Arsenic measurement in cobalt matrix using MS/MS mode with oxygen mass-shift


Katsuo Mizobuchi
Agilent Technologies, Japan
Keywords
arsenic, high purity metals, cobalt, zirconium, oxygen mass-shift
Introduction
Measuring the purity of materials such as high purity metals is of interest across advanced technology industries, to support the development of new materials and/or improve the performance of existing products. ICP-MS is widely used for determining elemental impurities in these materials due to its unique features: High sensitivity, low DLs, multi element analysis capability, wide dynamic range, fast analysis and minimal sample preparation requirements.
For many applications, the errors caused by spectral interferences in quadrupole ICP-MS have been adequately addressed by the introduction of CRC technology. However, the analysis of trace contaminants in high purity materials presents a particular challenge due to the high matrix levels and the need to determine impurities at the trace level. For example, the determination of As in Co is difficult for quadrupole ICP-MS due to the signal from CoO+ that overlaps the only isotope of arsenic at m/z 75. Although only about 0.01% of the Co ions in the plasma are present as CoO+ ions, the Co concentration in a 1000 ppm solution is 6 or 7 orders of magnitude higher than the trace levels of As that are of interest in this application. Consequently the CoO+ interference is still very significant relative to the As+ signal. This note describes the measurement of trace As in a 1000 ppm Co solution using an Agilent 8800 ICP‑QQQ in MS/MS mass-shift mode, using oxygen as the reaction gas.
Experimental
Instrumentation: Agilent 8800 #100.
Plasma conditions: Preset plasma/HMI-mid.
CRC conditions: O2 gas at 0.3 mL/min,
Octopole bias = -5 V, KED = -7 V.
Acquisition conditions: Three oxygen (O2) mass-shift operational modes were compared:
- Single Quad mode A with low mass cut off at m/z < 59
- Single Quad mode B with low mass cut off at m/z ≒ 59
- MS/MS mode with Q1 as an amu filter, Q1 = 75 and Q2 = 91
Sample: SPEX CLCO2-2Y
(SPEX CertiPrep Ltd., UK) was used as
1000 ppm Co solution.
Results and discussion
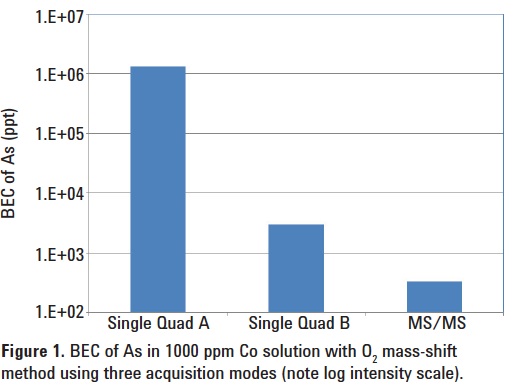
BEC of As in 1000 ppm Co solution using O2 mass-shift method
From the equation and reaction enthalpy below, it can be seen that arsenic reacts readily with O2 cell gas via an O-atom transfer reaction. This creates the reaction product ion AsO+ at m/z 91, moving the analyte away from the CoO+ interference on As+ at m/z 75.
As+ + O2 → AsO+ + O ΔHr = -1.21 eV
The reaction enthalpy for CoO+ with the O2 cell gas is much less favorable, so the overlap from the CoO+ polyatomic interference is successfully avoided. To evaluate the effectiveness of
MS/MS mode for this application, the 8800 ICP-QQQ was operated in three acquisition modes: MS/MS mode and two “Single Quad” modes, in which Q1 functions as a bandpass filter rather than a unit (1 amu) mass filter. In Single Quad mode A, Q1 was set to allow most of the plasma-formed ions to enter the cell; in Single Quad mode B, Q1 was set with a low mass cutoff around m/z 59 to allow only ions with a mass greater than 59 to enter the cell (most 59Co+ ions are rejected); and finally in MS/MS mode Q1 was set to allow only ions at m/z 75 to enter the cell (all 59Co+ rejected).
The BECs for As obtained using the three acquisition modes are shown in Figure 1. MS/MS mode achieved the lowest BEC for As of 330 ppt in 10000 ppm Co. The BEC obtained by the Single Quad modes were orders of magnitude higher, which suggests the occurrence of the following undesired reactions in the cell and indicates the incomplete rejection of Co+ by Single Quad mode B:
59Co+ + O2 → 59CoO+ + O;
59CoO+ + O2 →59CoO2+ + O
Note that this sequential reaction chemistry leads to a relatively intense signal for CoO2+, because the number of precursor ions for the reaction (the Co+ ions from the plasma) is so high (10000 times higher intensity than the CoO+ signal in the plasma). Consequently, in Single Quad mode, the CoO+ overlap cannot be successfully avoided by moving the As+ to its AsO+ product ion at m/z 91 using O2 cell gas, because CoO2+ (also at m/z 91) is formed relatively easily when a large number of Co+ ions are allowed to enter the cell.
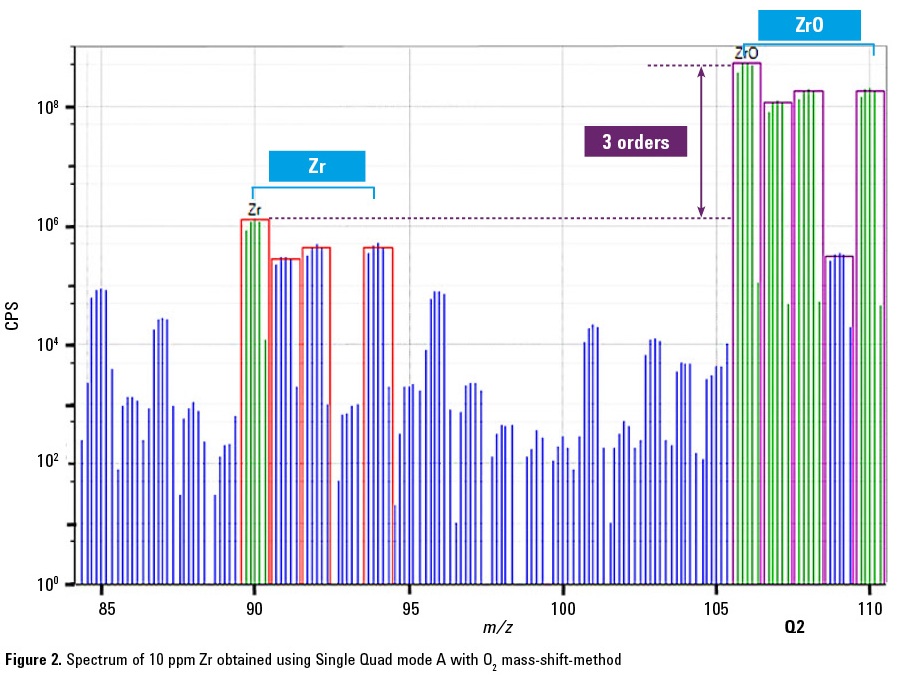
AsO+ in the presence of zirconium
To successfully avoid interferences using the mass-shift method, the mass of the analyte product ion must itself be free from interference. For example, in this application the AsO+ product ion is measured at m/z 91 where it could be overlapped by an isotope of zirconium (91Zr+). The presence of Zr in a sample may therefore cause an error in the results for As measured as AsO+ using O2 reaction mode on ICP-QMS. The potential effect of co-existing Zr on AsO+ measurement using ICP-QMS was investigated.
Figure 2 is a spectrum of 10 ppm Zr obtained using Single Quad mode A with O2 mass-shift. Zr reacts with O2 very efficiently (ΔHr = -3.84) and is converted to ZrO+. However not all the Zr+ ions are converted to ZrO+ so some Zr+ remains, interfering with the measurement of AsO+ at m/z 91. In contrast, in MS/MS mode the 91Zr+ ion is rejected by Q1, so the potential overlap on the AsO+ product ion at m/z 91 is removed.
Conclusions
Trace levels of arsenic in a 1000 ppm cobalt matrix can be successfully measured (BEC of 330 ppt) using the 8800 ICP-QQQ operating in MS/MS mass-shift mode, with oxygen as the reaction gas. There are two main advantages of using MS/MS compared to ICP-QMS:
- In MS/MS mode, Co+ is prevented from entering the cell by Q1, which is set to m/z 75. In ICP-QMS, CoO2+ is formed in the cell via a chain reaction, and will interfere with AsO+ at m/z 91.
- In MS/MS mode, the potential 91Zr+ overlap on the AsO+ product ion at m/z 91 is eliminated, as 91Zr+ ions (and all other ions apart from m/z 75) are rejected by Q1.