Ultratrace measurement of calcium in ultrapure water




Albert Lee, Vincent Yang, Jones Hsu,
Eva Wu and Ronan Shin,
BASF Taiwan Ltd., Taipei, Taiwan
Katsuo Mizobuchi, Agilent Technologies, Japan
Keywords
semiconductor, process chemicals, ultra pure water, UPW, calcium, method of standard additions, hydrogen on-mass
Introduction
In the semiconductor industry, the control of metal impurities in the process chemicals used in the manufacture of semiconductor devices is critical to achieve the required product performance and yield. As device performance is continually increasing, the required impurity control becomes ever more stringent. For example, metal content of the ultra-pure water (UPW) used in the manufacturing process must be at the sub-ppt level. ICP-MS is the standard technique used for the trace metals analysis of semiconductor chemicals and devices. The most common instrument and measurement technique used in the semiconductor industry is single quadrupole ICP-MS (ICP-QMS) with cool plasma. The cool plasma technique [1], developed in the mid 1990’s, enables the quantification of key contaminant elements at the single ppt level. Collision and reaction cell ICP-QMS, developed from 2000 onwards, enabled the direct analysis of more complex semiconductor matrices, but did not improve on the DLs or BECs of cool plasma for low-matrix samples. To achieve measurement at the sub-ppt level, reduction of the BEC is required. As outlined in this paper, the Agilent 8800 ICP-QQQ provides new reaction cell technology that enables a significant reduction in the BEC that can be achieved for Ca, to 100 ppq.
Experimental
Instrumentation: Agilent 8800 #200.
Plasma conditions: For the ultra-trace measurement of Ca, cool plasma operating conditions were used (Table 1). The sample was self-aspirated at a carrier gas flow rate of 0.7 L/min.
Reagents and sample preparation: A Ca standard was prepared in UPW acidified with 0.1% high purity HNO3. This was used to make 50 ppt and 100 ppt additions to a UPW blank acidified with 0.1% high purity HNO3.
Results and discussion
Ultra-low BEC for Ca using MS/MS mode
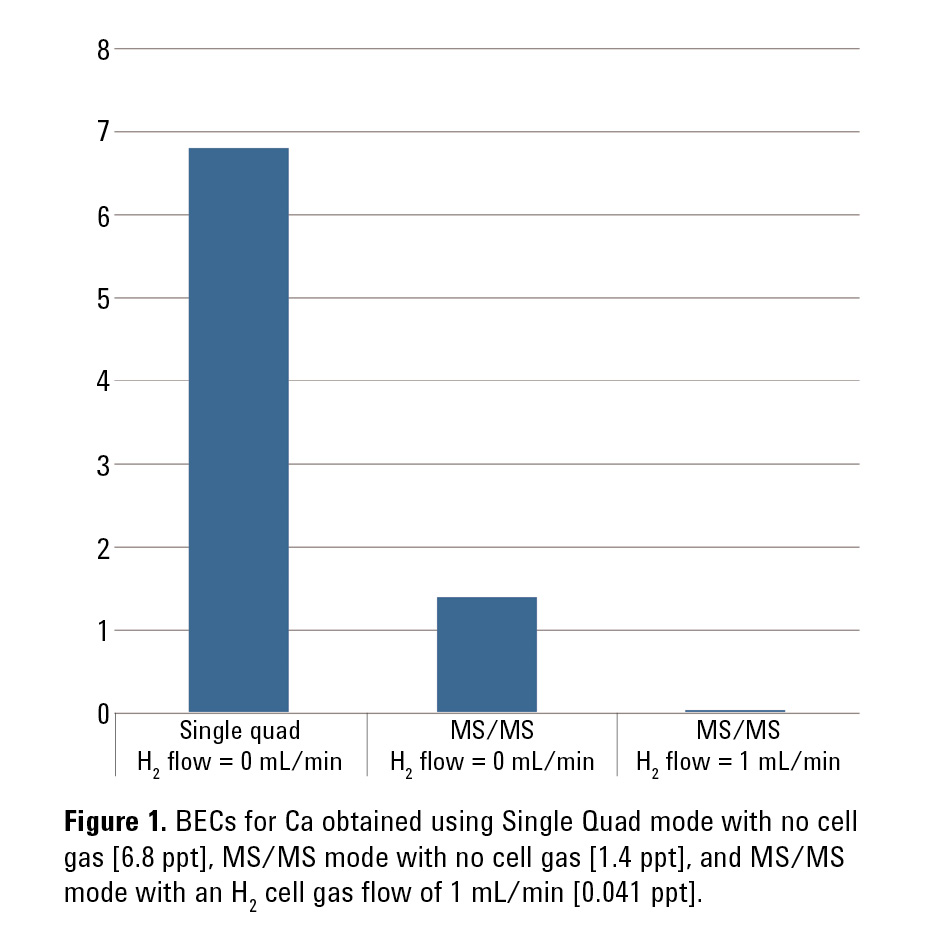
Figure 1 shows the BECs obtained for Ca, measured at its major isotope of 40Ca, using the method of standard additions (MSA) under three different operating conditions on the 8800 ICP-QQQ: Single Quad mode with no cell gas, MS/MS mode with no cell gas, and finally MS/MS mode with a H2 cell gas flow of 1 mL/min. The Single Quad mode uses operating conditions with Q1 acting as an ion guide, to emulate the Agilent 7700 ICP-QMS. The obtained BEC of 6.8 ppt is similar to that routinely achieved with the Agilent 7700 operated in cool plasma mode.
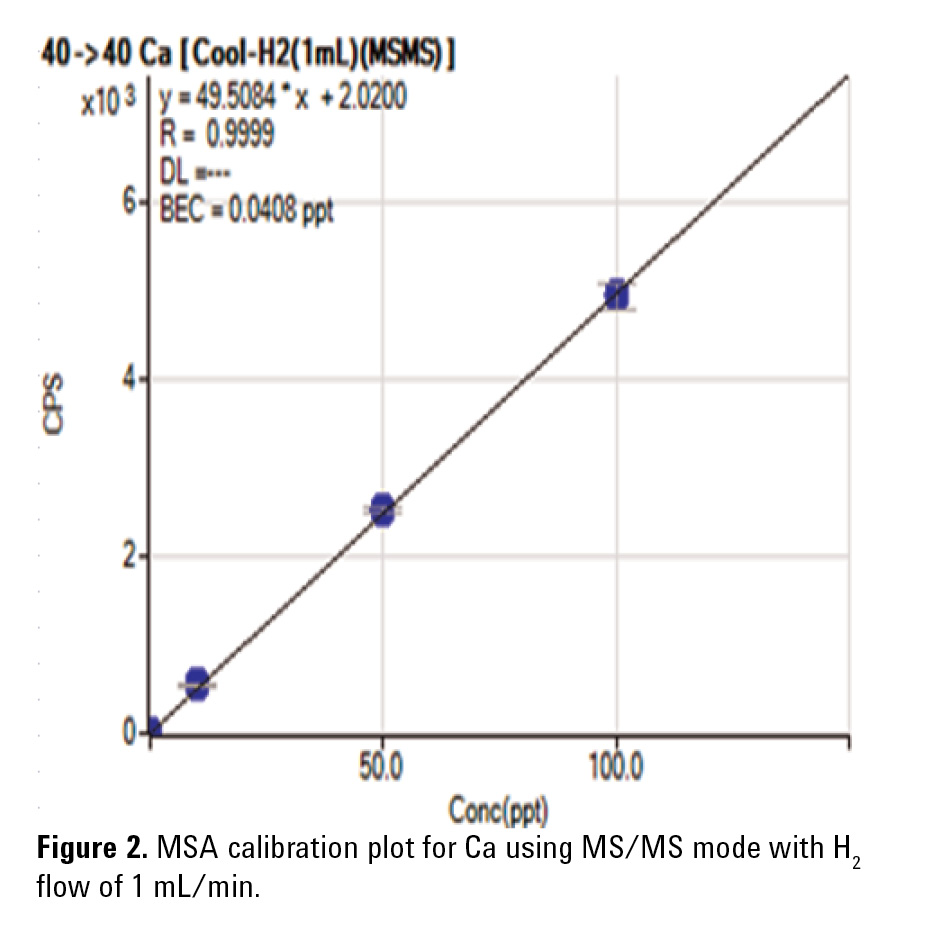
Using MS/MS mode (without cell gas) improved the Ca BEC to 1.4 ppt. MS/MS mode with H2 at 1 mL/min in the cell further improved the BEC down to 0.041 ppt (41 ppq). The obtained MSA plot is shown in Figure 2. The Agilent 8800 ICP-QQQ in MS/MS mode with H2 cell gas achieved a BEC for Ca in UPW two orders of magnitude lower than the BEC obtained using conventional ICP-QMS.
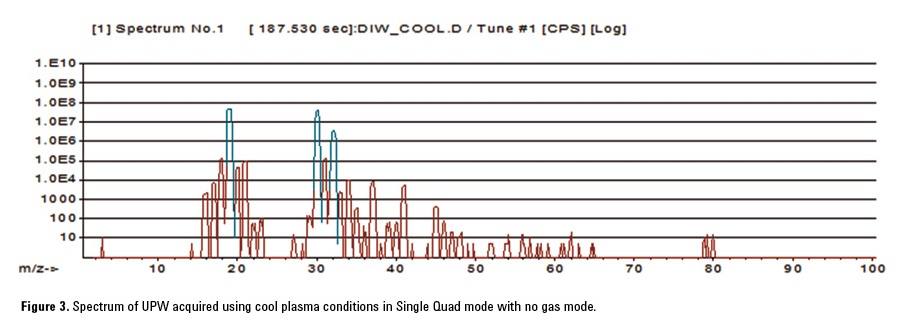
Figure 3 shows the spectrum obtained for UPW using cool plasma conditions in Single Quad mode with no cell gas. As can be seen, Ar+ (m/z 40) is suppressed under the lower temperature plasma conditions, but two intense background peaks are observed at m/z = 19 and 30. These are (H2O)H+ and NO+ respectively. In Single Quad mode, all ions formed in the plasma, including these two intense ions, pass through to the cell. Even with no gas added to the cell, a reaction occurs in the cell which causes a new interfering ion at m/z = 40. The likely reaction occurring in the cell is: NO+ + Ar → Ar+ + NO (charge transfer reaction), which increases the BEC for Ca by several ppt. Although the ionization potential (IP) of NO (IP = 9.26 eV) is lower than that of Ar (IP = 15.7 eV), a metastable ion, NO+, exists close to the ionization potential of Ar [2]. So it is reasonable to assume that the charge transfer reaction shown occurs in the cell.
With MS/MS mode on the 8800 ICP-QQQ, Q1 rejects all non-target ions such as NO+ and (H2O)H+, preventing unwanted reactions from occurring in the cell, which lowers the Ca BEC. The addition of H2 in the cell also removes any residual 40Ar+ that is formed even under cool plasma conditions.
References
- K. Sakata and K. Kawabata, Reduction of fundamental polyatomic ions in inductively coupled plasma mass spectrometry, Spectrochimica Acta, Part B, 1994, 49, 1027.
- R. Marx, Y.M. Yang, G. Mauclaire, M. Heninger, and S. Fenistein, Radioactive lifetimes and reactivity of metastable NO+(a3Σ+,v) and O2 +(a4 II,v) , J.Chem. Phys., Vol. 95, No. 4, 2259-2264, 1991.