DIRECT DETERMINATION OF V, Cr, Ge AND As IN HIGH-PURITY 20% HYDROCHOLORIC ACID





Junichi Takahashi
Agilent Technologies, Japan
Keywords
semiconductor, RCA Standard Clean, silicon wafer, hydrochloric acid, vanadium, chromium, germanium, arsenic, ammonia on-mass, ammonia mass-shift, oxygen mass-shift
Introduction
Since the 1970s, the RCA Standard Clean (SC) method has been used extensively in many countries for cleaning silicon wafer surfaces. SC-2 refers to a mixture of HCl and H2O2 that is used to remove ionic and metallic contaminants from the surface of silicon wafers. Because cleaning solutions are in direct contact with semiconductor devices, ultra high purity is required for these solutions. The SEMI standard Tier-D protocol for HCl defines the contaminant level to be
Experimental
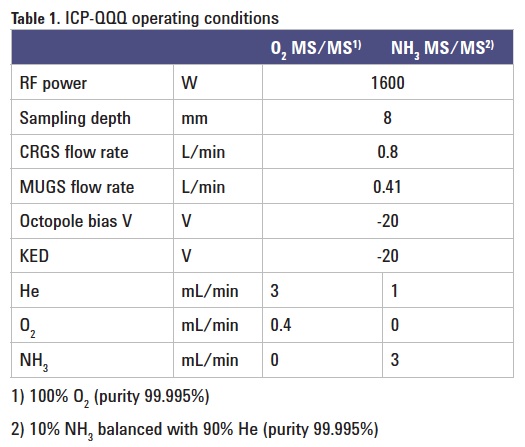
Instrumentation: Agilent 8800 #200. Operating parameters are given in Table 1.
Reagents: 20% TAMAPURE-AA-100 HCl (metallic impurities are guaranteed to be below 100 ppt) was purchased from Tama Chemicals Co., Ltd. (Kanagawa, Japan). The undiluted HCl was introduced directly into the ICP-QQQ.
Results and discussion
Determination of BECs of V, Cr, Ge and As in high purity HCl
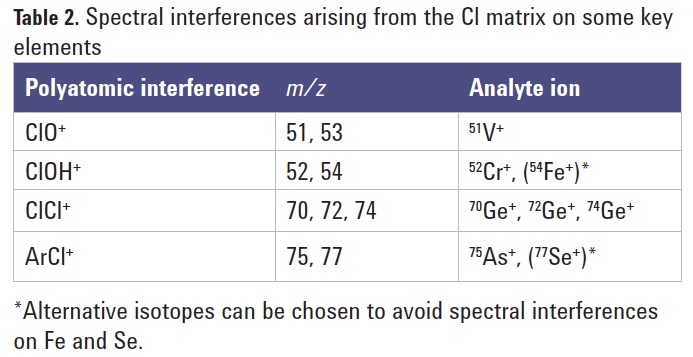
ICP-QMS with a CRC using He collision mode can successfully eliminate some polyatomic ions such as ArCl [1], and the use of NH3 as a reaction gas also works to remove the ClO+ ion for the determination of V. However, ICP-QMS has some serious limitations when highly reactive cell gases (such as NH3) are used in the CRC. Principal among these limitations is the fact that all ions enter the CRC, so predicted reaction pathways can be disrupted and new reaction product ion overlaps can be formed if the analyte levels in the sample change. ICP-QQQ with MS/MS removes this limitation, as the first quadrupole mass filter (Q1) allows precise selection of the ions that are allowed to enter the cell. This ensures that reaction processes and product ions are strictly controlled, dramatically improving detectability of the analyte ions shown in Table 2.
The MS/MS acquisition mode using O2 or NH3 as the reaction gas enables the determination of trace 51V (measured directly as V+ using NH3 cell gas),
Cr as 52Cr16O+ (using O2),
Ge as 74Ge14NH2 + (using NH3) and
As as 75As16O+ (using O2). In the case of As, the 91Zr+ ion is removed by Q1 (which is set to the As+ precursor ion mass of
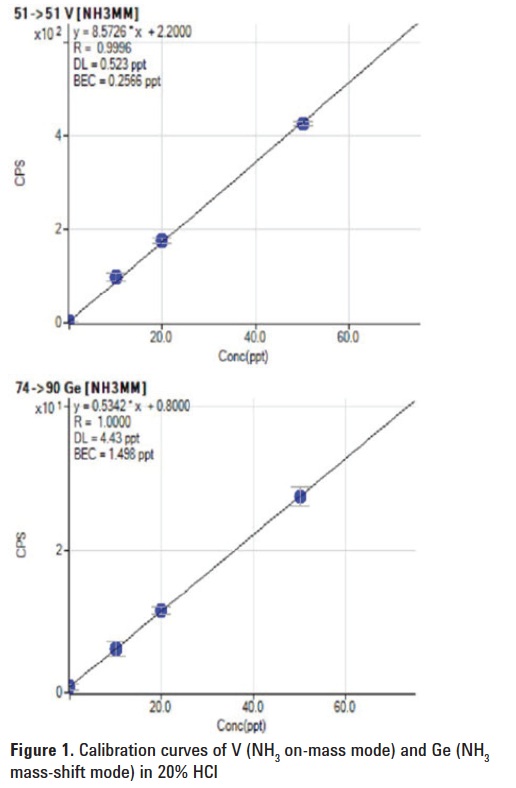
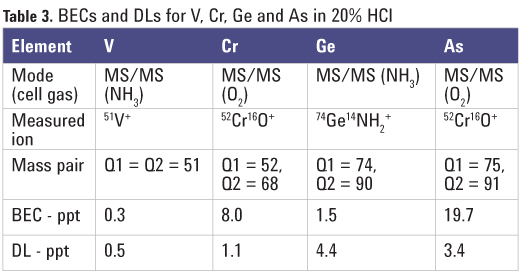
m/z 75), so the potential overlap from Zr on the AsO+ product ion at m/z 91 is also removed. The complete cut-off of cluster ions by Q1 also eliminates the possibility that 14NH235Cl is created in the cell, so the potential new product ion interference on 51V is avoided. Representative calibration curves for V and Ge are shown in Figure 1. BECs and DLs determined by the ICP-QQQ for V, Cr, Ge and As are given in Table 3.
Investigation of arsenic contamination
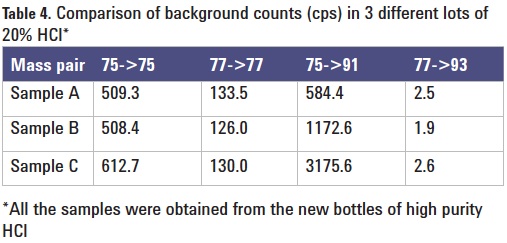
As the BEC for arsenic in high purity HCl was relatively high (Table 3), the signal count at m/z 91 was investigated further. The signals of the mass-pairs 75/75, 77/77, 75/91 and 77/91 were measured by ICP-QQQ with MS/MS, the mass pair number represents the set mass of Q1 followed by the set mass of Q2, so an MS/ MS mode acquisition of mass pair 75/91 represents a mass-shift mode with Q1 = 75 and Q2 = 91, for example. The four mass pairs were measured in HCl blanks from three different lots, and the results are shown in Table 4. The following observations were made:
- The ratio of the signal of 75/75 to 77/77 is around four, which is close to the ratio of the abundance of 35Cl to 37Cl, i.e. 3.13.
- The ratio of the signal of 75/91 to 77/93 is 200–1000, which is far in excess of the ratio of 35Cl to 37Cl.
- While the signals of 75/75 and 77/77 are similar for the three HCl blanks, those of 75/91 and 77/93 vary.
Finding #1 suggests that the remaining signal on 75/75 and 77/77 was mostly from ArCl+. This is a reasonable assumption since ArCl+ doesn’t react with O2 very efficiently so most ArCl+ remains at the original masses of 75 and 77. Finding #2 suggests that the signal of 75/91 is not due to ArCl+. Assuming that all counts of 77/93 arise from 40Ar37Cl, the contribution of 40Ar35Cl to the signal of 75/91 in the HCl blank is estimated to be just 7-8 cps, which is two orders of magnitude lower than the signal that is actually observed. Observation #3, together with #1 and #2, suggests the high count obtained for 75/91 in HCl is due to As impurity in the acid.
Reference
- Direct analysis of trace metallic impurities in high purity hydrochloric acid by Agilent 7700s ICP-MS, Agilent application note, 5990-7354EN.