Analysis of sulfur, phosphorus, silicon and chlorine in N-methyl-2-pyrrolidone






Naoki Sugiyama
Agilent Technologies, Japan
Keywords
N-methyl-2-pyrrolidone, NMP, semiconductor, process chemicals, sulfur, phosphorus, silicon, chlorine, method of standard additions, oxygen mass-shift
Introduction
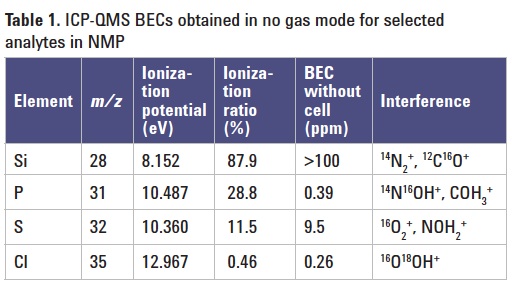
N-Methyl-2-Pyrrolidone (NMP), chemical formula: C5H9NO, is a stable, water-soluble organic solvent that is widely used in the pharmaceutical, petrochemical, polymer science and especially semiconductor industries. Electronic grade NMP is used by semiconductor manufacturers as a wafer cleaner and photo resist stripper and as such the solvent comes into direct contact with wafer surfaces. This requires NMP with the lowest possible trace metal (and non-metal) contaminant levels. ICP-MS is the technique of choice for the measurement of trace metal impurities in semiconductor process chemicals. It is a challenge, however for ICP-MS to measure non-metallic impurities such as sulfur, phosphorus, silicon, and chlorine in NMP. The low ionization efficiency of these elements greatly reduces analyte signal, while the elevated background signal (measured as background equivalent concentration, BEC) due to N-, O-, and C-based polyatomic ions formed from the NMP matrix makes low-level analysis even more difficult (Table 1).
Experimental
Instrumentation: Agilent 8800 #200 with narrow injector (id =1.5 mm) torch
(G3280-80080) typically used for the analysis of organic solvents. A C-flow 200 PFA nebulizer (G3285-80000) was used in self-aspiration mode. An option gas flow of 20% O2 in Ar was added to the carrier gas to prevent carbon build up on the interface cones.
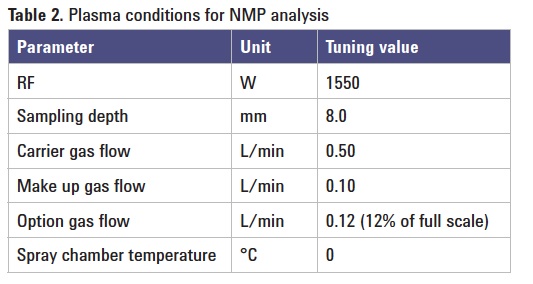
Plasma conditions: NMP analysis requires hotter plasma conditions than normal. This was achieved by reducing Make-up Gas (MUGS) by 0.2 L/min. Plasma tuning conditions are summarized in Table 2.
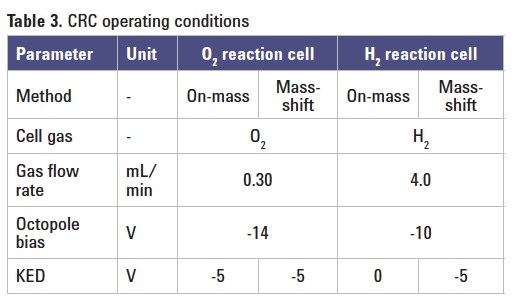
CRC conditions: Table 3 summarizes the cell tuning parameters (gas flow rate and voltages) used.
Reagents and sample preparation:
Electronic industry grade NMP was distilled at 120 °C and acidified by adding high purity HNO3 to a concentration of 1% w/w.
Results and discussion
NMP was analyzed directly using the method of standard additions (MSA). Three replicate measurements (ten replicates for the blank) were acquired for S, P, Si and Cl using an integration time of 1 s per isotope.
P and S measurement in NMP
The mass-shift method using O2 worked well for P and S measurement in NMP. The reactions of P and S with O2 are exothermic, indicated by the negative value for ΔH, as shown below; therefore P+ and S+ are efficiently converted to their oxide ions, PO+ and SO+. P and S can be measured as the product ions, avoiding the original spectroscopic interferences on their elemental masses, m/z 31 and m/z 32.
P+ + O2 → PO+ + O ΔHr = -3.17 eV
S+ + O2 → SO+ + O ΔHr = -0.34 eV
In MS/MS mode, Q1 rejects 36ArC+ before it can enter the cell, preventing it from overlapping SO+. This allows ICP-QQQ to control the reaction chemistry pathways and reaction product ions, ensuring that the analyte product ion is measured free from overlap, regardless of the levels of other co-existing analyte (or matrix) elements.
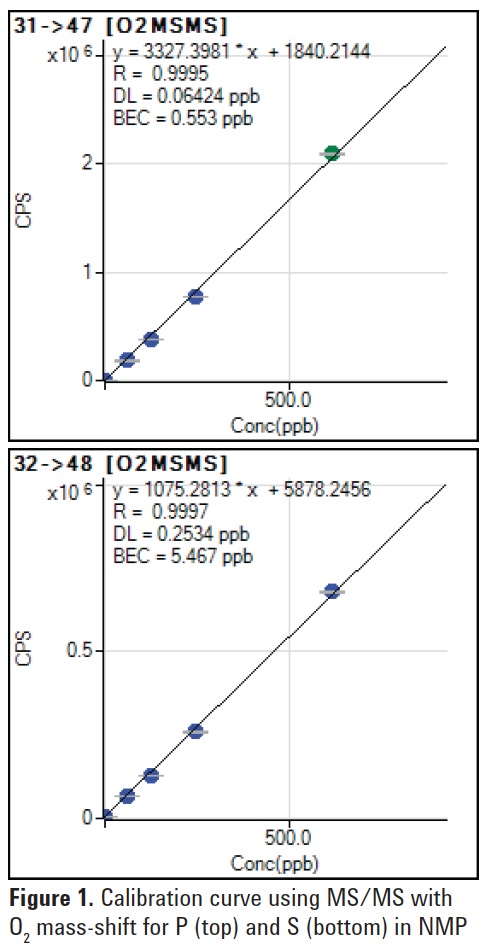
MS/MS mode with the O2 mass-shift method achieved BECs of 0.55 ppb and 5.5 ppb for P and S respectively in NMP. The low BECs and linear calibration plots achieved in MS/MS mode also prove that the matrix-based interferences do not react with O2, allowing the analytes to be separated from the interferences (see Figure 1).
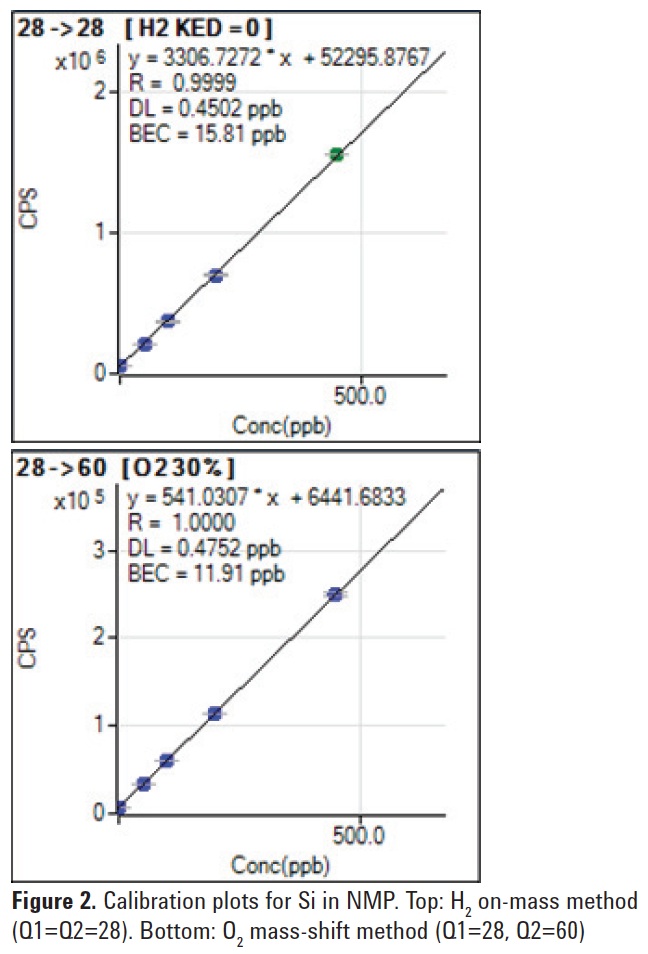
Si measurement in NMP
H2 cell gas was applied to the measurement of Si in NMP. The reaction kinetics for Si and its major interferences with H2 cell gas are shown below. The reaction rate data suggests that Si does not react with H2 cell gas (endothermic reaction indicated by the positive value for ΔH), and so could be measured in NMP using the direct, on-mass method. While the reaction of Si+ with H2 is endothermic, the reactions of the major interfering ions on Si at mass 28 (N2 + and CO+) are exothermic, and these interferences are therefore neutralized or reacted away.
Si+ + H2 → SiH+ + H ΔHr = 1.30 eV
N2 + + H2 → HN2 + + H ΔHr = - 0.60 eV
CO+ + H2 → COH+ +H ΔHr = - 1.63eV
The results obtained are shown in
Figure 2 (top). The H2 on-mass method achieved a BEC of 15.8 ppb for Si in NMP.
Oxygen cell gas was also tested of the measurement of Si in NMP. As shown below, the reaction of Si+ with O2 to form SiO+ is endothermic. However, collisional processes in the cell provide additional energy which promotes the reaction, enabling the O2 mass-shift method to be applied.
Si+ + O2 → SiO+ + O ΔHr = 0.11 eV
Unfortunately a major interference on
Si at m/z 28 (CO+) also reacts with O2, so
the BEC achieved using the O2 mass-shift
method to measure Si as
SiO+ (Q1 = 28, Q2 = 44) was not satisfactory. Fortunately, another Si reaction product ion (SiO2 +) also forms and this can be measured at m/z 60 (Q1 = 28, Q2 = 60) giving a BEC of 11.9 ppb
for Si in NMP (Figure 2, bottom).
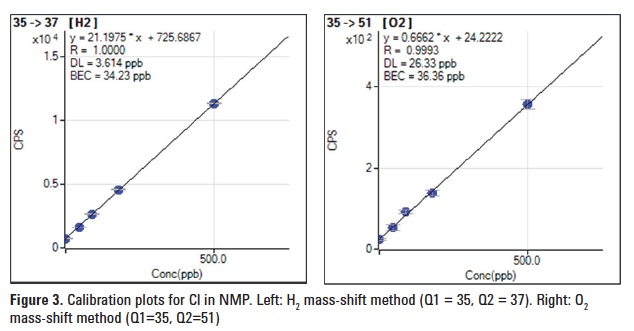
Cl in NMP
Cl+ reacts exothermically with H2 to form HCl+ as shown below. HCl+ continues to react via a chain reaction to form H2Cl+.
Cl+ + H2 → HCl+ + H ΔHr = -0.17eV
HCl+ + H2 → H2Cl+ + H ΔHr = -0.39eV
Figure 3 (left) shows calibration plots obtained for Cl in NMP using the H2 mass-shift method. The plot obtained using the O2 mass-shift method (Figure 3, right) is also shown for comparison. A slightly better BEC of 34.2 ppb was achieved with much higher sensitivity for Cl in NMP using the H2 mass-shift method.
More information
Trace level analysis of sulfur, phosphorus, silicon and chlorine in NMP using the Agilent 8800 Triple Quadrupole ICP-MS, 2013, Agilent application note, 5991-2303EN.