SAMHSA-Compliant LC/MS/MS Analysis of Opiates (Morphine and Codeine) in Urine with Agilent Bond Elut Plexa PCX and Agilent Poroshell 120
Irina Dioumaeva, John M. Hughes
Agilent Technologies, Inc.
Application Note
Forensic Toxicology
Abstract
New guidelines from the US Substance Abuse and Mental Health Services Administration (SAMHSA), effective October 2010, allowed LC/MS/MS methods to be used for confirmation of initial drug tests [1]. LC/MS/MS methods are often less complicated than previously employed GC/MS methods because they do not typically require a derivatization step. We present a method for analysis of opiates that meets the most recent SAMHSA guidelines to demonstrate linearity, limit of detection (LOD), accuracy and precision, as well as measurement of matrix effects, extraction recovery, and overall process efficiency. This is one of a suite of six simplified methods covering all classes of SAMHSA-regulated drugs and using premier Agilent products, including Agilent Bond Elut Plexa PCX mixed-mode polymeric SPE, Agilent Poroshell 120 EC-C18, 2.7 µm superficially porous LC column, Agilent 1200 Infinity LC system, and Agilent 6460 Triple Quadrupole LC/MS system with Agilent Jet Stream Technology (AJST) enhanced electrospray source.
Introduction
Opiates (morphine and codeine) are natural alkaloids found in the resin of the opium poppy. Codeine is currently the most widely used opiate in the world. In addition to detection of morphine and codeine, guidelines from SAMHSA require the confirmation method to demonstrate the ability to distinguish these drugs from structurally related compounds, such as the semisynthetic opioids: hydromorphone, oxymorphone, hydrocodone, oxycodone, and the codeine metabolite norcodeine [2].
Both morphine and codeine are extensively metabolized in the body. Morphine is metabolized primarily into morphine-
3-glucuronide and morphine-6-glucuronide. Codeine’s major metabolites are morphine, codeine-6-glucuronide, and norcodeine. Because both morphine and codeine are found in urine largely in the form of glucuronide conjugates, SAMHSA requires measurement of the total concentration of each compound. A full conversion of glucuronides back to parent species must be performed prior to analysis. The most reliable conversion method ensuring complete recovery of free opiates is acid hydrolysis. Frequently used enzymatic hydrolysis often leads to incomplete recovery of parent compounds which could lead to false negative results [3].
The SAMHSA-established confirmation cutoff concentration for morphine and codeine is 2,000 ng/mL [1]. Because high concentrations of opiates can be expected in some urine samples, we chose to use a higher capacity 3 mm id Poroshell 120 column instead of a 2 mm id column for all Agilent SAMHSA methods. With superficially porous 2.7 µm particles, Poroshell 120 provides similar efficiency to sub-2 µm UHPLC columns but with about 40% less back pressure. It, therefore, allows users of even 400 bar LC systems to increase resolution and to shorten both analysis and re-equilibration times by applying a higher flow rate.
The extraction method described in this application note provides reproducible high recoveries of morphine and codeine due to the unique properties of the Agilent Bond Elut Plexa polymer. Unlike other polymeric sorbents, Plexa possesses an amide-free hydroxylated particle surface that excludes protein binding. This results in minimized ion suppression and maximum sensitivity. Fast flow and reproducible performance are due to the narrow particle size distribution with no fines to cause blockages.
With a low sample injection volume of 2 µL and no sample preconcentration, the method demonstrates excellent signal‑to-noise (S/N) ratios for both morphine and codeine (>150:1 at 200 ng/mL, 10% of the SAMHSA confirmation cutoff) due to the enhanced sensitivity of the Agilent 6460 Triple Quadrupole LC/MS with the AJST electrospray source.
Previous methods from Agilent used the Agilent 6410 Triple Quadrupole LC/MS system and other SPE/LC products and procedures [4,5].
Experimental
Analytes
Drug standards were purchased from Cerilliant Corporation as 1 mg/mL (morphine, codeine, hydromorphone, norcodeine, hydrocodone, oxycodone, oxymorphone, and morphine‑3‑glucucronide) and 100 µg/mL (morphine-D6 and
codeine-D6) solutions in methanol.
Materials and instrumentation
SPE
- Agilent Bond Elut Plexa PCX cartridges, 30 mg, 3 mL
(p/n 12108303) - Agilent vacuum manifold VacElut 20 (p/n 12234100)
- Agilent stopcock valves
(p/n 12234520) - Agilent 2-mL autosampler vials
(p/n 5182-0716) - Agilent screw caps for autosampler vials (p/n 5182-0717)
LC
- Agilent Poroshell 120 EC-C18,
3 × 50 mm, 2.7 µm (p/n 699975-302) - Agilent 1260 Infinity LC (G1379B microdegasser, 1312B binary pump in low delay volume configuration, G1367E autosampler, and G1330B thermostat)
MS
- Agilent 6460A Triple Quadrupole LC/MS system with AJS electrospray ionization source.
Sample preparation
Hydrolysis and sample pretreatment
- Spike 0.5 mL of urine with ISTD at 1000 ng/mL; use of 12 × 75 mm glass tubes is recommended.
- Add 125 µL concentration HCl.
- Incubate in the heating block at
95 ±5 °C for 90 minutes. - Cool. Add 2 mL 0.1 M sodium acetate buffer (pH 4.5).
- Neutralize with 250 µL 7 N KOH, vortex, and test pH; it should be <6.
- Centrifuge 20 minutes at 6,000 rpm.
Extraction
- Condition Bond Elut Plexa PCX column with 0.5 mL methanol – soak, then let drip.
- Load sample/supernatants.
- Wash 1: 1 mL 2% formic acid.
- Wash 2: 1 mL of methanol.
- Dry 5–10 minutes under vacuum
(10–15 in Hg). - Elute with 2 mL methanol: ammonium hydroxide (100:20), freshly prepared. Let eluate drip into collection vials, then apply low vacuum (2–3 in Hg).
- Evaporate to dryness at 40 °C.
- Reconstitute in 0.5 mL initial mobile phase (5% methanol, 95% water, 0.1% formic acid).
LC/MS/MS
LC conditions
Mobile phase A
0.1% formic acid in water
Mobile phase B
0.1% formic acid in methanol
Flow rate
0.8 mL/min
Gradient
Time (min) % B
0.0 5
0.5 5
1.5 25
2.5 55
2.6 90
5.6 90
5.7 5
Stop time
5.8 min
Post time
2 min
Max pump pressure
400 bar
Injection volume
2 µL
Injection with needle wash
Needle wash
Flush port 75:25 methanol:water for 10 s
Disable overlapped injection
No automatic delay volume reduction
MS conditions
ES source parameters
Ionization mode
Positive
Capillary voltage
3,000 V
Drying gas flow
10 L/min
Drying gas temperature
350 °C
Nebulizer gas
35 psi
Sheath gas flow
12 L/min
Sheath gas temperature
400 °C
Nozzle voltage
0 V
MS parameters
Scan type
Dynamic MRM
Pre-run script
SCP_MSDiverterValveToWaste()
{MH_Acq_Scripts.exe}
Time segments
#1: 1.0 min - diverter valve to MS
Delta EMV (+)
0 V
Results and Discussion
At low pH, morphine, codeine, and their derivatives were protonated at the tertiary amine group and were strongly retained on Plexa PCX polymeric sorbent by a combination of hydrophobic retention and a strong cation exchange.
A 100% methanol wash eliminated most matrix interferences without loss of opiates from the SPE column. A strong base was added to the organic eluent to break ionic interaction between the analytes and the strong cation exchange sorbent. The opiates recovery was optimized with 20% NH4OH added to methanol shortly before sample elution.
The Poroshell 120 EC-C18, 3 × 50 mm,
2.7 µm column provided excellent separation and peak shapes for opiates and potentially interfering compounds, with the analysis completed within 2.5 minutes (Figure 2). LC separation started with a low fraction of organic solvent (5%) to allow salts and other polar components of urine to elute at the beginning of the sample run. Each sample run started with diverting a first portion of flow (0 to 1 minutes) to waste to minimize source contamination. Data collection started at 1.0 minutes, immediately after the diverter valve switch. A flow rate of 0.8 mL/min allowed for short analysis and re‑equilibration times.
The only partially unresolved pair in the chromatogram in Figure 2 were codeine and norcodeine (peaks 4 and 5), but because these compounds have different precursor ions and mass transitions, any possibility of interference of norcodeine signals with codeine quantitation was excluded.
In a separate experiment, Plexa PCX was tested for the possibility of norcodeine methylation and conversion to codeine. Test results were negative; no codeine was detected in negative urine samples that were spiked with norcodeine and then extracted using the method described in this application note.
When testing for interferences, a dynamic MRM method using retention time and delta RT (time window) for a certain transition is recommended. However, when good separation from interferences is ensured, data collection for morphine and codeine and their ISTDs can be performed with normal MRM.
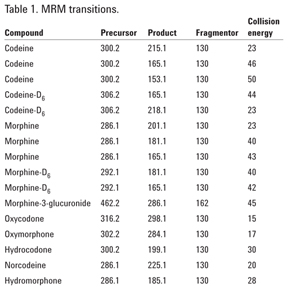
SAMHSA guidelines require the use of one quantifier and at least one qualifier ion for both target compound and ISTD. A third transition for the target analyte is provided (Table 1) for additional confidence. Agilent MassHunter Quantitative software calculates qualifier ion ratios, automatically highlighting those out of acceptable range.
When processed according to the protocol, urine samples spiked with morphine-ß-3-glucuronide at 10,000 ng/mL showed 97 to 99.2% conversion to morphine. MS parameters for the detection of morphine-ß-3-glucuronide are included in Table 1 for analysts interested in testing the hydrolysis
efficiency.
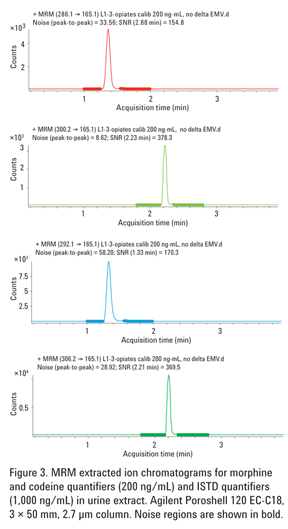
S/N ratios exceeding 150:1 were obtained for quantifier peaks of morphine and codeine at 200 ng/mL (Figure 3, panel 1 and 2 from the top). This illustrates the state-of-the-art performance of the Agilent 6460 Triple Quadrupole LC/MS system, capable of reliably detecting opiates at a small fraction of the SAMHSA cutoff.
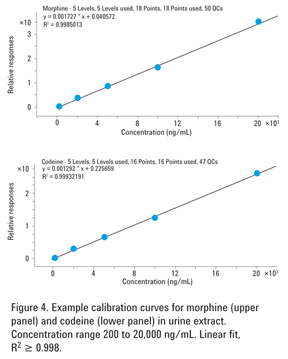
Figure 4 gives examples of calibration curves for extracted urine standards at five concentration levels. Calibration standards were prepared by spiking negative urine at 200, 1,000, 2,000, 10,000, and 20,000 ng/mL with morphine and codeine. Internal deuterated standard morphine-D6 and codeine-D6 were added at 1,000 ng/mL. Excellent linear fit (R2 ≥ 0.998) to each of the curves demonstrates linearity of the method across a broad dynamic range of concentrations, as required by SAMHSA guidelines.
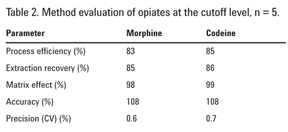
Method evaluation
Method performance metrics in Table 2 were calculated according to the principles laid out in Matuszewski et al. and widely accepted as an industry standard approach for LC/MS/MS methods [6]. The extraction procedure and LC/MS/MS measurement were performed for five replicates of negative urine spiked pre-extraction with morphine and codeine at the cutoff level, and five replicates of negative urine extract reconstituted in initial mobile phase and then fortified at 2,000 ng/mL (spiked post-SPE). The third measurement was of initial mobile phase (the reconstitution solvent) fortified to correspond to the cutoff concentration of 2,000 ng/mL in urine (spiked mobile phase).
Process efficiency (absolute recovery) is a ratio of a peak area of target analyte in urine sample spiked pre-SPE to its peak area in matrix-free spiked mobile phase. Extraction recovery is a ratio of a peak area of target analyte in urine extract spiked pre-SPE to its peak area in an extracted negative urine sample spiked post-SPE. Matrix effect is a ratio of a peak area of target analyte in urine spiked post-SPE to its peak area in spiked mobile phase. Accuracy is a ratio of a measured concentration calculated using the calibration curve to the expected concentration in a sample spiked with a known amount of target analyte. Precision or coefficient of variation (CV) is a measure of reproducibility and is calculated as a percent standard deviation over the mean of the five measurements
Table 2 shows high extraction recovery and process efficiency for morphine and codeine (approximately 85%). The high matrix effect value (98–99%) means only 1 to 2% signal reduction is due to ion suppression, thus, confirming the exceptional cleanliness of Plexa PCX-processed extracts. High accuracy (within 10% of the target) and excellent precision (CV<1%) are typical for the method.
Conclusions
The solid phase extraction procedure coupled with LC/MS/MS detection method described in this application note is SAMHSA-compliant and provides reproducible results for forensic toxicology or other analytical environments with similar requirements for legally defensible data. The hardware setup is the same as in other 2011 SAMHSA methods from Agilent. These methods are intended for all users of Agilent 1100 and Agilent 1200 Series LCs because the back pressure in the LC system does not exceed 400 bar. Source parameters can be easily modified to use this method with other models of Agilent Triple Quadrupole LC/MS systems. Electronic copies of the LC/MS/MS acquisition and quantitation methods are available from Agilent Technologies.
References
- SAMHSA (2010) Manual for Urine Laboratories, National Laboratory Certification Program, 1 October 2010. U. S. Department of Health and Human Services.
- R. Baselt (2008) Disposition of Toxic Drugs and Chemicals in Man. 8th edition. Atlas Books, Ashland, OH, USA.
- P. Wang, J. A. Stone, K. H. Chen, S. F. Gross, C. A. Haller, and A. H. Wu (2006) Incomplete recovery of prescription opioids in urine using enzymatic hydrolysis of glucuronide metabolites. Journal of Analytical Toxicology, 30: 570-575.
- P. Moorman and J. Hughes (2010) “Opiates (morphine and codeine) in Urine by LC/Triple Quadrupole Mass Spectrometry (LC/MS/MS)”. SOP, Agilent Technologies, Inc. Publication Number 5990-5875EN.
- J. Hughes and P. Moorman (2011) “Confirmation by Triple Quadrupole LC/MS/MS for HHs-compliant Workplace Urine Drug Testing”. Agilent Technologies, Inc. Seminar available from www.agilent.com/chem.
- B. K. Matuszewski, M. L. Constanzer, and C.M. Chavez-Eng (2003) Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Analytical Chemistry, 75: 3019-3030.
For More Information
These data represent typical results. For more information on our products and services, visit our Web site at www.agilent.com/chem.
©Agilent Technologies, Inc., 2013
February 15, 2013
View this Application Note in its entirety: 5990-9625EN