Removal of MH+ interferences in refined REE material analysis



Naoki Sugiyama
Agilent Technologies, Japan
Keywords
Rare Earth Elements, REE, geochemistry, mining, material science, lanthanum, barium, cerium, method of standard additions, MSA, oxygen mass-shift
Introduction
The measurement of Rare Earth Elements (REEs) is of great importance in geochemistry, mining and material science. Manufacturers of high purity REE materials need to quantify metal impurities, including trace levels of the other REEs, in the refined, single element REE matrix. ICP-MS is the technique of choice for the measurement of REEs, but most of the REE isotopes suffer from interference by polyatomic species (predominantly hydride ions, MH+ and oxide ions, MO+) derived from other, lower-mass REE elements. While MH+ interferences are lower in intensity than MO+ interferences, they present a more challenging problem for REEs that have no isotope free from interference. For example 139La+ is interfered by 138BaH+ and 140Ce+ by 139LaH+. These interferences are too close in mass to be resolved by high-resolution (HR-)ICP-MS [1]. In this paper, we describe the removal of the MH+ interferences using an Agilent 8800 ICP-QQQ in MS/MS mass-shift mode, with oxygen as the reaction gas.
Experimental
Instrumentation: Agilent 8800 #100. The standard glass nebulizer was replaced with a C-flow nebulizer (G3285-80000) for optimal washout between the high matrix samples.
Plasma conditions: Preset plasma/General purpose.
Ion lens tune: Soft extraction tune:
Extract 1 = 0 V, Extract 2 = -180 V.
CRC conditions: O2 gas at 0.3 mL/min,
Octopole bias = -5 V, KED = -5 V.
Acquisition parameters: MS/MS mode with O2 mass-shift method.
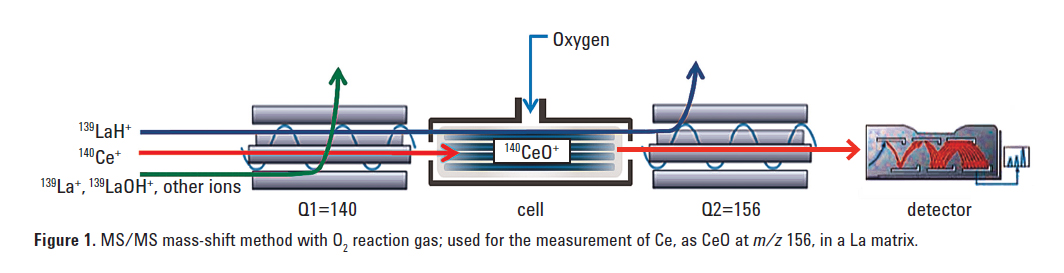
Figure 1 illustrates the mechanism of MS/MS O2 mass-shift mode used for measuring Ce in a La matrix sample. The major isotope of Ce at m/z 140 suffers an interference from 139LaH+. Q1 is set to
m/z 140, allowing only the analyte ion 140Ce+ and any other ions at m/z 140 to pass through to the cell. All other ions not at m/z 140 are rejected. In the cell, Ce reacts with oxygen to form CeO+ at m/z 156. Q2 is set to m/z 156, allowing CeO+ to pass to the detector. Since 139LaH+ does not react with O2 to form 139LaOH+, it remains as LaH+ at m/z 140 and is rejected by Q2. The same principle is used for the separation of 139La+ from 138BaH+ in a Ba matrix.
Results and discussion
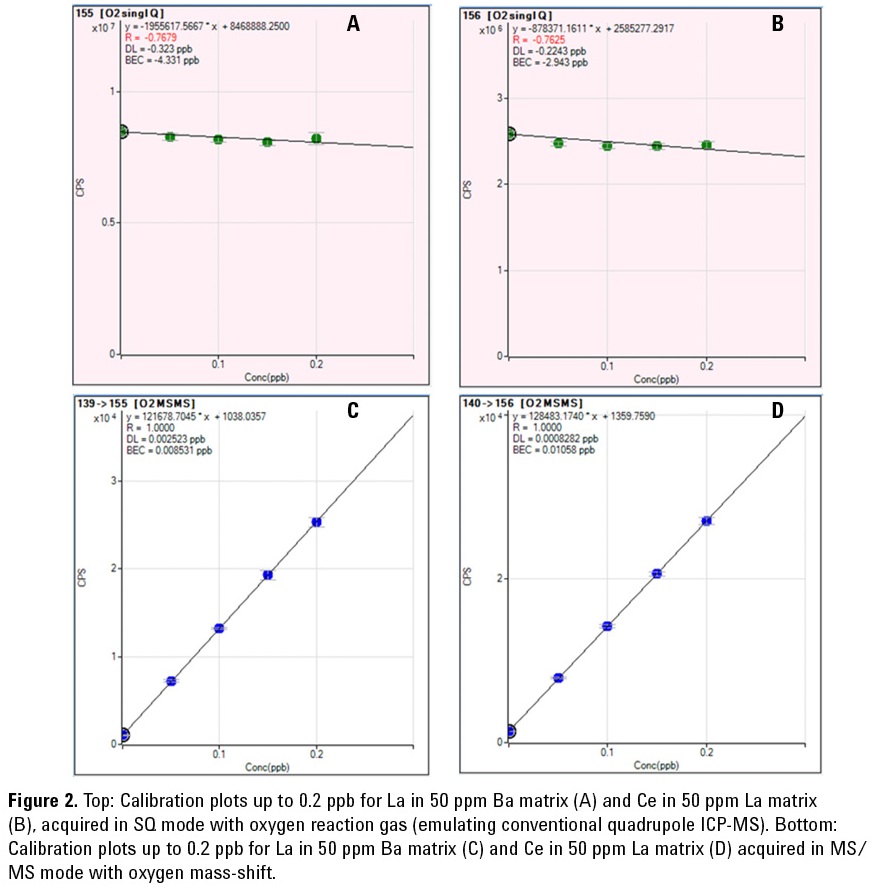
Using the Method of Standard Addition (MSA), the BECs and DLs of La in a matrix of 50 ppm Ba, and Ce in a matrix of
50 ppm La were determined. Data was acquired using MS/MS mode with O2
mass-shift, and also using Single Quad (SQ) mode with O2 reaction gas to emulate conventional quadrupole ICP-MS
(ICP-QMS) for comparison.
As shown in Figures 2A and 2B, SQ mode with O2 reaction gas suffers from interferences that prevent the measurement of La in the Ba matrix and Ce in the La matrix, respectively. In contrast, the calibration plots shown in Figures 2C and 2D demonstrate that MS/ MS mode with O2 mass-shift can successfully remove the matrix overlaps to permit the trace quantitation of La in a Ba matrix and Ce in a La matrix. The BECs and DLs achieved were 8.5 ppt and 2.5 ppt respectively for La in a 50 ppm Ba solution, and 10.6 ppt and 0.2 ppt respectively for Ce in a 50 ppm La solution.
Investigation of unexpected product ion observed at m/z 156 in the 50 ppm La matrix
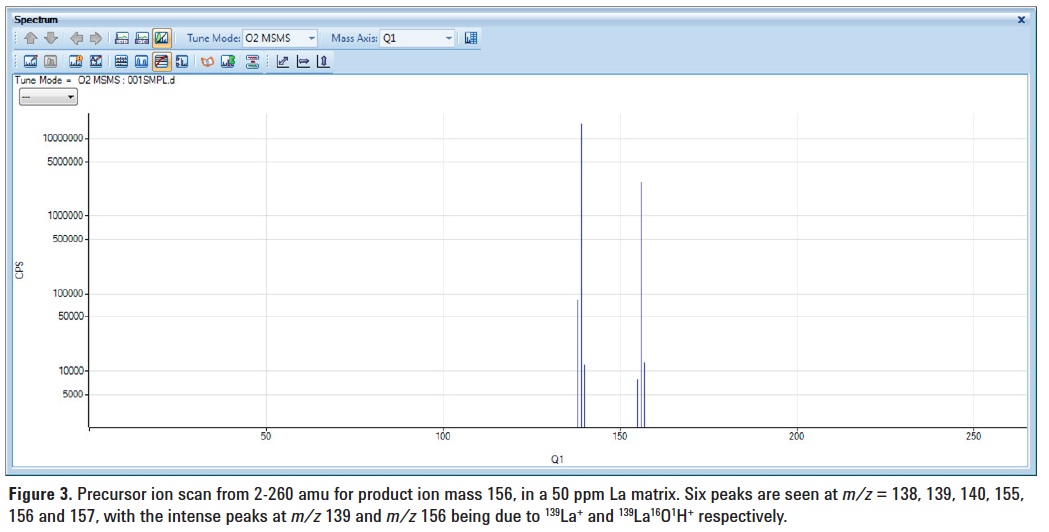
The background signals that contributed to the poor result obtained for Ce in the La matrix using SQ mode with O2 reaction gas (Figure 2B) were investigated by carrying out a precursor ion scan for product ion mass 156. The precursor ion scan capability of the 8800 ICP-QQQ provides a uniquely powerful approach to identifying the source of potential polyatomic and reaction product interferences. Oxygen cell gas was introduced into the cell and a precursor ion spectrum was obtained by scanning Q1 from 2 to 260 amu (Figure 3) with Q2 fixed at mass 156. From the spectrum, we can identify which precursor ions react with O2 to produce product ions at mass 156, overlapping 140CeO+ in SQ mode.
Figure 3 shows the precursor ion scan spectrum for product ion mass 156 for the 50 ppm La matrix, with intense peaks at
m/z 139 (139La+) and 156 (139La16OH+). In SQ mode, as with conventional ICP‑QMS, these ions all enter the cell, and with Q2 set to 156 amu, the 139La16OH+ polyatomic ions contribute to the signal measured at m/z 156 (140Ce measured as analyte product ion 140CeO+). These unwanted precursor ions cannot be rejected by a CRC operating as a bandpass filter in ICP-QMS, as they are too close in mass to the target analyte precursor ion. Only by using MS/MS mode on the 8800 ICP-QQQ, where Q1 operates as a unit mass filter, can non-target masses (like 139La16OH+ in this example) be prevented from entering the cell.
Reference
- Sabine Becker and Hans Joachim Dietze, Journal of Analytical Atomic Spectrometry, 1997, vol.12, p881.
More information
Removal of hydride ion interferences (MH+) on Rare Earth Elements using the Agilent 8800 Triple Quadrupole ICP-MS, Agilent application note, 5991-1481EN.