type 2 diabetes mellitus and stable ischemic heart disease. Circulation. 2011;123:1492- 1500.
- Frye RL, August P, Brooks MM, et al; BARI 2D Study Group. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503-2515.
- Boden WE, O’Rourke RA, Teo KK, et al; COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503-1516.
- Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. N Engl J Med. 1996;335:217-225.
- Hlatky MA, Boothroyd DB, Bravata DM, et al. Coronary artery bypass surgery compared with percutaneous coronary interventions for multivessel disease: a collaborative analysis of individual patient data from ten randomised trials. Lancet. 2009;373:1190- 1197.
- Farkouh ME, Domanski M, Sleeper LA, et al; FREEDOM Trial Investigators. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med. 2012;367:2375-2384.
- Toutouzas K, Patsa C, Vaina S, et al. A preliminary experience report: drug-eluting stents versus coronary artery bypass surgery in patients with a single lesion in the proximal left anterior descending artery suffering from diabetes mellitus and chronic stable angina. Hellenic J Cardiol. 2008;49:65-71.
- Heidenreich PA, McDonald KM, Hastie T, et al. Metaanalysis of trials comparing beta-blockers, calcium antagonists, and nitrates for stable angina. JAMA. 1999;281:1927-1936.
- Bangalore S, Steg G, Deedwania P, et al; REACH Registry Investigators. β-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA. 2012;308:1340-1349.
- Chaitman BR, Skettino SL, Parker JO, et al; MARISA Investigators. Anti-ischemic effects and long-term survival during ranolazine monotherapy in patients with chronic severe angina. J Am Coll Cardiol. 2004;43:1375-1382.
- Rousseau MF, Pouleur H, Cocco G, Wolff AA. Comparative efficacy of ranolazine versus atenolol for chronic angina pectoris. Am J Cardiol. 2005;95:311-316.
- Chaitman BR, Pepine CJ, Parker JO, et al; Combination Assessment of Ranolazine In Stable Angina (CARISA) Investigators. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. JAMA. 2004;291:309-316.
- Stone PH, Gratsiansky NA, Blokhin A, et al; ERICA Investigators. Antianginal efficacy of ranolazine when added to treatment with amlodipine: the ERICA (Efficacy of Ranolazine in Chronic Angina) trial. J Am Coll Cardiol. 2006;48:566-575.
- Chaitman BR, Sano J. Novel therapeutic approaches to treating chronic angina in the setting of chronic ischemic heart disease. Clin Cardiol. 2007;30(2 suppl 1): I25-I30.
- Borer JS, Fox K, Jaillon P, Lerebours G; Ivabradine Investigators Group. Antianginal and antiischemic effects of ivabradine, an I(f) inhibitor, in stable angina: a randomized, double-blind, multicentered, placebocontrolled trial. Circulation. 2003;107:817-823.
- Ruzyllo W, Tendera M, Ford I, Fox KM. Antianginal efficacy and safety of ivabradine compared with amlodipine in patients with stable effort angina pectoris: a 3-month randomised, double-blind, multicentre, noninferiority trial. Drugs. 2007;67:393-405.
- Sasaki H, Naka K, Kishi Y, et al. Nicardipine may impair glucose metabolism in hypertensive diabetic patients. Diabetes Res Clin Pract. 1994;26:67-75.
- Holzgreve H, Nakov R, Beck K, Janka HU. Antihypertensive therapy with verapamil SR plus trandolapril versus atenolol plus chlorthalidone on glycemic control. Am J Hypertens. 2003;16:381-386.
- Morrow DA, Scirica BM, Chaitman BR, et al; MERLIN- TIMI 36 Investigators. Evaluation of the glycometabolic effects of ranolazine in patients with and without diabetes mellitus in the MERLINTIMI 36 randomized controlled trial. Circulation. 2009;119:2032-2039.
- Kipnes MS, Bays HE, Staehr P, et al. A study to assess the metabolic effects of ranolazine when added to ongoing non-insuln medical therapy in subjects with type 2 diabetes mellitus (T2DM) [abstract]. Diabetes. 2011;60:A316. 1149-P.
- Ranolazine monotherapy in subjects with type 2 diabetes mellitus. ClinicalTrials.gov website. NCT01472185. https://clinicaltrials.gov/
ct2/show/NCT01472185. Accessed March 30, 2015. - Effects of ranolazine on coronary flow reserve in symptomatic diabetic patients and CAD (RANDCFR). ClinicalTrials.gov website. NCT01754259. https://clinicaltrials.gov/ct2/
show/NCT01754259?term=NCT01754259&rank=1. Accessed March 30, 2015. - A phase 3 study of ranolazine in subjects with type 2 diabetes who are not well controlled on metformin alone. ClinicalTrials.gov website. NCT01555164. https://clinicaltrials.gov/ct2/
show/NCT01555164?term=NCT01555164&rank=1. Accessed March 30, 2015. - Ranolazine when added to glimepiride in subjects with type 2 diabetes mellitus. NCT01494987. ClinicalTrials.gov website. https://clinicaltrials.gov/ct2/
show/NCT01494987?term=NCT01494987&rank=1. Accessed March 30, 2015. - Single cohort 4-period study to assess pharmacokinetics of metformin alone and in combination with ranolazine. NCT01546597. ClinicalTrials.gov website. https://clinicaltrials.gov/ct2/
show/NCT01546597?term=NCT01546597&rank=1. Accessed March 30, 2015. - Single cohort, 2-period study to assess pharmacokinetics of metformin alone and in combination with ranolazine 500 mg. NCT01546558. ClinicalTrials.gov website. https://clinicaltrials.gov/ct2/
results?term=NCT01546558&Search=Search. Accessed March 30, 2015. - Vadnais DS, Wenger NK. Emerging clinical role of ranolazine in the management of angina. Ther Clin Risk Manag. 2010;6:517-530.
- Shavelle DM. Long term medical treatment of stable coronary disease. Heart. 2007;93:1473-1477.
- van Baak MA, Böhm RO, Arends BG, et al. Long-term antihypertensive therapy with betablockers: submaximal exercise capacity and metabolic effects during exercise. Int J Sports Med. 1987;8:342-347.
- Thadani U, Ripley TL. Side effects of using nitrates to treat heart failure and the acute coronary syndromes, unstable angina and acute myocardial infarction. Expert Opin Drug Saf. 2007;6:385-396.
- Erdine S. How do compliance, convenience, and tolerability affect blood pressure goal rates? Am J Cardiovasc Drugs. 2012;12:295-302.
- Ingersoll KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of literature. J Behav Med. 2008;31: 213-224.
- Frishman WH. Importance of medication adherence in cardiovascular disease and the value of once-daily treatment regimens. Cardiol Rev. 2007;15:257-263.
- van Dulmen S, Sluijs E, van Dijk L, et al. Patient adherence to medical treatment: a review of reviews. BMC Health Serv Res. 2007;7:55.