Feasibility study of fluorine detection by ICP-QQQ



Noriyuki Yamada
Agilent Technologies, Japan
Keywords
fluorine-containing polyatomic ions, barium, oxygen on-mass, ammonia mass-shift
Introduction
Fluorine (19F) cannot be directly detected by conventional quadrupole ICP-MS (ICP-QMS) because of severe water-derived interferences at m/z 19 from 1H316O+ and 1H18O+, and extremely low sensitivity due to the fact that it is very difficult to convert fluorine atoms to the positive ions that are measured in ICP-MS. The interference problem can be resolved by high resolution ICP-MS, but the sensitivity issue remains a challenge because almost no F atoms are ionized in an argon plasma due to F having an ionization potential (17.423 eV) that is higher than that of Ar (15.760 eV).
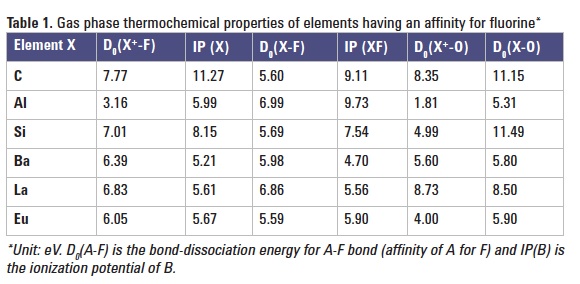
However, fluorine-containing polyatomic ions (XF+) can be formed in the plasma and they may be used to determine fluorine. Candidate ions are those with a high bond-dissociation energy for the X+-F bond and low ionization potential of X or XF. Since oxygen is present in the plasma (from the water matrix or from air entrainment), the formation of XO+ or XO often competes against that of XF+. Therefore, a low bond-dissociation energy for X+-O and X-O bonds (low affinity of X+ and X for O) is also desirable for the efficient formation of XF+. Barium was selected as "X" for this feasibility study, based on its thermochemical properties (Table 1).
Experimental
Instrumentation: Agilent 8800 #200 with a Micromist nebulizer.
Plasma conditions and ion lens tune:
RF power = 1500 W;
Sampling depth = 8 mm;
CRGS flow rate = 1.00 L/min;
Sample uptake rate 0.33 mL/min;
100 ppm Ba uptake rate = 0.03 mL/min;
MUGS flow rate = 0.32 L/min;
Extract 1 = -150 V, Extract 2 = -4 V.
CRC conditions: O2 gas at 1 mL/min (100%), Octopole bias = -60 V, Energy discrimination = -10 V in O2 mode;
10% NH3/90% He flow rate 8.5 mL/min (85%), Octopole bias = -20 V, Energy discrimination = -10 V in NH3 mode.
Acquisition parameters: MS/MS O2 on-mass and MS/MS NH3 mass-shift. Integration time per mass for BaF and BaF(NH3)3 = 1 sec; integration time per mass for BaF(NH3)4 = 10 sec.
In order to produce BaF+ in the plasma, Ba solution was mixed online with fluorine standards per a fixed mixing ratio of 1:10. The mixing occurred just before the nebulizer. BaF+ was efficiently formed under general plasma conditions with the BaO+/Ba+ ratio at about 11%. Under hotter plasma conditions, the formation of BaF+ decreases because it tends to break apart. Under cooler plasma conditions, the formation of BaF+ also decreases because of the formation of BaO+ or, possibly, BaO. The signal intensity of BaF+ was proportional to the concentration of Ba, which was fixed at about 10 ppm (after mixing).
Interference removal using MS/MS mode
138Ba19F+ (m/z=157) suffers an interference from 138Ba18O1H+. O2 and NH3 were tested as reaction gases to reduce the interference. It was found that O2 reacts with BaOH+ more efficiently than it reacts with BaF+ in high energy reaction mode (octopole bias < -50 V). Therefore, using MS/MS mode, a mass pair (Q1 → Q2) =
(157 → 157) was selected to detect BaF+ in O2 mode. With Q1 set to 157 amu, 138Ba+ was prevented from entering the cell and forming new interferences through unwanted reactions.
NH3 was found to react with BaF+ at a high NH3 flow rate to form BaF(NH3)n+, where n = 2, 3, 4. The most abundant complex ion was BaF(NH3)3+ at
m/z = 208, but BaF(NH3)4+ at m/z = 225 was preferable in terms of signal to background ratio or BEC.
Mass pairs (Q1 → Q2) = (157 → 208) and (157 → 225) were selected in NH3 mode.
Results and discussion
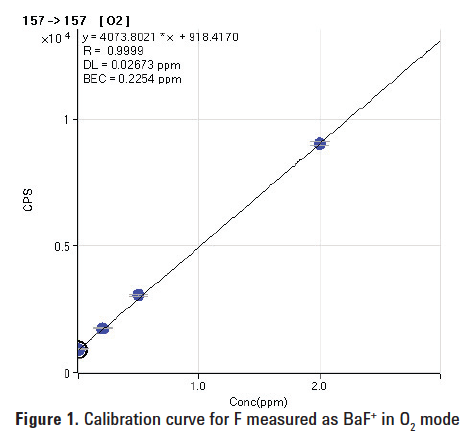
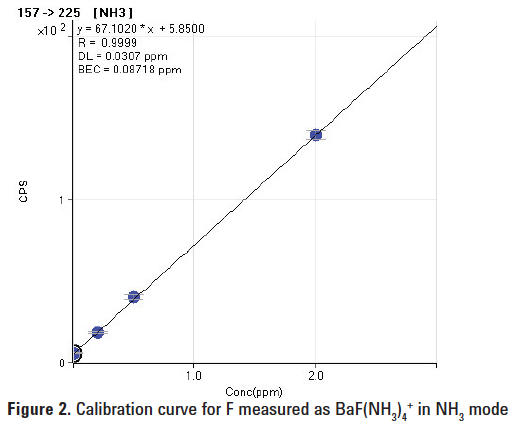
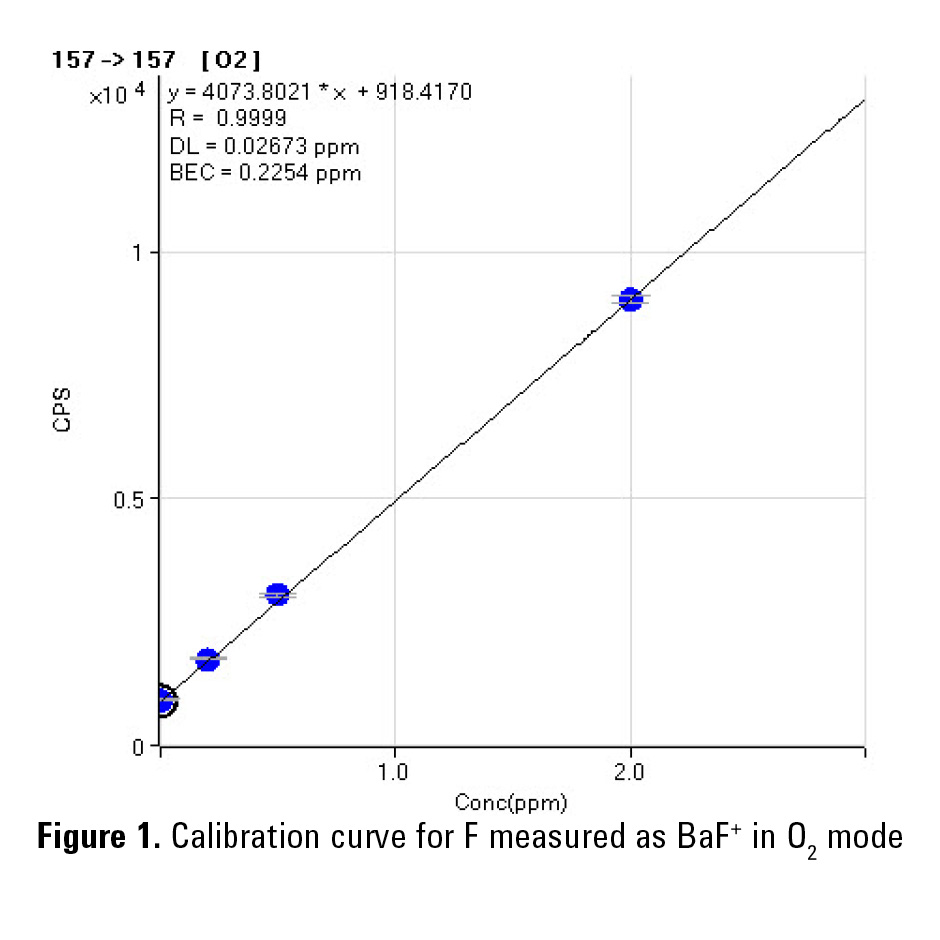
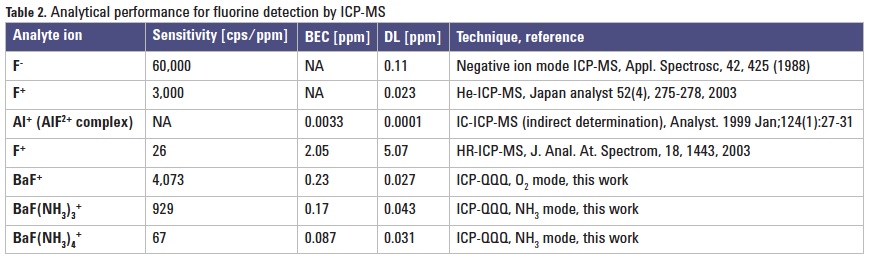
Figures 1 and 2 show calibration curves up to 2 mg/L (ppm) for fluorine in deionized water. The lowest detection limit (27 ppb) was obtained in O2 mode. The lowest BEC (87 ppb) was obtained by measuring BaF(NH3)4+ in NH3 mode. Table 2 shows the BEC and DL results for F obtained from this study in comparison with the literature values.
Conclusions
Based on this preliminary study, it is clear that the controlled reaction chemistry that is possible with MS/MS mode on the 8800 ICP-QQQ can provide a novel approach to the measurement of F by ICP-MS. In addition to demonstrating detection limits that are comparable with published data measured using conventional quadrupole ICP-MS or high-resolution ICP-MS, the 8800 ICP-QQQ also allows unprecedented flexibility to monitor specific reaction transitions, making it invaluable for method development.