Pharmaceuticals in Whole Blood Using Mini-Extraction Sample Prep and Poroshell 120
Joan Stevens
Agilent Technologies, Inc.
Application Note
Small Molecule Pharmaceuticals
and Generics
Abstract
A convenient analytical method for determination of pharmaceuticals in various therapeutic categories in whole blood involves the addition of acetonitrile and salts to a small amount of blood. The mixture is shaken and centrifuged for extraction/partitioning, which removes water and proteins from the sample. An aliquot of the organic layer is cleaned by dispersive solid-phase extraction (SPE) employing SPE sorbent and salts, to remove endogenous matrix components. Analytes are then isolated from spiked samples with recoveries above 80% on average, and RSDs typically below 10% for a wide range of substances. This mini-extraction approach in whole blood delivers successful separation for a variety of pharmaceuticals, with limits of detection below 10 ng/mL. The method is quick, easy, inexpensive, and effective.
Introduction
Determination of pharmaceuticals in biological matrixes is commonly employed in ADME (DMPK), clinical and forensic analysis. The main techniques used for analysis are immunoassays, LC, and GC. Mass spectral chromatographic methods are the first choice for many applications, based on their flexibility, selectivity, sensitivity, qualitative, and quantitative capabilities. Analysis of pharmaceuticals in biological samples requires sample preparation that can range from simple protein precipitation (PPT) to more complex solid- phase extraction (SPE). There is a need in classic sample preparation for a method to determine multi-classes of pharmaceuticals in biological samples. Polymeric or mixed-mode SPE sorbents that can isolate acidic, neutral and basic drugs by hydrophobic and, or ion-exchange interactions address this need, but there is always room for sample preparation techniques that are rapid and inexpensive to implement.
Previously reported methods provide analysis of multi-residue pesticides in foods. They are known as QuEChERS (a quick, easy, cheap, effective, rugged, and safe sample preparation approach) [1]. The authors reported outstanding recoveries for a wide range of pesticide classes. Since its inception, there have been many reported articles employing QuEChERS for the analysis of a wide range of compounds including, but not specific to, antibiotics [2], toxins [3], contaminants [4], and pharmaceuticals [5].
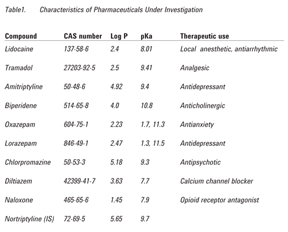
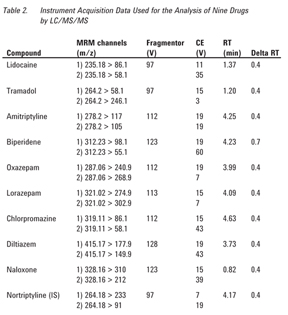
In this note we describe an extension of the work presented by Plössl et al. [5] for the determination of pharmaceuticals in whole blood employing a modified mini-extraction procedure with LC/MS/MS analysis. The experiments presented in this application note used human whole blood containing either EDTA or citrate as an anticoagulant and were evaluated with both nonbuffered and buffered extraction salts used in the QuEChERS methodology, namely nonbuffered, AOAC 2007.01 and EN 15662. Modifications to the acetonitrile (extraction solvent) used in the first step (extraction/partitioning) were also evaluated. The experiments were performed using nine different pharmaceuticals (lidocaine, tramadol, amitriptyline, biperidene, oxazepam, lorazepam, chlorpromazine, diltiazem, and naloxone), with a broad range of hydrophobicity and dissociation constants (Table 1). Agilent Poroshell 120 is a good column for this analysis, in part because it has standard 2-µm frits and is more forgiving for more complex samples relative to a sub‑2‑µm column. Poroshell 120 has mass transfer such that it acts very much like a sub-2-µm particle LC column, without the high back pressure associated with a sub-2-µm column. The efficient mass transfer equates with faster analysis time and higher throughput with optimum resolution.
Experimental
All reagents and solvents were HPLC analytical grade. The compounds were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA).
A stock solution of 1 M ammonium acetate (NH4OAc) pH 5 was made by dissolving 19.27 g NH4OAc powder in 250 mL Milli-Q water. The pH was adjusted to 5 with acetic acid monitored with a pH meter. The solution was stored at 4 °C. MeOH:H2O (20:80) containing 5 mM NH4OAc
pH 5 was made by combining 200 mL MeOH and 800 mL Milli-Q water, adding
5 mL 1 M NH4OAc, pH 5 stock solution. The 5 mM NH4OAc in ACN was prepared by adding 5 mL 1 M NH4OAc, pH 5 stock solution to 1 L ACN, sonicating well.
Standard and internal standard solutions (2.0 mg/mL) were made in MeOH and stored at –20 °C. A QC spiking solution of 5.0 µg/mL was made fresh daily in 1:1 ACN:H2O (0.1% FA). A 0.5 and 5.0 µg/mL standard solution in 1:1 ACN:H2O
(0.1% FA) was made for the preparation of calibration curves in the matrix blank extract with appropriate dilution. Five µg/mL nortriptyline in 1:1 ACN:H2O (0.1% FA) was used as the internal standard (IS).
Equipment
- Agilent 1260 Infinity LC with Diode Array
- Agilent 6460 Triple Quadrupole LC/MS system with Electrospray Ionization
- Agilent Bond Elut QuEChERS AOAC Extraction kit (p/n 5982-6755)
- Bond Elut QuEChERS EN Extraction kit (p/n 5982-6650)
- Bond Elut QuEChERS Non-Buffered Extraction kit (p/n 5982-6550)
- Bond Elut QuEChERS AOAC Dispersive SPE kit for General Fruits and Vegetables (p/n 5982-5022)
- Bond Elut QuEChERS EN Dispersive SPE kit for General Fruits and Vegetables (p/n 5992-5021)
- Bond Elut Ceramic Homogenizers
(p/n 5982-9312) - Sorvall ST 16R Centrifuge (Thermo IEC, MA, USA)
- Micro centrifuge 5415D Eppendorf (Brinkman Instruments, Westbury, NY, USA)
- Geno Grinder 2010 (SPEX CertiPrep, Inc., Metuchen, NJ, USA)
- DVX 2500 Multi-Tube Vortexer (VWR International, West Chester, PA, USA)
HPLC conditions
Column
Agilent Poroshell 120 EC-C18, 2.1 × 100 mm, 2.7 µm (p/n 695775-902)
Flow rate
0.4 mL/min
Column temperature
30 °C
Injection
10 µL
Mobile phase
A. 5 mM Ammonium acetate, pH 5 in 20:80 MeOH:water
B. 5 mM Ammonium acetate, pH 5 in ACN
Needle wash
1:1:1:1 ACN:MeOH:IPA:H2O (0.2% FA)
Gradient
20 to 75% B over 5.5 min
MS conditions
ESI
Positive mode
GT
300 °C
GF
7 L/min
Nebulizer
40 psi
SGT
400 °C
SFG
12 L/min
Capillary
3500 V
NV
500 V
Other MS conditions relating to the analytes are listed in Table 2.
General procedure
- Add 1 mL of whole blood to a centrifuge tube.
- Spike with appropriate volume from a concentrated stock mixture to yield 25, 50, and 100 ng/mL of the component mix.
- Add 20 µL of IS stock solution, yield 100 ng/mL (nortriptyline), and two ceramic homogenizers.
- Vortex.
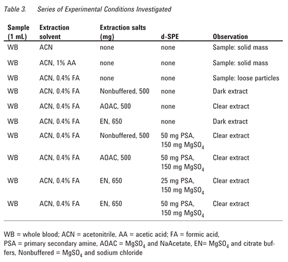
- Add 2 mL acetonitrile solution (with or without acid), see Table 3.
- Vortex.
- Add a premixed amount (see Table 3) of the extraction salts and vigorously shake.
- Centrifuge at 5,000 rpm for 5 minutes.
- Transfer 1 mL of the extract into a d-SPE tube (2 mL centrifuge tube) containing 50 mg PSA and 150 mg MgSO4 for matrix cleanup.
- Vortex for 1 minute.
- Centrifuge at 18,000 rpm for 3 minutes.
- Transfer 200 µL aliquot of the extract into a LC vial containing 800 µL of water.
- Vortex and analyze.
The entire series of experiments are in Table 3. A matrix-matched calibration curve from 10 to 250 ng/mL was employed to determine recovery.
Results and Discussion
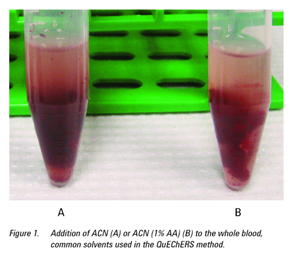
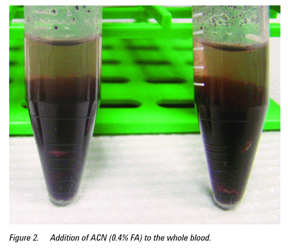
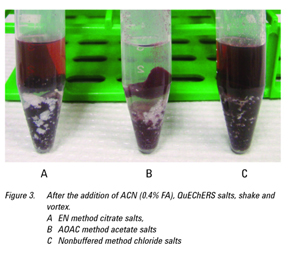
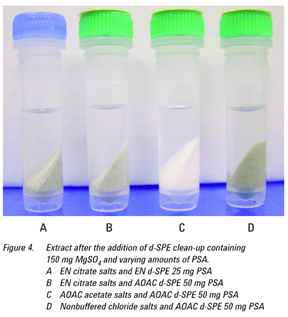
The experiments showed that the use of ACN (0.4% FA) as the extraction solvent offered a better lysed sample versus the other extraction solvents where the sample became a solid mass (see Figures 1 and 2). The AOAC-buffered salts yielded the cleanest extract, visually (Figure 3) and was chosen for use with the d-SPE containing
50 mg PSA, 150 mg MgSO4 for the extraction of the pharmaceuticals in whole blood (Figure 4). It is worth noting that the
d-SPE step does in fact offer substantial cleanup for all the extracted samples, especially from the EN and nonbuffered salt extracts, which initially showed a significant amount of red blood cells remaining in the extract.
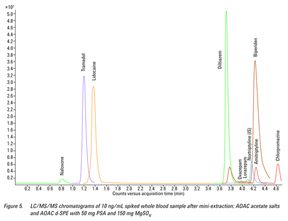
The mini-extraction procedure is based on the principles behind the QuEChERS methodology. It provides an alternative to more complicated techniques, offering a simplified sample preparation technique for complex matrixes such as whole blood. This type of sample preparation technique is extremely complementary to the powerful selectivity of LC/MS/MS multiple reaction monitoring (MRM) mode. The whole blood extract appeared to be clean and free of impurities, indicating that the blank whole blood extract did not contribute any interferences with target compounds. Figure 5 shows the chromatogram of a 10 ng/mL spiked whole blood sample after the mini-extraction procedure.
Linearity and limit of quantification (LOQ)
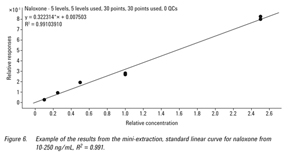
The linear calibration range evaluated for all the pharmaceuticals was 10 to 250 ng/mL. Matrix blank extracts were prepared for the calibration curves. Calibration curves, spiked in the matrix blank extracts, were made at 10, 25, 50, 100, and 250 ng/mL. The nortriptyline (IS) was used at 100 ng/mL. The calibration curves were generated by plotting the relative responses of analytes (peak area of analyte/peak area of IS) to the relative concentration of analytes (concentration of analyte/concentration of IS). Figure 6 is an example of the regression equation and correlation coefficient (R2) observed for the nine pharmaceuticals from whole blood.
Recovery and reproducibility
The recovery and reproducibility were evaluated by spiking standards in the whole blood sample at 25, 50, and
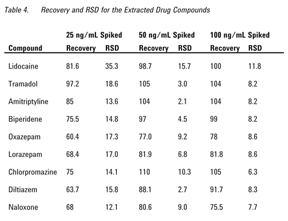
100 ng/mL. These QC samples were quantitated against the matrix-spiked calibration curve. The analysis was performed in six replicates at each level. The recovery and reproducibility (RSD) data are shown in Table 4.
It can be seen from the results that all the pharmaceuticals give acceptable recoveries (average > 90%) and precision (average of 7% RSD). We have observed a small degree of matrix interference at low levels of concentration, < 25 ng/mL, with the pharmaceuticals investigated.
Conclusion
Mini-extraction sample preparation is a simple, easy, and cost-effective approach, requiring minimal sample preparation expertise, solvent, or equipment. The mini-extraction approach for the extraction of pharmaceuticals from whole blood offers an alternative sample preparation technique that can be easily implemented by laboratories. Although matrix interference was observed at low-level concentrations for some of the pharmaceuticals, improvements in the method can include a dispersive SPE that contains additional solid phase extraction materials to facilitate matrix removal. The Poroshell 120 EC-C18 column offers different selectivity and exceptional peak shape across the wide range of pharmaceuticals used in this study.
References
- M. Anastassiades, S. J. Lehotay, D. Štajnbaher, F. J. Schenk. J. AOAC Int. 86, 4121 (2003).
- G. Stubbings, T. Bigwood. Anal. Chim. Acta, 637, 68 (2009).
- R. R. Rasmussen, I. M. L. D. Storm, P. H. Rasmussen, J. Smedsgaard, K. F. Nielsen. Anal. Bioanal. Chem. 397, 765 (2010).
- D. Smith, K. Lynam. GC/µECD Analysis and Confirmation of PCBs in Fish Tissue with Agilent J&W DB-35ms and DB-XLB GC Columns. Application note, Agilent Technologies, Inc. Publication number 5990-6236EN (2010).
- F. Plössl, M. Giera, F. Bracher. J. Chrom. A. 1135, 19 (2006).
For More Information
These data represent typical results. For more information on our products and services, visit our Web site at www.agilent.com/chem.
©Agilent Technologies, Inc., 2013
July 3, 2013
View this Application Note in its entirety: 5990-8789EN