Measurement of titanium in clinical samples:
Possible application to monitoring patients with joint replacements



Glenn Woods
Agilent Technologies (UK) Ltd.
Keywords
titanium, biological, serum, urine, joint-replacement, Seronorm, ammonia mass-shift
Introduction
Although titanium (Ti) has little or no direct biological role, it is widely used in dental, artificial/replacement joints and surgical reconstruction applications due to its high strength, light weight and the fact it is biocompatible. It is also used extensively as a pigment and abrasive polishing agent
(as TiO2) and is often found in foods and toothpaste due to its inert nature (this form is usually passed unaltered in faecal matter and is not normally transported or expressed through body fluids).
More recent applications with certain types of metal-on-metal (rather than ceramic or polymer based) joint replacements can lead to the release of wear metal particles or ions within the body of the patient. These can become highly concentrated in the synovial fluid (lubricating fluid of the joint), pass into the bloodstream and be expressed through urine. Unusually “high” concentrations of Ti can indicate a premature failure of a Ti-based joint and such failure can lead to infection or constant pain for the patient. It is therefore important to reliably determine the concentration of Ti within biological fluids at normal endogenous levels in order to obtain a basal concentration. An increase from this concentration could indicate an imminent failure of the joint.
Experimental
The determination of Ti in biological matrices is challenging for conventional ICP-MS, due to its low natural concentration and the presence of spectral interferences on all the Ti isotopes e.g. sulfur (as SO), P (as PO) and Ca. It is possible to use reaction chemistry with NH3 cell gas in the CRC of a quadrupole ICP-MS (ICP-QMS), to mass-shift the Ti+ to a higher mass product ion, leaving the interfering species behind. However, the use of highly reactive cell gases in ICP-QMS is prone to severe errors, as there is no way to control the ions that enter the CRC. This means that the reaction chemistry and the product ions created can change dramatically, with even slight differences in sample matrix or co-existing analyte concentrations. For this application, the 8800 ICP-QQQ was used to provide controlled reaction chemistry with ammonia as the reaction gas and measuring Ti as the TiNH2(NH3)4+ cluster ion at the M + 84 amu transition.
Instrumentation: Agilent 8800 #100.
Plasma conditions: Preset plasma/General purpose.
Ion lens tune: Soft extraction tune:
Extract 1 = 0 V, Extract 2 = -170 V.
CRC conditions:
NH3 gas (10% in He) at 1.7 mL/min,
Octopole bias = -8 V, KED = -8 V.
Samples and sample preparation: Certified reference materials of human serum and urine were purchased from Seronorm (Norway). They were prepared in duplicate by 10x dilution into a basic diluent consisting of NH4OH (0.5%),
H4-EDTA (0.01%), BuOH (2%) &
Triton X-100 (0.01%) in ultrapure water. No further matrix matching was applied for the standards.
Results and discussion
Selection of product ion for Ti measurement
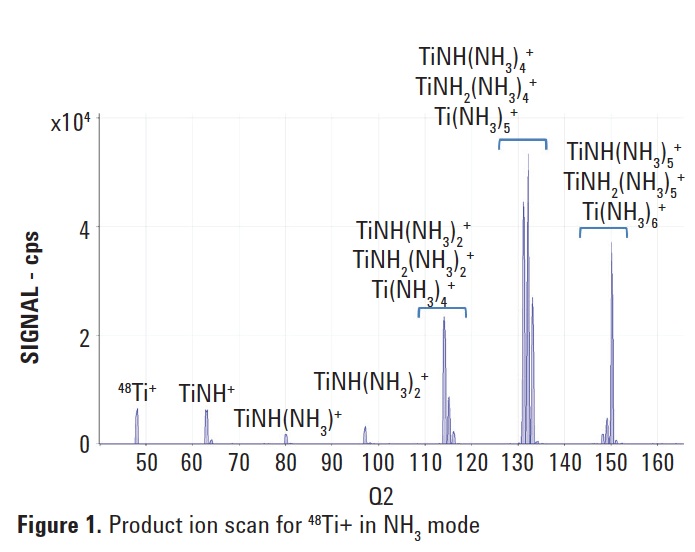
In order to select the most appropriate
Ti cluster ions in NH3 mode, a product ion scan was performed for the 48Ti isotope by introducing a 10 ppb Ti solution (Figure 1). Q1 was set to m/z 48, allowing only ions at the mass of the target precursor ion to enter the cell; Q2 was scanned over a selected mass range to measure all the product ions formed in the cell by NH3 reactions with 48Ti. Based upon this scan, the two most abundant cluster ions
(Q1 + 84 amu [TiNH2(NH3)4] and
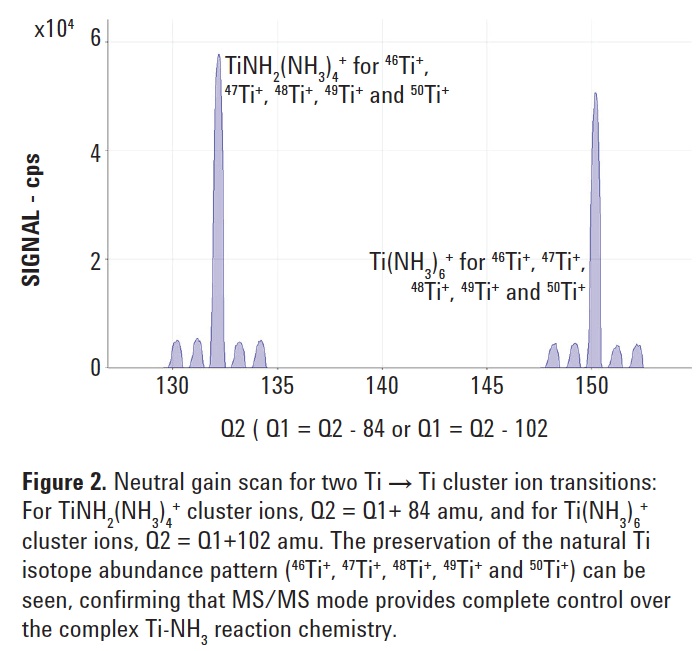
Q1 + 102 amu [Ti(NH3)6]) were selected for further study. For each of the two reaction transitions identified above, neutral gain scans (where Q1 and Q2 are scanned synchronously, with a set mass difference between them
(Q2 = Q1 + 84 and Q2 = Q1 + 102 in this case)) were performed. These scans are shown in Figure 2 confirming the correct natural isotopic abundances for the different Ti isotopes. Without MS/ MS capability, it would be impossible to preserve the isotopic information for this element due to the relatively complex nature of the Ti-ammonia adducts. The instrument cell conditions were optimized using simple HNO3 acidified Ti standards and applied to the analysis of the CRMs.
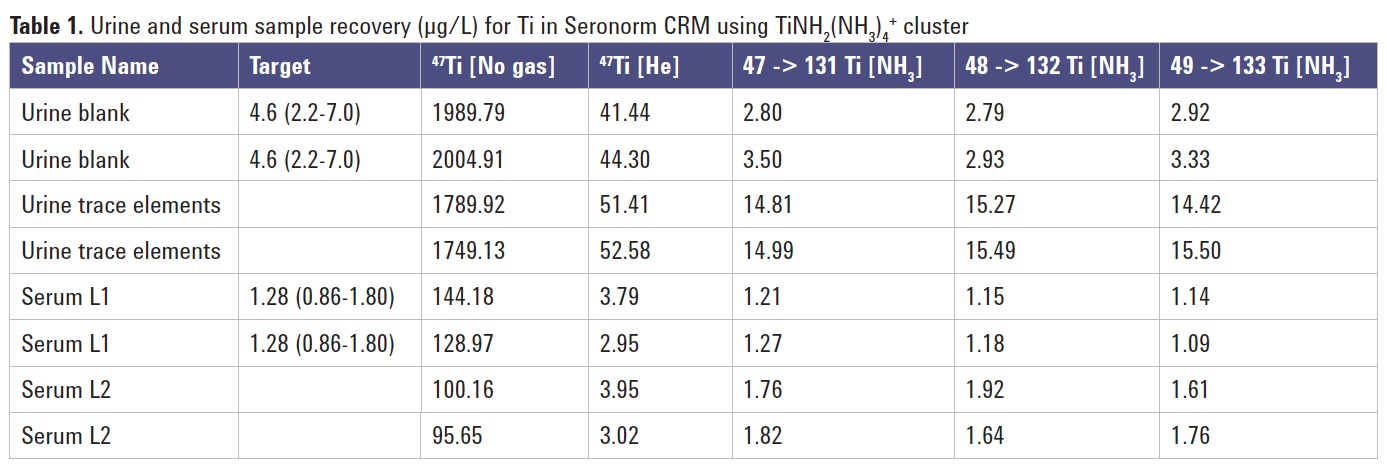
Table 1 displays the results for both serum and urine sample types measured against a single calibration. The 8800 ICP-QQQ was operated also under no gas and He mode to provide comparative data, and three Ti isotopes were monitored for the same cluster ion transition, to give confirmation of the results.
Conclusions
Titanium was only certified in two of the four materials measured but the 8800 ICP-QQQ data were all comfortably within the measured ranges when operating under ammonia MS/MS mode, in contrast to no gas and He mode data. Importantly, the three Ti isotopes measured under ammonia MS/MS mode all gave equivalent data; this could indicate applicability of the method to the use of isotope-based analysis such as isotope dilution (ID) or isotope tracer analysis. The use of ammonia combined with MS/MS greatly simplifies the analysis of Ti in biological media for several isotopes. Furthermore, because MS/MS mode provides control over the reaction chemistry, no special attention needs to be paid to specific matrix matching regardless of the fluid investigated.