LC/MS/MS of Buprenorphine and Norbuprenorphine in Whole Blood Using Agilent Bond Elut Plexa PCX and an Agilent Poroshell 120 Column
Irina Dioumaeva
Agilent Technologies, Inc.
Application Note
Forensic Toxicology
Abstract
Determination of buprenorphine and norbuprenorphine in whole blood by forensic toxicology laboratories requires an analytical method capable of reliable detection of these compounds at concentrations below
1 ng/mL. A simple sample cleanup procedure coupled with an LC/MS/MS method using mass transitions 468.2 → 55.1 and 414.2 → 83.1 allows for a limit of detection (LOD) below 0.1 ng/mL for both analytes. Typical calibration curves are linear in the range of 0.2 to 20 ng/mL for each analyte, with R2 values equal or higher than 0.999. High sensitivity is achieved by using Agilent products, including an Agilent Bond Elut Plexa PCX mixed mode polymeric SPE sorbent, an Agilent Poroshell 120 EC-C18 2.7 µm superficially porous LC column, an Agilent 1200 Infinity LC system, and an Agilent 6460 Triple Quadrupole LC/MS System with Agilent Jet Stream Technology (AJST) enhanced electrospray source.
Introduction
Buprenorphine is a semisynthetic opioid with a structure similar to morphine, although buprenorphine is much more hydrophobic (Figure 1). Buprenorphine is converted to norbuprenorphine, its major active metabolite [1,4]. Concentrations of buprenorphine and norbuprenorphine in blood are very similar, and in more than 50% cases, are below 1 ng/mL [9], presenting a challenge for an analyst. In addition, MS/MS detection of these compounds is complicated by the rigidity of the molecular structures of the analytes, resulting in very low amounts of collision-induced fragments. To achieve sensitivity below 1 ng/mL, analytical methods for determination of these compounds need not only excellent MS performance, but also an efficient sample cleanup procedure providing high recoveries and low ion suppression. We used an extraction method that delivered detection limits below 0.1 ng/mL, easily achieved due to the cleanliness of SPE‑processed whole blood extracts. Unlike other polymeric sorbents, all members of the Agilent Bond Elut Plexa family possess an amide-free hydroxylated particle surface that excludes protein binding. This results in minimized ion suppression and maximum sensitivity. Fast flow and reproducible performance are due to the narrow particle size distribution with no fines to cause blockages.
Good separation of analytes and excellent peak shapes achieved with this method are distinctive features of the Agilent Poroshell 120 column family. With superficially porous 2.7 µm particles, these columns provide similar efficiency to sub-2 µm UHPLC columns, but with approximately 40% less backpressure. This allows users of even 400 bar LC systems to increase resolution and to shorten analysis and re‑equilibration times by applying a higher flow rate.
New ion transitions identified as the most abundant and used in this work for quantitation are 468.2 > 55.1 (buprenorphine) and 414.2 > 83.1 (norbupenorphine). With only 0.5 mL of blood, a low sample injection volume of
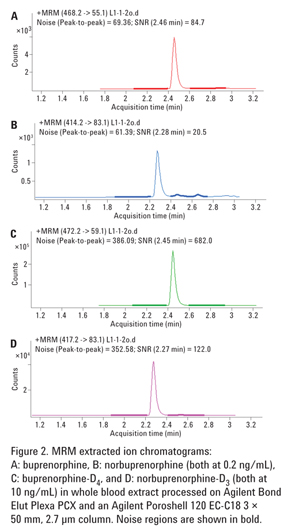
10 µL and preconcentration of only 5× at the extraction step, the method demonstrates excellent signal-to-noise ratios at 0.2 ng/mL:84:1 for buprenorphine and 20:1 for norbuprenorphine (Figure 2).
Experimental
Analytes
Drug standards were purchased from Cerilliant Corporation as 1 mg/mL (buprenorphine, norbuprenorphine) and
100 µg/mL (buprenorphine-D4 and norbuprenorphine-D3) solutions in methanol.
Materials and instrumentation
SPE
- Agilent Bond Elut Plexa PCX cartridges 30 mg, 3 mL (p/n 12108303)
- Agilent vacuum manifold VacElut 20 (p/n 12234100)
- Agilent stopcock valves
(pn 12234520) - Agilent silanized autosampler vials 2 mL (p/n 5183-2072)
- Agilent vial inserts, 250 µL, deactivated glass, with polymer feet (p/n 5181-8872)
- Agilent screw caps for AS vials
(p/n 5182-0717)
LC
- Agilent Poroshell 120 EC-C18, 3 × 50 mm, 2.7 µm column (p/n 699975-302)
- Agilent 1260 Infinity LC system (G1379B microdegasser, 1312B binary pump in low delay volume configuration, G1367E autosampler, G1330B thermostat)
MS
- Agilent 6460A Triple Quadrupole LC/MS system with AJS electrospray ionization source.
Sample preparation
Pretreatment
- Spike 0.5 mL of blood with ISTD at
10 ng/mL, or prepare 10 ng/mL solution of ISTD in 0.1 M phosphate buffer (pH 6.0) and add 0.5 mL of this buffer to each blood sample. Use of methanol-rinsed and air-dried glass tubes 12 × 75 mm is recommended. - After adding ISTD, add 2 to 2.5 mL phosphate buffer (so that blood is diluted at least 1:5).
- Vortex and centrifuge to obtain a good pellet.
Extraction
- Condition Bond Elut Plexa PCX cartridge with 0.5 mL methanol, soak, then let drip.
- Load sample/supernatants with a Pasteur glass pipette.
- Wash 1: × 2 mL 2% formic acid.
- Wash 2: mL of 70 MeOH:30 of 2% formic acid.
- Dry 5-10 minutes under vacuum
(10-15 in Hg). - Elute with 1.5 mL of 80 ethyl acetate:20 isopropanol: 5 NH4OH eluent. Add NH4OH shortly before elution. Apply eluent in 2 aliquots and soak the sorbent bed with each aliquot. Soak for approximately 0.5 minute with the stopcock valves closed, then let the eluate drip into the collection vials under gravity. When the dripping stops, apply low vacuum to extract eluate from the smallest pores.
- Evaporate to dryness under a stream of nitrogen at 45 °C.
- Reconstitute in 0.1 mL initial mobile phase (15% methanol, 85% water, 0.1% formic acid), vortex, and transfer into vial inserts with polymer feet.
LC/MS/MS
LC conditions
Mobile phase A
0.1% formic acid in water
Mobile phase B
0.1% formic acid in methanol
Flow rate
0.8 mL/min
Gradient:
Time (min) % B
0.0 15
2.0 70
2.1 95
5.5 95
5.51 15
Stop time
5.6 min
Post time
2 min
Max pump pressure
400 bar
Injection volume
10 µL
Injection with needle wash
Needle wash
Flush port 95 methanol:5 water for 10 s
Disable overlapped injection
No automatic delay volume reduction
MS conditions
ES source parameters
Ionization mode
positive
Capillary voltage
2,800 V
Drying gas flow
10 L/min
Drying gas temperature
350 °C
Nebulizer gas
35 psi
Sheath gas flow
12 L/min
Sheath gas temperature
350 °C
Nozzle voltage
0 V
MS parameters
Scan type
MRM
Prerun script
SCP_MSDiverterValveToWaste()
{MH_Acq_Scripts.exe}
Time segments
#1: 1.8 min - diverter valve to MS
Delta EMV (+)
400 V
Table 1 shows the MRM transitions for one quantifier and one qualifier product ion for the target compounds, and their deuterated internal standards.
Results and Discussion
At low pH, buprenorphine and norbuprenorphine are protonated at the tertiary amine group and strongly retained on Agilent Bond Elut Plexa PCX polymeric sorbent by a combination of hydrophobic retention and strong cation exchange.
A 100% methanol wash led to partial loss of analytes from the SPE column. The optimum wash that efficiently removed most matrix interferences without loss of analytes proved to be 70 MeOH:30 2% formic acid. A strong base is added to the organic eluent to break the ionic interaction between the analytes and the strong cation-exchange sorbent. The recovery of buprenorphine and norbuprenorphine was optimized with 5% NH4OH added to the combination eluent (80 ethyl acetate: 20 isopropanol) shortly before sample elution. Two-step elution with a soaking procedure is recommended to enhance the solvent-analyte interaction and improve analyte recoveries.
Due to high hydrophobicity, buprenorphine and norbuprenorphine can adhere to glassware, LC tubing, and injector parts, which is why we recommend a 95% MeOH column rinse in the LC method and
95 MeOH:5 water flushing solution for the flushport needle rinse. Deactivated vials/inserts and MeOH‑rinsed/air-dried glassware (both tubes and bottles for STD/ISTD dilutions) also ensure reproducible results.
The LC separation intentionally begins with a relatively low fraction of organic solvent (15%) to allow salts and other polar components of blood to elute at the beginning of the sample run. A flow rate of 0.8 mL/min allows for a short retention and re-equilibration time. Each sample run begins with diverting a first portion of flow
(0 to 1.8 minutes) to waste to minimize source contamination. Data collection begins at 1.8 minutes, immediately after the diverter valve switch.
Chromatograms for buprenorphine and norbuprenorphine at the LOQ of 0.2 ng/mL and corresponding deuterated internal standards at 10 ng/mL are shown in
Figure 2.
The high stability of molecular ions of both buprenorphine and norbuprenorphine presents a challenge for MS/MS detection [3,9]. It led many researchers to quantitation in SIM mode [2,8], or in SRM mode by monitoring a molecular ion > molecular ion transition at relatively high collision energy without fragmentation [3,9]. Compared to a more selective quantitation by a parent-product transition, this approach is less reliable. It results in a much higher signal-to-noise (S/N) ratio and, therefore, in a higher lower limit of quantification (LLOQ). MS-MS transitions most commonly used for buprenorphine/norbuprenorphine quantification were 468 to 414, 396 m/z for buprenorphine, and 414 to 396, 340 and
101 m/z for norbuprenorphine
[2, 3, 4, 5, 6, 7]. A new stable fragmentation pattern achieved with an Agilent 6460 Triple Quadrupole LC/MS System (Table 1) at high collision energy levels allows for a reliable quantitation with an LLOQ of 0.2 ng/mL for both analytes. The most abundant fragment of buprenorphine is the methylocyclopropyl (C4H7) group with m/z 55.1. Its identification is confirmed by a fragment of buprenorphine-D4 with m/z 59.1. The most abundant product of norbupenorphine fragmentation (m/z 83.1) probably comes from the branched side chain of the parent ion and includes the tert-butyl group (CH3)3C. Compared to most commonly used fragmentation products obtained at their optimum collision energies, m/z 55.1 is a 8× more abundant product of buprenorphine than m/z 396.2, while 83.1 is a 2× more abundant product of norbuprenorphine than m/z 101.1.
MRM transitions listed in Table 1 are for one quantifier and one qualifier product ion for both target compounds and their deuterated ISTDs. Agilent MassHunter software automatically calculates qualifier ion ratios, highlighting those out of the acceptable range. Either normal or dynamic MRM acquisition modes can be used with this method.
S/N ratios at the LLOQ level of 0.2 ng/mL were 84:1 for buprenorphine and 20:1 for norbuprenorphine Figures 2, A and B). This illustrates the efficiency of a sample cleanup procedure and the excellent sensitivity of the 6460 Triple Quadrupole, capable of detecting these analytes with LODs way below 0.1 ng/mL.
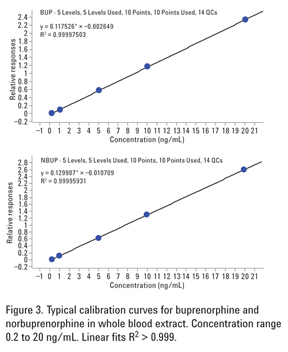
Figure 3 shows typical calibration curves for buprenorphine and norbuprenorphine in extracted whole blood standards at five concentration levels. Calibration standards were prepared by spiking whole blood with analytes at 0.2, 1, 5, 10, and 20 ng/mL. Deuterated internal standards were added at 10 ng/mL. Excellent linear fit (R2 > 0.999) to each of the curves demonstrates linearity of the method. No weighting was applied, and the origin was included in the curve fit.
Table 2 shows recovery (accuracy) and precision (CV, or RSD) data collected for five samples of whole blood fortified with
1 ng/mL of each analyte. Quantitation was performed against calibration curves obtained from the spiked matrix standards (Figure 3).
Conclusions
A simple, solid phase extraction procedure coupled with an LC/MS/MS detection method allows determination of buprenorphine and norbuprenorphine in whole blood at concentrations below
0.2 ng/mL. This method is intended for users of Agilent 1100 and 1200 LC series since the backpressure in the LC system does not exceed 400 bar. Source parameters can be easily modified to use this method with other models of Agilent Triple Quadrupole LC/MS System instruments. Low detection limits are achieved due to cleanliness of sample extracts and robust MS detection using newly identified ion transitions with abundant fragmentation products.
References
- Baselt, R. (2008) Disposition of Toxic Drugs and Chemicals in Man. 8th edition. Atlas Books, Ashland, OH, USA.
- Concheiro, M., Shakleya, D. M. and Huestis, M. A. (2009) Simultaneous quantification of buprenorphine, norbuprenorphine, buprenorphine-glucuronide and norbuprenorphine-glucuronide in human umbilical cord by liquid chromatography-tandem mass spectrometry Forensic Science International, 188 (1-3): 144–151.
- Kronstrand, R., Selden, T. G. and Josefsson, M. (2003) Analysis of buprenorphine, norbuprenorphine, and their glucuronides in urine by liquid chromatography–mass spectrometry. Journal of Analytical Toxicology, 27: 464–470.
- Miller, E. I., Torrance, H. J. and Oliver, J. S. (2006) Validation of the Immunalysis microplate ELISA for the detection of buprenorphine and its metabolite norbuprenorphine in urine. Journal of Analytical Toxicology, 30:115–119.
- Moody, D. E., Slawson, M. H., Strain, E. C., Laycock, J. D., Spanbauer, A. C. and Foltz, R. L. (2002) A liquid chromatographic–electrospray ionization-tandem mass spectrometric method for determination of buprenorphine, its metabolite, norbuprenorphine, and a coformulant, naloxone, that is suitable for in vivo and in vitro metabolism studies. Analytical Biochemistry, 306: 31–39.
- Moore, C., Coulter, C. and Crompton, K. (2007) Determination of buprenorphine, norbuprenorphine and their glucuronides in urine using LC/MS/MS. Agilent application note 5989-7072EN. Agilent Technologies, Inc.
- Øiestad, E. L., Johansen, U., Øiestad, A. M. L. and Christophersen, A. S. (2011) Drug screening of whole blood by ultra-performance liquid chromatography-tandem mass spectrometry. Journal of Analytical Toxicology, 35: 280-293.
- Scislowski, M., Piekoszewski, W., Kamenczak, A. and Florek, E. (2005) Simultaneous determination of buprenorphine and norbuprenorphine in serum by high-performance liquid chromatography–electrospray ionization-mass spectrometry. Journal of Analytical Toxicology, 29: 249–253.
- Selden, T., Roman, M., Druid, H. and Kronstrand, R. (2011) LC–MS–MS analysis of buprenorphine and norbuprenorphine in whole blood from suspected drug users. Forensic Science International, 209: 113–119.
For More Information
These data represent typical results. For more information on our products and services, visit our Web site at www.agilent.com/chem.
©Agilent Technologies, Inc., 2013
February 20, 2013
View this Application Note in its entirety: 5990-9930EN