Ultra trace copper analysis in a semiconductor grade organometallic titanium complex



Akio Hashizume, Toshiya Shingen, ADEKA Corporation, Japan
Katsuo Mizobuchi, Agilent Technologies, Japan
Keywords
semiconductor, organometallic, copper, titanium, ammonia mass-shift
Introduction
Most quadrupole ICP-MS (ICP-QMS) instruments use CRC technology to resolve spectroscopic interferences. Helium collision mode is widely accepted due to its versatility and ease of use for multi-element analysis of complex and variable samples. While the performance achievable with He mode meets the requirements for most applications, there are some applications, for example impurity analysis of semiconductor materials, that require improved interference removal capability. For these applications, a reactive cell gas (reaction mode) may be used, but the use of highly reactive cell gases in quadrupole ICP-MS is prone to unexpected interferences and overlaps, especially when the matrix is complex, or other analytes are present at varying concentrations. The new Agilent 8800 Triple Quadrupole ICP-MS (ICP‑QQQ) eliminates the variability associated with reactive cell gases in ICP-QMS, by using the first quadrupole (Q1) to control the ions that enter the CRC. This ensures that the reactions are predictable and the product ion spectrum is simple and consistent.
This report describes the measurement of trace Cu in a semiconductor grade organometallic Ti complex used in advanced semiconductor processing. It is a challenging application for quadrupole ICP-MS since both isotopes of copper, 63Cu and 65Cu, suffer interference from TiO and TiOH ions, and the use of reactive cell gases to avoid the overlap leads to a very complex product ion spectrum, particularly for organic samples. We demonstrate that the Agilent 8800 ICP-QQQ, operating in MS/MS mass-shift mode using ammonia as a reaction gas, was able to separate Cu+ from the Ti-based interferences and measure Cu at low ppt levels in a matrix of 500 ppm Ti. Results were also acquired using MS/MS He collision mode, for comparison.
Experimental
Instrumentation: Agilent 8800 #200 with narrow injector (id = 1.5 mm) torch
(G3280-80080) used for organic solvent analysis. A low flow PFA nebulizer
(G3285-80002) was used in self-aspiration mode. An option gas flow of 20% O2 balanced in Ar was added to the carrier gas via the standard option-gas line to prevent carbon build up on the interface cones.
Operating conditions: Table 1 summarizes plasma, ion lens and cell tuning conditions.
Acquisition conditions: MS/MS mode was used; cell gas was either NH3 or He.
Sample and sample preparation: Semiconductor grade organometallic
Ti complex (ADEKA Corp., Japan) was diluted with high purity IPA (Tokuyama Corp., Japan) to 500 ppm Ti solution. A spiked standard was prepared from the multi-element standard, xstc-331, purchased from SPEX CertiPrep Ltd. (UK).
Results and discussion
He collision mode
The He cell gas flow rate was optimized for the lowest BEC of Cu in a 500 ppm Ti solution. As the BEC for 63Cu was lower than the BEC for 65Cu due to the higher abundance of the 63 isotope, and the more significant interference from TiO+ at m/z 65, Cu was determined on-mass at m/z 63. In MS/MS mode, this is achieved by the acquisition conditions:
Q1 = 63; Q2 = 63 (63, 63).
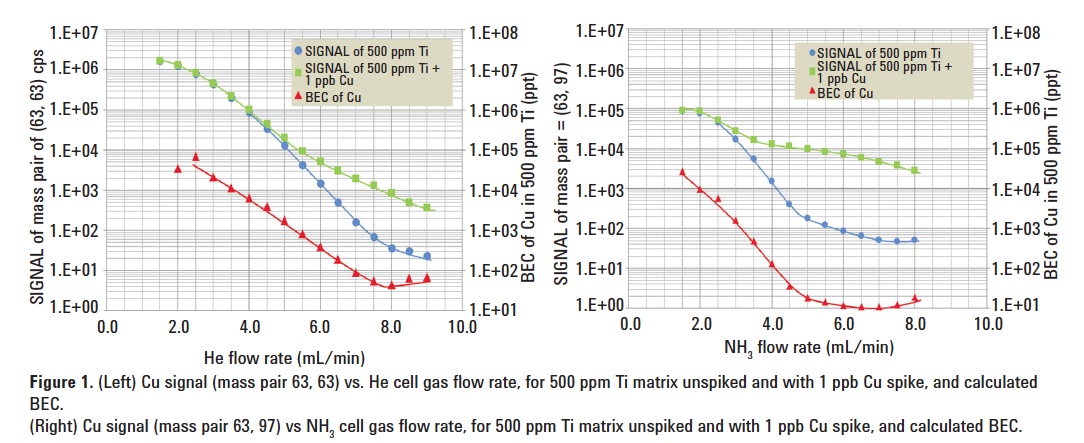
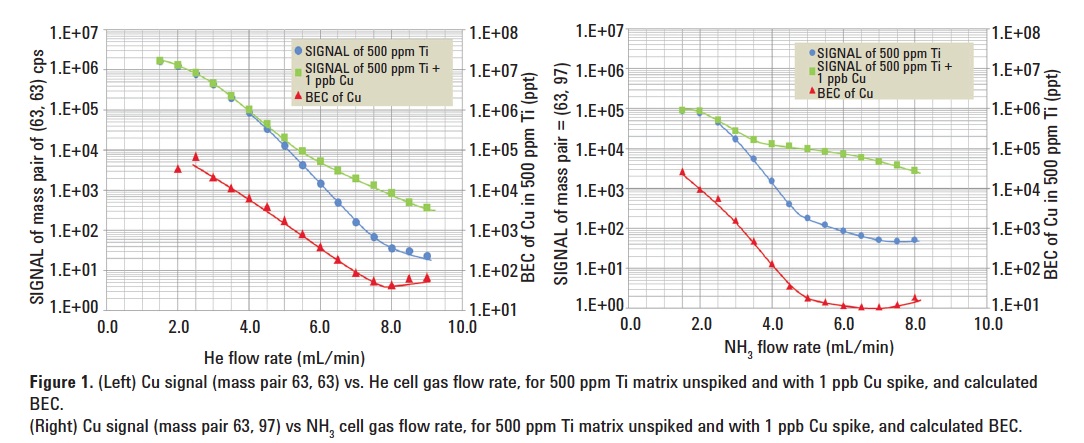
Two solutions were analyzed: 500 ppm Ti solution and 500 ppm Ti + 1 ppb Cu spike. Figure 1 (left) shows the signal at m/z 63 obtained from the analysis of the two solutions, plotted as a function of He flow rate. The BEC calculated from these signals is also given in the figure. It shows that the lowest Cu BEC in He mode was 46 ppt, achieved at a flow rate of 8.0 mL/min He.
NH3 reaction cell mode
Cu+ reacts efficiently with NH3 to form NH3 cluster ions with the general form Cu(NH3)n+. TiO+ does not follow the same reaction pathway as Cu+, so the Cu product ion can be measured free from Ti overlap. Based on a preliminary study, one of the intense product ions,
Cu (NH3)2+, was selected to measure Cu separated from the original TiO+ interference. A mass pair of Q1 = 63, Q2 = 97 was used with NH3 as the reaction gas. Figure 1 (right) shows the result. A BEC of 11 ppt for Cu in 500 ppm Ti solution was achieved in NH3 mode (10% NH3/He mixed gas), at a flow rate of 6.5 mL/min NH3.
Conclusions
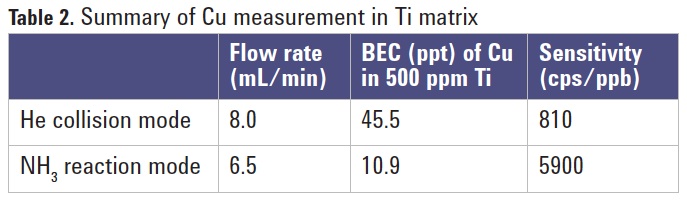
Table 2 summarizes the analytical performance achieved by the
8800 ICP-QQQ operating in MS/MS mode with He collision and NH3 reaction gas. As can be seen, NH3 reaction mode is more effective than He collision mode for the removal of the TiO+ interference on Cu. The BEC obtained for Cu in a Ti matrix by NH3 reaction mode is four times lower than He mode, with seven times higher sensitivity.