DETERMINATION OF Ti, V AND Cr IN 9.8% SULURIC ACID




Junichi Takahashi
Agilent Technologies, Japan
Keywords
semiconductor, process chemicals, sulfuric acid, H2SO4, titanium, vanadium, chromium, ammonia mass-shift, oxygen mass-shift
Introduction
High purity H2SO4 is frequently used in the manufacturing of semiconductor devices, in processes such as the removal of organic substances from the surface of silicon wafers. The required metallic impurity level is lower than 100 ppt in the concentrated (usually 98%) acid. ICP-MS is the technique of choice for the measurement of trace metal impurities in semiconductor process chemicals. There are, however, some limitations for the measurement of elements such as Ti, V and Cr in H2SO4. Because of its high viscosity of 27 cP, it is not possible to introduce H2SO4 directly into the ICP without dilution. A 10 times dilution in UPW is normally applied, thus the BEC of the calibration curve must be lower than 10 ppt in the 9.8% H2SO4 solution measured. In addition, spectral interferences from SO+, S2+ and ArS+ originating from H2SO4 make it difficult to determine elements such as Ti and Cr at low concentration even by quadrupole ICP-MS (ICP-QMS) equipped with collision/reaction cell (CRC). As outlined in this report, the Agilent 8800 ICP-QQQ with MS/MS mode allows the successful determination of the most problematic elements including Ti, V and Cr in H2SO4.
Experimental
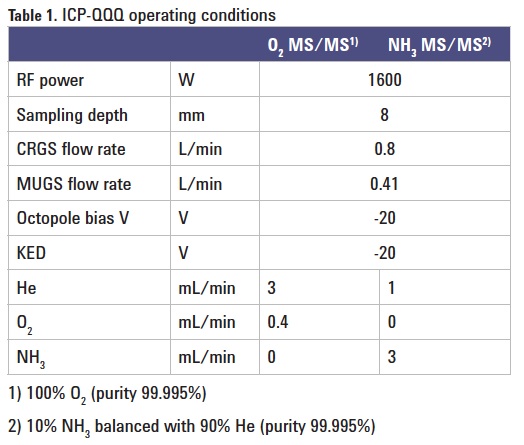
Instrumentation: Agilent 8800 #200. Operating parameters are given in Table 1.
Reagents and sample preparation: Highly purified H2SO4, TAMAPURE-AA-100 (98% H2SO4) was purchased from Tama Chemicals Co., Ltd. (Kanagawa, Japan). 5g of H2SO4 was diluted by a factor of 10 in a chilled PFA bottle.
Results and discussion
Of the potential polyatomic interferences formed from the H2SO4 matrix, the SO+ ion is very stable and difficult to eliminate because its dissociation energy is as high as 5.44 eV. In addition, its ionization potential is 10.3 eV, which is almost the same as that of S, 10.36 eV. The spectral interferences caused by SO+ and
SOH+ overlap with 48Ti (32S16O),
51V (33S18O, 34S16OH and 32S18OH) and
52Cr (34S18O). Quadrupole ICP-MS operating in He collision mode provides BECs of 60 ppt for 47Ti (the BEC for the preferred isotope 48Ti is much higher), 3 ppt for V and 8 ppt for Cr in 9.8% H2SO4. The BEC of Ti, in particular, is not acceptable for producers and users of semiconductor grade H2SO4.
Appropriate reaction gases to remove SO+ successfully in ICP-QMS are difficult to find. NH3 can reduce SO+ by two orders of magnitude but the background signal remains too high for this application. Additionally, cluster ions of NH3 such as NmHn produced by the reaction between Ar+ and the NH3 cell gas lead to new reaction product ion interferences that increase the background at m/z 51, for example.
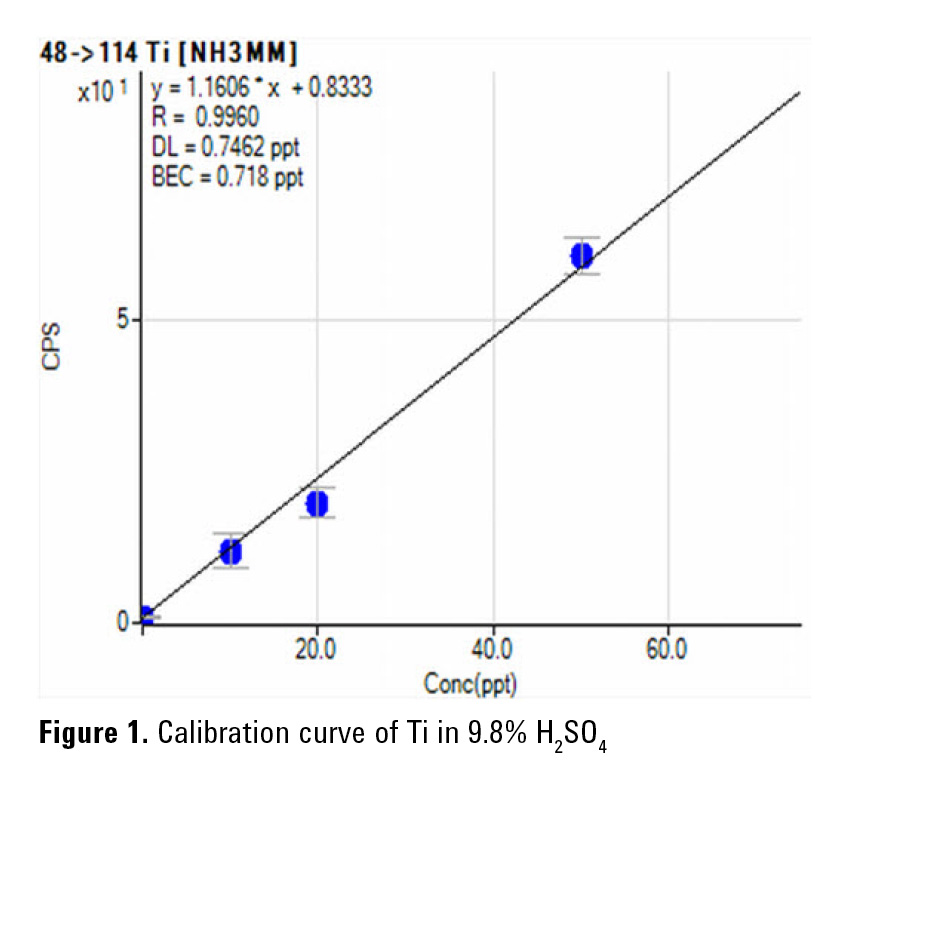
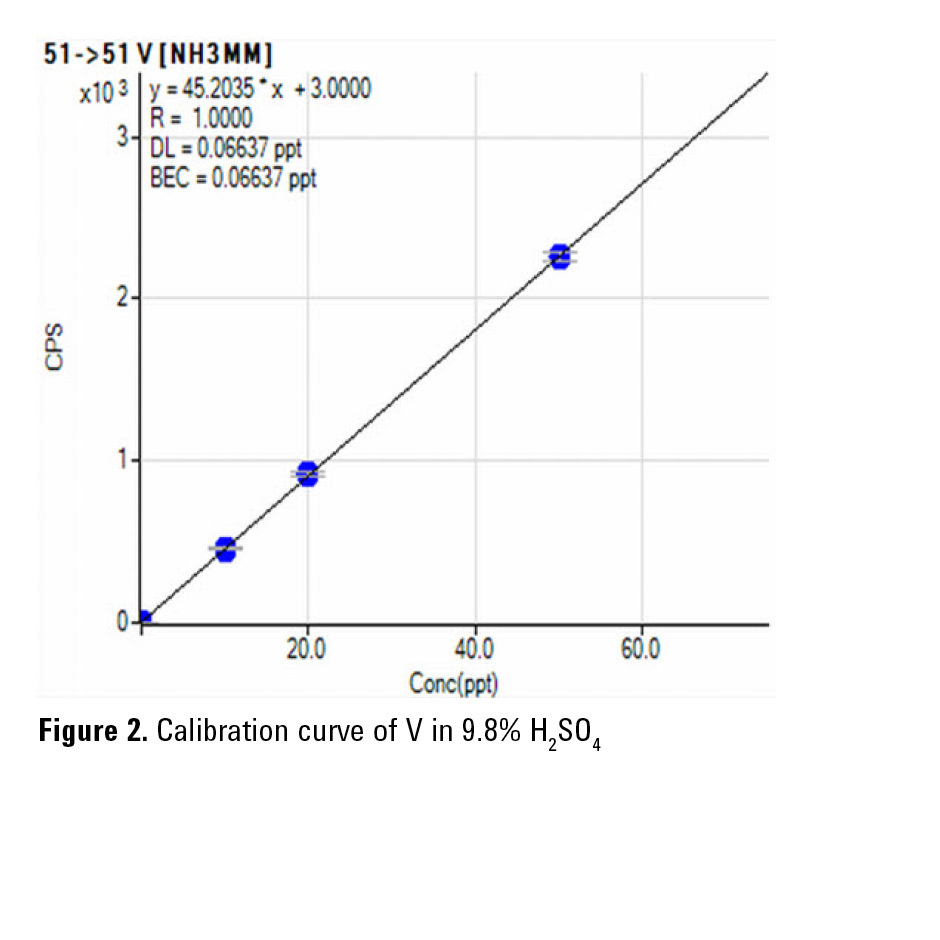
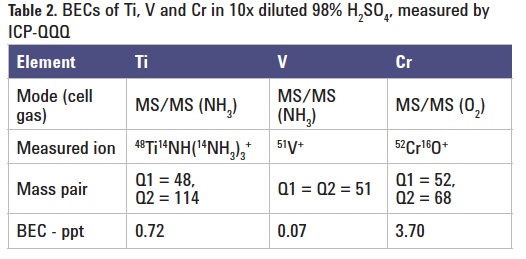
The 8800 ICP-QQQ operating in MS/MS mass-shift mode with NH3 or O2 reaction gas provides reliable and consistent measurement of Ti as 48Ti14NH(14NH3)3 (Figure 1) and Cr as 52Cr16O in H2SO4. Furthermore, in MS/MS mode, the Ar+ ion is removed by Q1, preventing it from reacting with NH3 to form new product ion interferences in the cell. This reduces the background at m/z 51 improving the BEC for V, as shown in Figure 2. The final BECs obtained by ICP-QQQ in 9.8% high purity H2SO4 are summarized in Table 2.
Conclusions
ICP-QQQ operating in MS/MS mode provides a reliable means for manufacturers of high purity H2SO4 to guarantee all metallic impurity concentrations at less than 100 ppt in the concentrated acid.
More Information
Determination of challenging elements in ultrapure semiconductor grade sulfuric acid by Triple Quadrupole ICP-MS, Agilent application note, 5991-2819EN.